INTRODUCTION
Heart failure (HF) is not a single medical condition, but a clinical syndrome characterized by various symptoms, including shortness of breath and extreme fatigue. Other possible signs include peripheral edema, pulmonary rales, and elevated jugular venous pressure. HF arises from pathological changes in heart structure or function, leading to increased intracardiac pressure or inadequate blood flow during both rest and activity, often accompanied by elevated levels of natriuretic peptides[1,2]. There are four types of HF according to the 2022 American Heart Association (AHA)/American College of Cardiology (ACC)/HF Society of America guideline for the management of HF based on left ventricular ejection fraction (LVEF): HF with preserved ejection fraction (LVEF ≥ 50%), mildly reduced EF (LVEF 41%-49%), reduced EF (HFrEF, LVEF < 40%), and HF with improved EF (referring to those patients who had an initial LVEF < 40% and there was an increase with at least 10% compared to the first evaluation[3].
As reported in the PARADIGM-HF study, the risk of all endpoints increases as LVEF declines. Specifically, for every 5-point drop in LVEF, the risk of cardiovascular death or hospitalization due to HF complications rises by 9%, while the risk of death from any cause increases by 7%[4]. The primary causes of HF typically include coronary artery disease, valvular heart disease, and arrhythmias. However, this review aims to highlight the growing recognition of cardiomyopathies as a significant cause of HF, with a particular focus on a specific genetic cardiomyopathy, namely nexilin (NEXN) cardiomyopathy.
CURRENT KNOWLEDGE ABOUT CARDIOMYOPATHIES
Cardiomyopathy is a condition characterized by structural and functional abnormalities in the heart muscle, occurring in the absence of coronary artery disease, hypertension, valvular heart disease, or congenital heart defects that could otherwise explain signs and symptoms of HF[5]. Cardiomyopathy phenotypes are characterized by various morphological and functional traits, such as left and/or right ventricular hypertrophy or dilation, myocardial scarring, or fatty infiltration. Additional defining features include family history, arrhythmias, genetic testing, biochemical markers, and other coexisting medical conditions. Based on these characteristics, cardiomyopathies are classified into several types: Dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), arrhythmogenic right ventricular cardiomyopathy, restrictive cardiomyopathy, and non-dilated left ventricular cardiomyopathy[5,6].
Cardiomyopathies are generally categorized into two main groups based on the organ affected. Primary cardiomyopathies predominantly impact the heart muscle and can be hereditary (genetic), non-genetic, or acquired. In contrast, secondary cardiomyopathies result from pathological damage to the myocardium due to systemic illnesses with multi-organ involvement[7,8]. Standard treatment protocols are recommended for patients with HFrEF, regardless of the underlying etiology. However, certain cardiomyopathies may benefit from targeted therapies that address their specific causes. These treatments may include enzyme replacement therapy, transthyretin stabilizers, gene silencing, or immunotherapy, potentially improving patient outcomes[9]. This paper will focus exclusively on the genetic forms of DCM and HCM, exploring the nuances of these cardiomyopathies in the context of HF, and specifically examining NEXN cardiomyopathy. Currently, clinical data on patients with NEXN mutations are limited, and the relationship between this gene and DCM or HCM remains under investigation[10]. Reported manifestations of NEXN-related cardiomyopathy range from transient DCM[11] to severe reductions in LVEF requiring heart transplantation[12].
DCM and HF
DCM is characterized by left or both ventricular dilation and systolic dysfunction that cannot be attributed to abnormal loading conditions or coronary artery disease. It is the leading cause of HFrEF, with an estimated prevalence of 1 in 250 individuals and an incidence reported with 5-7 cases per 10000 persons[13], many patients eventually requiring a heart transplant[6]. Recent findings suggest that DCM might be more prevalent than previously believed, potentially surpassing HCM in frequency[13]. Without treatment, the prognosis is highly unfavorable, with 1-year survival of 70%-75% and at 5 years half of the patients are deceased[14]. Pathophysiologically, DCM is marked by dilation and a spherical deformation of the LV, which reduces stroke volume and cardiac output, while also impairing diastolic function by increasing end-diastolic pressure[15]. Generally, female patients with DCM tend to have a more favorable prognosis compared to males, potentially due to less severe LV dysfunction[16].
The initial signs of HF in DCM typically appear between ages 30 and 40. Usually patients with DCM present with typical symptoms of HF such as dyspnea, fatigue, peripheral edema, but some of them describe symptoms of arrhythmia such as palpitations, syncope/presyncope or even have as first manifestation of the disease a cardiac arrest. In a minority of cases the disease is diagnosed due to incidental findings (abnormal electrocardiogram and murmur) or during the family screening. Older age is an additional risk factor for mortality, with a 5-year mortality rate of approximately 50%[17]. Accurate diagnosis of DCM requires ruling out common causes of LV dysfunction. Less common cardiomyopathies, such as LV non-compaction cardiomyopathy (LVNC) or peripartum cardiomyopathy, may mimic DCM phenotypically[18]. LVNC is still a subject of disagreement, being classified as a hereditary cardiomyopathy by the ACC[7] contradictorily to the European Society of Cardiology Guidelines which consider that LVNC is a morphological trait[5]. The LV hypertrabeculation pattern may exhibit similarities with other forms of cardiomyopathy, and individuals with DCM may display morphological characteristics associated with this phenotype[19].
A LVEF of 35% or below is not always sensitive or specific in predicting sudden cardiac death (SCD). Therefore, additional imaging predictors, along with clinical factors such as age and genetics, are necessary. Indicators such as cardiac fibrosis and global longitudinal strain offer additional predictive value beyond LVEF[20]. For instance, global longitudinal strain may be reduced even when LVEF is normal in individuals with sarcomeric mutations associated with DCM[21]. Cardiac magnetic resonance imaging is recommended for all DCM patients, utilizing late gadolinium enhancement (LGE), T1/extracellular volume (ECV), and T2 parameters. This imaging technique reveals structural abnormalities, storage disorders, edema, inflammation, and scarring (fibrosis). Elevated LGE levels and strain anomalies, particularly in the LV and left atrium (LA), provide valuable information for assessing SCD and HF risk[5,22].
Despite significant advances in HF treatment, mortality rates for DCM remain high, primarily due to HF progression and arrhythmic events leading to SCD[18]. DCM is the most common cardiomyopathy progressing to HF, often resulting in HFrEF, with HF being a major cause of death in these patients[9]. DCM patients may follow one of three clinical trajectories: Recovery of both heart structure and function after initiating specific treatment, a symptom-free period with improved or stabilized LVEF, or progression to end-stage HF requiring a heart transplant or LV assist devices[23].
Prior to the era of guideline-directed medical therapy, data indicated that less than 20% of DCM patients experienced substantial clinical improvement, with 77% dying within two years of diagnosis[24]. In contrast, guideline-directed medical therapy patients had a significantly better prognosis, with a two-year survival rate of 92% and fewer hospitalizations due to HF complications[25], but there are various elements that can influence prognosis [the type of mutation(s), age of symptoms appearance, severity of symptoms, comorbidities, arrhythmia burden, fibrosis expanse, functional capacity, the degree of impairment in left and right ventricular contractility, LV enlargement, QRS duration, etc.] that suggest the need for development of a tailored prognostic calculator[26]. Optimal medical therapy (OMT), including agents such as angiotensin-converting enzyme inhibitors/angiotensin receptor-neprilysin inhibitors, beta-blockers, mineralocorticoid receptor antagonists, and sodium-glucose co-transporter 2 inhibitors, combined with loop diuretics for fluid retention, provides documented benefits for DCM patients[1,27-32]. Implantable devices, particularly implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy (CRT) devices, also offer significant advantages[1,9].
Patients with non-ischemic DCM and symptomatic HFrEF (LVEF ≤ 35%, New York Heart Association class II-III) despite over three months of OMT may benefit from an ICD to reduce SCD risk (class IIa level of recommendation)[1]. The DANISH trial found that ICD implantation did not improve overall survival, but reduced SCD risk by 50%[33]. Addressing LV mechanical dyssynchrony, which affects 15%-30% of DCM patients, can significantly improve morbidity and mortality; therefore, CRT should be considered for symptomatic HF patients with LVEF ≤ 35% and QRS duration greater than 130 ms, particularly those with left bundle branch block (LBBB) morphology (class IIa level of recommendation)[1,34,35]. The COMPANION study demonstrated that upgrading to a CRT with defibrillator in non-ischemic DCM patients already receiving OMT resulted in a more significant reduction in all-cause mortality[1,35]. Looking ahead, the emerging field of “imagenetics”, which combines gene editing techniques with advanced cardiac imaging and artificial intelligence, is expected to greatly enhance the treatment and management of DCM patients[36].
HCM and HF
HCM is characterized by morphological features such as a thickened LV wall, which may occur with or without right ventricular hypertrophy, or an increased LV mass[5]. The primary diagnostic tool for HCM is transthoracic echocardiography, which identifies unexplained LV wall hypertrophy in the absence of other systemic or cardiac conditions, such as arterial hypertension or aortic valve stenosis[7]. Typically, HCM presents as asymmetric hypertrophy affecting the interventricular septum, with 40%-70% of patients developing obstructive HCM. This obstruction is diagnosed when there is an intraventricular gradient greater than 30 mmHg at rest or during activity. Conversely, non-obstructive HCM, with intracavitary gradients less than 30 mmHg, affects 30%-60% of patients[37-39]. Currently, HCM is understood to primarily involve the sarcomere, but the relationship between genotype and phenotype is complex, influenced by both genetic and non-genetic factors[40]. Severe LV hypertrophy can be associated with various genetic conditions unrelated to cardiac sarcomere mutations. When diagnosing HCM, it is crucial to consider differential diagnoses such as glycogen or lysosomal storage disorders, mitochondrial mutations, amyloidosis, fatty-acid metabolism disorders, mutations in rat sarcoma-mitogen-activated protein kinase pathway genes, neuromuscular disorders, and lipodystrophic syndromes[39]. Another condition with similar phenotypical characteristics to HCM is “athlete’s heart”, where it is important to determine whether LV hypertrophy is a physiological adaptation to intense exercise or a pathological condition[41].
HF in HCM is less common compared to DCM, with HF with preserved ejection fraction being the most prevalent form. Most patients with obstructive HCM experience HF, whereas only 10% of those with non-obstructive HCM show symptoms or signs of HF. Timely identification of HF development is crucial due to the poor prognosis associated with advanced HF in HCM[9,42]. HF in HCM differs significantly from traditional congestive HF. Advanced HCM often presents with minimal or no signs of pulmonary or peripheral congestion, meaning these patients are typically ambulatory and rarely require hospitalization for acute HF, with most exhibiting preserved LVEF[39,42,43].
Mitral valve anomalies are also common in HCM, affecting a significant number of patients. Besides systolic anterior motion, structural abnormalities such as elongated leaflets or defects in the sub-mitral apparatus can contribute to LV outflow tract obstruction. Patients unresponsive to maximal pharmacological treatment should be referred to specialized HCM centers, where surgical mitral valve repair combined with septal myomectomy is considered optimal[44,45]. Additionally, conditions such as coronary myocardial bridging, apical aneurysms, atrial remodeling, and autonomic dysfunction may exacerbate HF symptoms[46]. A study over nine years indicated that each 5-mm increase in LA diameter correlates with a 20% increase in all-cause mortality, though this has not been linked SCD risk[47]. LV aneurysms, often resulting from apical myocardial ischemia (sometimes due to myocardial bridging), increase the risk of HF and ventricular arrhythmias. While the correlation between apical aneurysms and SCD risk remains debated, there are recommendations for anticoagulants in patients with large LV aneurysms[46,48].
Until recently, treatment for HCM focused on symptom management with beta-blockers, calcium channel blockers, lifestyle adjustments, septal reduction surgery, or ICDs for primary and secondary prevention of SCD[49]. Patients with HCM and LBBB which develop end stage/dilated HCM could gain benefit from CRT with New York Heart Association class improvement, LV revers remodeling and increase in LVEF[50]. Advances in understanding the pathophysiology and molecular mechanisms of HCM have led to new treatment options, including myosin modulators, such as mavacamten and aficamten[49]. These innovations have significantly reduced mortality in HCM patients to 0.5% annually[51]. For patients without LV outflow tract obstruction who develop severe HF and have poor prognoses due to hypokinetic or restrictive phenotypes and inadequate responses to traditional HF treatments, heart transplantation may be the only viable option[46,52].
Risk stratification for SCD remains a primary concern for HCM patients. The European Society of Cardiology recommends using the HCM Risk-SCD score, which includes variables such as family history of SCD, LV outflow tract gradient, maximal LV wall thickness, LA diameter, age, and evidence of non-sustained ventricular tachycardia. A score below 4% indicates low risk for SCD in 5 years, 4%-6% suggests intermediate risk with potential ICD consideration, and a score above 6% indicates high risk, warranting ICD implantation[39]. The 2020 ACC/AHA guidelines introduced additional predictors for risk stratification: Extensive fibrosis (≥ 15% of LV mass) on LGE-cardiac magnetic resonance (CMR), presence of LV aneurysms, and systolic dysfunction (LVEF < 50%). These are to be considered alongside unexplained syncope, LV wall thickness ≥ 30 mm in adults, family history of SCD related to HCM, and non-sustained ventricular tachycardia. If any of these conditions are present, the patient has a class 2a indication for receiving an ICD, with a class 2b recommendation for those with extensive LGE on CMR[53].
GENETIC CAUSES OF CARDIOMYOPATHIES
Over the past thirty years, our understanding of the etiology of cardiomyopathies has advanced significantly, largely due to genetic studies. HF, one of the leading causes of death in developed nations, is predominantly caused by hereditary factors in most cases of primary cardiomyopathies. Although continuously evolving genetic technologies aid in diagnosing these conditions, distinguishing between pathogenic (P), likely-pathogenic (LP), and benign variants remains challenging. Additionally, even among family members, there can be considerable variability in penetrance, age of onset, and clinical manifestations of a particular phenotype when a variant is identified. Given the critical importance of this field, targeted genetic diagnostics could provide solutions for developing personalized treatments for affected patients[6].
Genetic testing for genes associated with mendelian cardiomyopathies has become a routine part of clinical care for patients and their families. Initial testing should focus on genes strongly associated with the observed traits. However, if initial testing fails to identify a cause despite a strong suspicion of a monogenic origin, further sequencing may be necessary. Expanding the gene panel may not necessarily increase the likelihood of identifying P/LP variants, but it may identify variants of uncertain significance[54]. When a genetic cause is identified in one family member, other relatives should be tested for the same variant[5]. HCM results from various mutations affecting genes responsible for producing contractile proteins within the cardiac sarcomere, with over 400 distinct variations currently recognized. Eleven genes are directly linked to HCM, with myosin heavy chain 7 (MYH7) and myosin-binding protein C being the most frequently observed. The remaining nine genes are associated with fewer HCM cases and include troponin T (TNNT), TNNI, regulatory myosin light chain, essential myosin light chain, titin, α-tropomyosin, α-actin, MYH6, and muscle LIM protein[7]. The variability in HCM characteristics can be linked to disease-causing genes as well as potential influences from environmental factors and gene modifiers[40], such as angiotensin-converting enzyme and components of the renin-angiotensin-aldosterone system, including angiotensinogen, angiotensin II receptor type 1, and aldosterone[55]. As genomic interaction technologies advance, we can anticipate emerging treatments aimed at correcting or disabling aberrant genes and transcripts associated with cardiomyopathies[5]. Sarcomeric mutations are responsible for the decreased effective contractile function caused by the reduced myofilament calcium sensitivity in both HCM and DCM subjects. Beyond the contractile impairment mechanism mentioned above, force transduction defect by disfunction of mechanosensing is another deficient pathway involved in the pathogenesis of HF in cardiomyopathies, mainly by mutations in titin, desmin and dystrophin proteins[56].
Genetic causes of DCM have an important role in the pathophysiology of the disease, DCM being recognized as familial in 30%-40% of cases. The inheritance pattern of familial DCM is typically autosomal dominant, suggesting monogenic or mendelian disease, but autosomal recessive, X-linked and mitochondrial inheritance are also observed. Due to incomplete and age-dependent penetrance, clinical expression differs substantially, even within the same family[50]. The genetic basis of DCM is associated with an increased susceptibility to SCD. Specific genes, such as lamin A/C, RNA-binding motif protein 20, filamin C, and desmoplakin, are linked to higher risk. The 2022 European Heart Rhythm Association consensus recommends that initial genetic testing for DCM should include genes with definitive and moderate evidence of causing the disease. This includes BCL2-associated athanogene 3, desmin, filamin C, lamin A, MYH7, phospholamban, RNA-binding motif protein 20, sodium voltage-gated channel alpha 5 subunit, TNNC1, TNNT2, titin, and desmoplakin. Additionally, actin alpha cardiac muscle 1, actinin alpha 2, junctophilin 2, NEXN, TNNI3, α-tropomyosin, and vinculin genes should also be considered for testing[57].
Notably, while the NEXN gene is categorized with moderate evidence in DCM, there is no solid evidence linking it to HCM, and it is consequently not included in HCM panels. The Z-disk plays a crucial role in the structure and function of striated muscle cells, particularly in the heart. It anchors thin actin filaments, providing structural integrity to sarcomeres, and facilitates the reception and transmission of biochemical signals between cells[58]. Research has increasingly focused on the Z-disk in HF due to the identification of mutations in genes encoding its components that can lead to cardiomyopathies. NEXN, a cardiac Z-disk protein encoded by the NEXN gene (MIM 613121) on chromosome 1, was first identified in 1998 by Ohtsuka et al[59].as a novel filamentous actin-binding protein. Variants in the NEXN gene have been associated with cardiomyopathies (DCM, MIM 613122, and HCM, MIM 613876), sudden infant death syndrome, LVNC[60] and idiopathic ventricular fibrillation[12]. Both homozygous and heterozygous mutations are implicated in cardiomyopathy development, though heterozygous variants generally result in less severe manifestations. We present herein the case of a patient with rapid progression towards DCM with severe dysfunction of LV associated with an NEXN in-frame mutation.
CLINICAL CASE
Patient presentation and initial workup
A 63-year-old male with a medical history of grade 3 arterial hypertension, type 2 diabetes mellitus, dyslipidemia, and mild obstructive sleep apnea is evaluated by a cardiologist for a routine assessment. The patient is on a chronic medication regimen that includes aspirin (100 mg once daily), atorvastatin (20 mg once daily), bisoprolol (2.5 mg once daily), indapamide (1.5 mg once daily), telmisartan (40 mg twice daily), and empagliflozin/metformin (5/1000 mg twice daily). The patient reports intermittent palpitations but has no history of angina or dyspnea. One month ago, he experienced a syncope, which emergency physicians attributed to a vasovagal episode. His family history reveals a first-degree cousin who died suddenly at the age of 40, but no additional medical information is available for other family members.
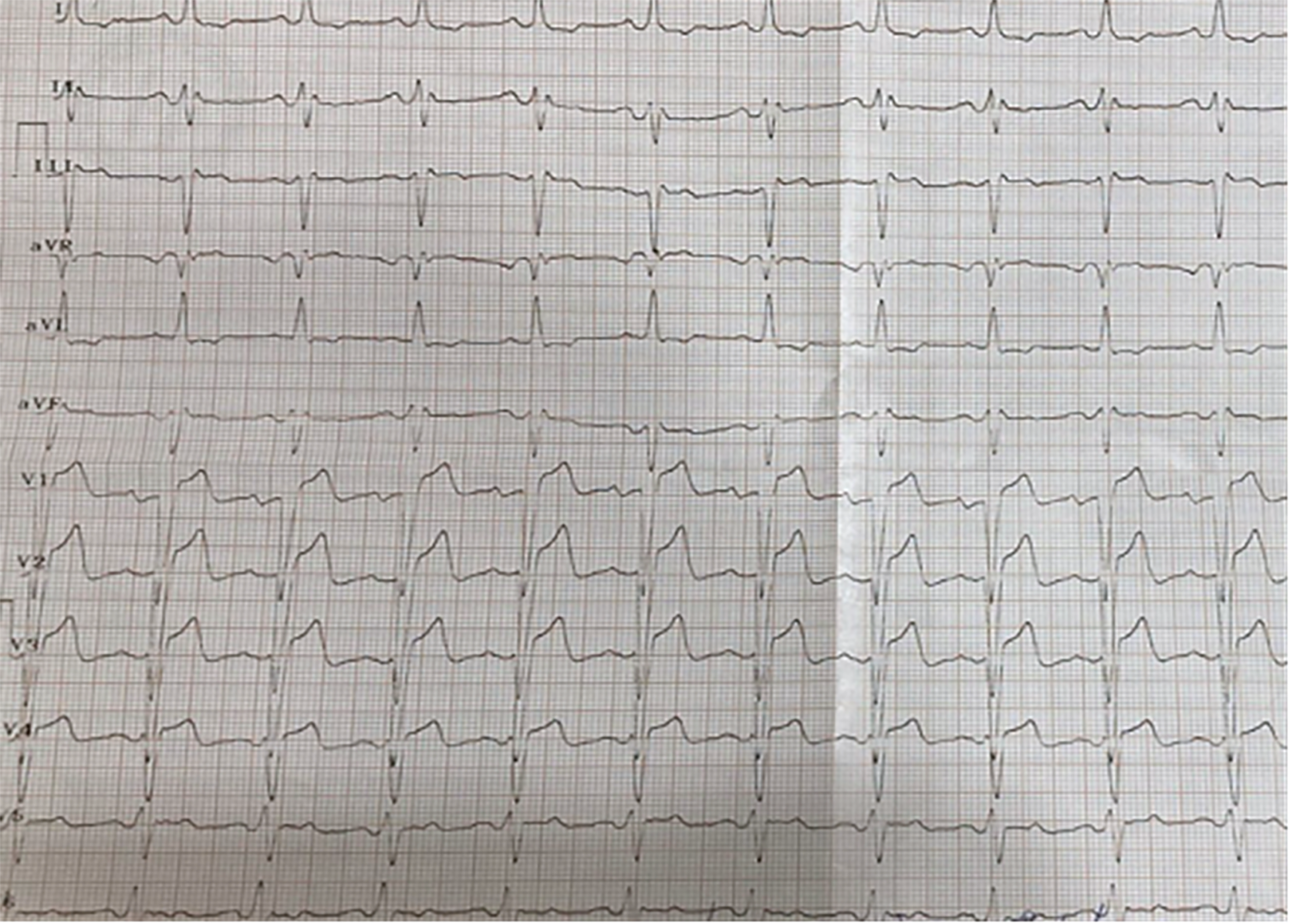
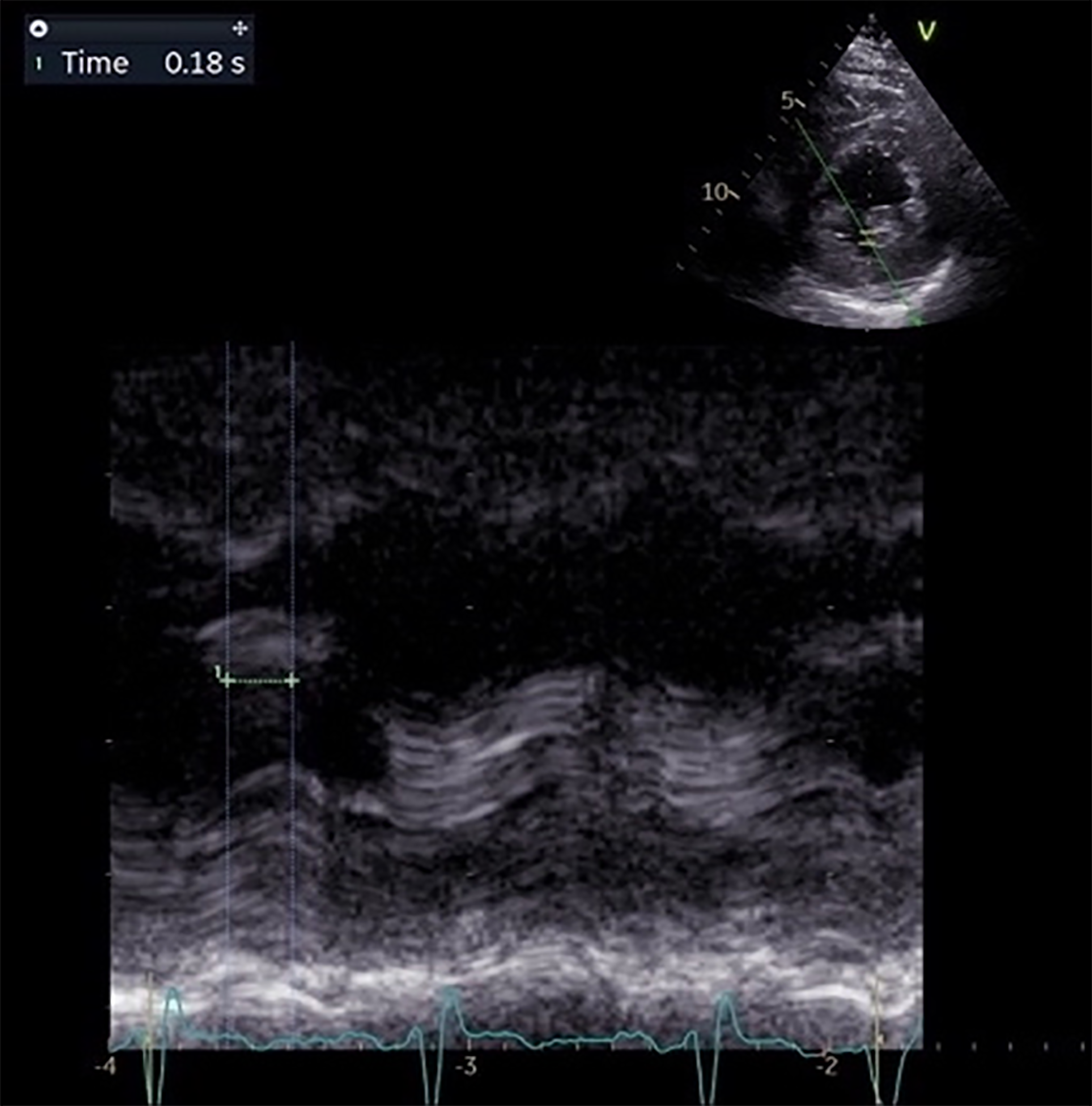
Physical examination revealed no abnormalities. A 12-lead electrocardiogram showed sinus rhythm with an intraventricular conduction delay, evidenced by a QRS duration of 130 milliseconds, resembling a LBBB (Figure 1). A cardiac ultrasound at that time indicated a normal LV diameter (53 mm) and LV volumes (62 mL/m²), slightly increased wall thickness (interventricular septum and posterior wall of 14 mm), preserved LVEF of 54%, and no intraventricular asynchrony (septal-to-posterior wall motion delay of 110 milliseconds). After six months, the patient was re-evaluated, now reporting dyspnea and fatigue with moderate exertion. Follow-up cardiac echocardiography revealed a dilated LV (81 mL/m²), diffuse hypokinesia of the LV walls, and severe LV dysfunction (LVEF of 30%). Significant intraventricular asynchrony was noted, with a septal-to-posterior wall motion delay of 180 milliseconds (Figure 2), apical rocking, and septal flash. Blood tests showed elevated N-terminal fragment brain natriuretic peptides at 463 pg/mL.
Figure 1
The 12-lead electrocardiogram showing sinus rhythm and non-specific intraventricular conduction delay.
Figure 2 Transthoracic echocardiography in anatomic M-mode, parasternal short axis at papillary muscles view.
Septal to posterior wall motion delay: 180 ms.
Diagnostic
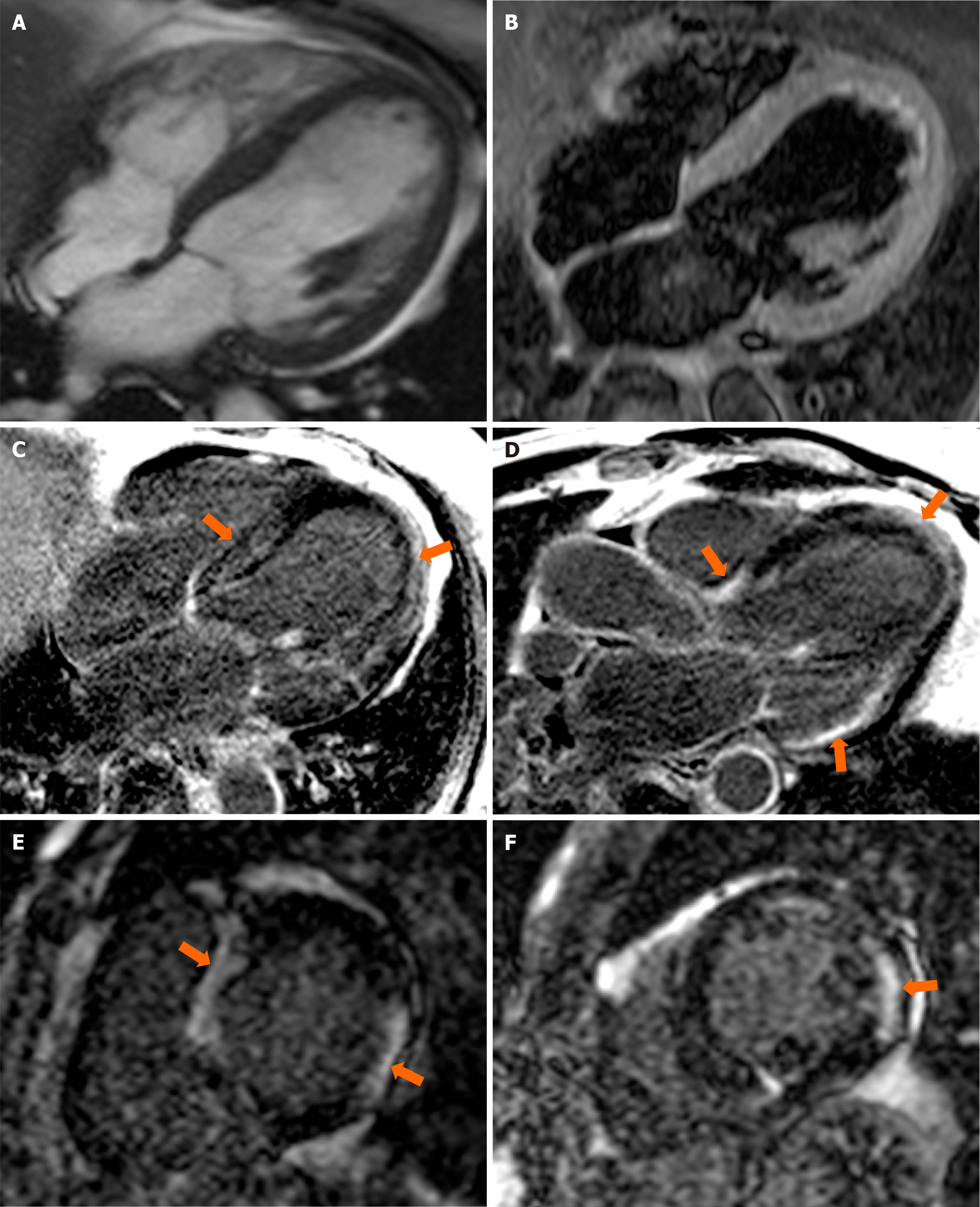
Given the unfavorable clinical and echocardiographic progression over the past six months, a comprehensive evaluation was conducted, including coronary angiography, which ruled out ischemic causes as no significant angiographic lesions were identified. The evaluation continued with CMR, revealing a dilated (indexed telediastolic LV volume of 114 mL/m²) and hypertrophied LV (98 g/m²), with systolic dysfunction secondary to diffuse hypokinesia of the LV walls and intraventricular dyssynchrony. The right ventricular was not dilated and exhibited normal systolic function. Extensive areas of focal sub-epicardial, mid-myocardial, and sub-endocardial fibrosis were observed in the LV walls, predominantly in the interventricular septum and inferolateral wall (Figure 3). There was no evidence of myocardial edema or scarring suggestive of myocarditis. These findings were consistent with a diagnosis of DCM with LV systolic dysfunction and extensive focal myocardial fibrosis, indicating a possible genetic etiology.
Figure 3 Contrast enhanced cardiovascular magnetic resonance.
A: Balanced steady state-free precession cine diastolic frame in 4-chamber (4C) view, showing dilated left ventricle with mild hypertrophy; B: T2 weighted sequence in 4C view showing homogenous signal of the myocardial - suggestive of no myocardial oedema; C-F: Late gadolinium enhancement in 4C, 3C and short axis views respectively, showing extensive areas of intramyocardial hyperenhancement without coronary topography (orange arrows).
The patient was referred for genetic testing using an extensive cardiomyopathy panel, which identified a heterozygous in-frame deletion variant in the NEXN gene [NEXN c.1949_1951del, p.(Gly650del)], classified as LP based on several criteria: The established genotype-phenotype association, the rarity of the variant in control populations, the identification of the variant as homozygous in one individual with autosomal recessive NEXN-related disease, and functional assay data. Next-generation sequencing was employed for the genetic analysis, utilizing the blueprint genetics cardiomyopathy panel, which identified only this variant. A total of 217 genes were sequenced, including 180 cardiomyopathy-related genes and 37 mitochondrial genes, with optimal coverage and sensitivity. Variant classification followed the Blueprint Genetics Variant Classification Schemes, which are modified from the American College of Medical Genetics/Association for Molecular Pathology guidelines (2015)[61]. Genetic counseling was offered to the patient and the family, as these are at a 50% risk of inheriting the variant. The patient has one asymptomatic daughter with normal electrocardiogram and echocardiography who declined genetic testing.
Patient management
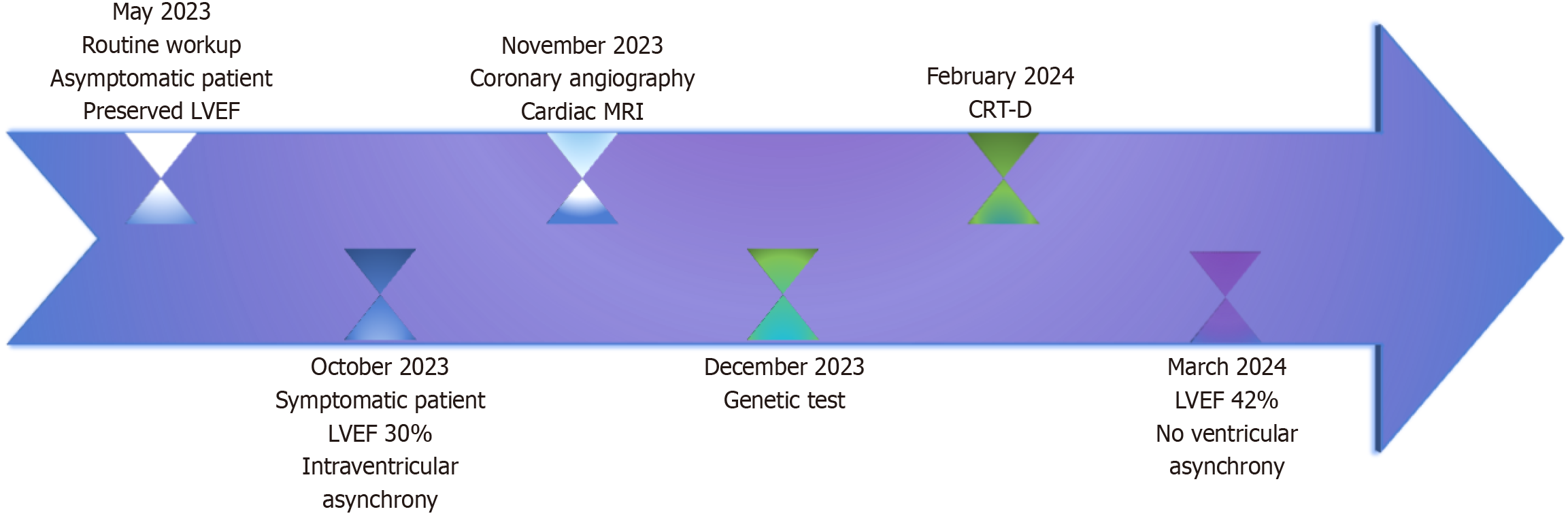
Given the clinical and paraclinical data and the patient’s rapid progression toward HF with reduced LVEF, the case was reviewed by a heart team. This team included an expert in cardiomyopathy and HF, as well as an electrophysiologist, to determine the optimal treatment strategy. Pharmacological treatment was optimized to include sacubitril/valsartan (initiated at a dose of 49/51 mg twice daily, titrated up to the target dose of 97/103 mg twice daily with good tolerance), a beta-blocker (bisoprolol 10 mg once daily), a mineralocorticoid receptor antagonist (spironolactone 25 mg once daily), and a sodium-glucose co-transporter 2 inhibitor (empagliflozin 10 mg once daily, in combination with metformin) according to the latest HF guidelines[1]. The treatment regimen was complemented with aspirin and a statin. Given the patient’s DCM with severe systolic dysfunction (LVEF 30%) and wide QRS complex (130 ms), a CRT with defibrillator was implanted as primary prevention for SCD (IIa class of recommendation, level A evidence). Follow-up at three months post-resynchronization therapy and optimized HF treatment showed an improvement in LVEF to 42% (assessed by Simpson biplane) and a successful reduction in intraventricular asynchrony. Periodic evaluations, including clinical examinations, repeated electrocardiograms, and echocardiographic assessments, were performed at our clinic. A summary timeline of the key steps in the patient’s management is illustrated in Figure 4.
Figure 4 Timeline depicting the essential stages of the patient’s diagnosis.
CRT with defibrillator - cardiac resynchronization therapy device with defibrillator support. LVEF: Left ventricular ejection fraction; MRI: Magnetic resonance imaging; CRT-D: Cardiac resynchronization therapy with defibrillator.
DISCUSSION
Cardiomyopathies represent a significant group of causes for HF. Understanding the underlying causes and mechanisms of HF in each cardiomyopathy case is crucial for developing targeted treatments that can lead to improved outcomes. Despite this, data on NEXN cardiomyopathy as a cause of HF remain limited, which is why this paper focuses on the topic. Almost two decades ago, Hassel et al[10] demonstrated that NEXN is a component of the Z-disk, and its loss leads to DCM in a zebrafish model. NEXN not only binds to actin but also interacts with ryanodine receptor 2 and junctophilin 2, both essential for T-tubule formation and calcium homeostasis[62]. In the same study, Hassel et al[10] identified three variants [p.(Tyr652Cys), Gly650del, and p.(Pro611Thr)] associated with DCM in adults. In a mouse model, the homozygous G645del variant resulted in the expression of only about 30% of NEXN compared to wild type and induced progressive DCM, underscoring the crucial role of NEXN in cardiac function[10]. Liu et al[62,63] demonstrated that global NEXN knockout mice (using Sox2-cre-loxP) and specific cardiomyocyte knockout NEXN mice (using XMLC2-Cre or Tnnt2-cre) developed severe, progressive DCM with cardiac dysfunction and increased fibrosis. In 2021, the ClinGen DCM Working Group evaluated NEXN as having moderate evidence supporting its role in this gene-disease relationship[64]. Less commonly, NEXN mutations have been associated with other phenotypes, such as HCM[65], LVNC[60,66], and sudden infant death syndrome or idiopathic ventricular fibrillation[12]. ClinVar (as of April 2024) lists at least 31 NEXN variants classified as P or LP, with most being loss-of-function variants (nonsense, splice site, or frameshift mutations).
Supplementary Table 1 lists reported NEXN variants associated with cardiac phenotypes. According to the ClinGen DCM working group, 14 NEXN gene variants have been identified in DCM patients. Of 1000 DCM patients analyzed in Germany, six carried the p.(Gly650del) variant, which is associated with late-onset DCM (ages 35-51, average 51 years). One patient with both p.(Gly586del) and p.(Gly650del) variants (shared haplotype) developed progressive DCM within 10 years[10]. The ClinGen HCM Gene Curation Expert Panel and Hereditary Cardiovascular Disease Gene Curation Expert Panel have found limited evidence supporting the gene-disease relationship for HCM, with only a few specific missense HCM-related variants described in 2010 and one additional variant reported as disease-causing. High-frequency NEXN variants were ultimately classified as B[65].
The case report detailed above, involving a DCM diagnosis, identified the G650del variant, which results in the deletion of a highly conserved amino acid in the C-terminal IGcam domain of NEXN (glycine at position 650) without altering the reading frame. This variant is significantly more prevalent in DCM patients (6/994) compared to the general population (168/1613646, odds ratio: 57.9, P < 0.0001)[10]. Although there is substantial evidence supporting the pathogenicity of the NEXN p.(Gly650del) variant, the allele frequency in the Genome Aggregation Database version 4 (168 out of 1613646 alleles) suggests that, while this mutation is LP when homozygous or in combination with other disease-causing NEXN variants, it may act as a variably expressive or mildly penetrant variant of dominant DCM. According to American College of Medical Genetics/Association for Molecular Pathology guidelines (2015), this mutation is classified as LP based on one strong (PS3) and one moderate (PM4) criterion: PS3-strong (both in vitro and in vivo studies have shown that human hearts with this variant exhibit disrupted sarcomeres and abnormal Z-discs, also observed in zebrafish embryos with severe cardiac dilation)[10], and PM4-moderate (protein length change due to in-frame deletion in a non-repeat region), as well as PP3 (multiple in silico computational programs support a deleterious effect on the gene). Compared to the limited case reports and case-controls in the literature displaying NEXN variants[12], our patient experienced rapid progression of LV dysfunction within six months of diagnosis, with diffuse intramyocardial fibrosis. This highlights that both homozygous and heterozygous NEXN variants can be associated with severe phenotypes. Future considerations may include personalized treatments for patients with NEXN-related DCM. Recent studies suggest that gene replacement therapy in NEXN knockout mice with G645del restored cardiomyocyte function and extended lifespan, indicating that targeted gene therapy could address various loss-of-function NEXN variants[67].
This case emphasizes the need for further data to refine the management of individuals with NEXN variants and to enhance genetic counseling for their families. Even if current guidelines recommend that clinically stable patients with cardiomyopathies are followed-up every 1 to 2 years[5], in certain cases including here subjects with mutations in NEXN gene and extensive fibrosis, maybe the reevaluations should be done more frequently to promptly pinpoint asymptomatic progression of the disease. The key message for practicing physicians is to personalize the management of each cardiomyopathy patient including tailored monitoring and treatment including all the elements that could make a change (symptoms evolution, genetic mutations, fibrosis expense, electric or conduction disturbances).