Published online Feb 26, 2025. doi: 10.4330/wjc.v17.i2.100952
Revised: January 25, 2025
Accepted: February 8, 2025
Published online: February 26, 2025
Processing time: 177 Days and 23.9 Hours
Cardiac myxoma is a benign neoplasm and one of the most common types of primary cardiac tumors. Synchronous cardiac myxoma and other malignancies are extremely rare, and only limited cases have been reported.
We describe a young patient with newly diagnosed locally advanced laryngeal cancer, with a synchronous cardiac tumor detected on staging scans. An echocardiogram showed the typical appearance of myxoma in the left atrium. Early cardiac surgery was performed in view of its obstructive features and post cardiac surgery recovery was uneventful. The patient was scheduled for subsequent oncological treatment for the laryngeal cancer. However, due to rapid progression of the advanced laryngeal malignancy, he was placed on supportive care.
To our knowledge, this is the first reported case of synchronous cardiac myxoma with laryngeal malignancy. Individualized treatment strategy should be adopted to manage synchronous tumors in a multidisciplinary approach. The most life-threatening condition needs be treated first. Single resection, staged operations or simultaneous resection of both tumors have been reported with good outcomes.
Core Tip: Cardiac myxoma is the one of the most common types of primary cardiac tumors. It is most frequently located in the left atrium, and surgical resection is indicated to relieve hemodynamic obstruction or to prevent embolic events. Cases of synchronous cardiac myxoma with other malignancies have been rarely reported. Herein we report probably the first case in a patient with left atrial myxoma with concurrent locally advanced laryngeal cancer, for whom early cardiac surgery was performed. A multidisciplinary approach is crucial to manage synchronous tumors, and the most life-threatening condition needs to be treated first. Optimal results have been reported after surgical resection of myxoma. The long-term prognosis, however, is mainly determined by the nature of the concurrent malignancy.
- Citation: Zhu L, Neo JYZ, Punjabi LS, Lai SH, Chua YL. Large left atrial myxoma with synchronous laryngeal squamous cell carcinoma: A case report. World J Cardiol 2025; 17(2): 100952
- URL: https://www.wjgnet.com/1949-8462/full/v17/i2/100952.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i2.100952
Cardiac myxoma is the one of the most common types of primary cardiac tumors. However, the presence of concurrent cardiac myxoma and another malignancy is extremely rare. The treatment strategy varies according to the features of the myxoma and the nature and stage of the other malignancies, with evidence mainly limited to case reports. We here report a unique case of a left atrial myxoma and synchronous laryngeal squamous cell carcinoma. Following a literature review, we also summarize the clinical features and management approaches in cases of cardiac myxoma with synchronous malignancies.
A 48-year-old male presented to the emergency department with odynophagia and voice change for 3 months.
The patient had intermittent blood stains in the sputum every few days and had lost 5 kg of weight. He did not have shortness of breath or fever.
He had no other past medical history, and was not receiving long-term medication.
He had a 20 pack-year smoking history. There was no family history of malignancy.
He was afebrile without stridor. His blood pressure was 111 mmHg/79 mmHg, and heart rate was 91 beats per minute. A laryngeal mass was detected on physical examination, with palpable bilateral cervical lymph nodes. Oxygen saturation was good in room air.
He was mildly anemic with a hemoglobin level of 10.8 g/dL. His platelet count and coagulation profile were in the normal range.
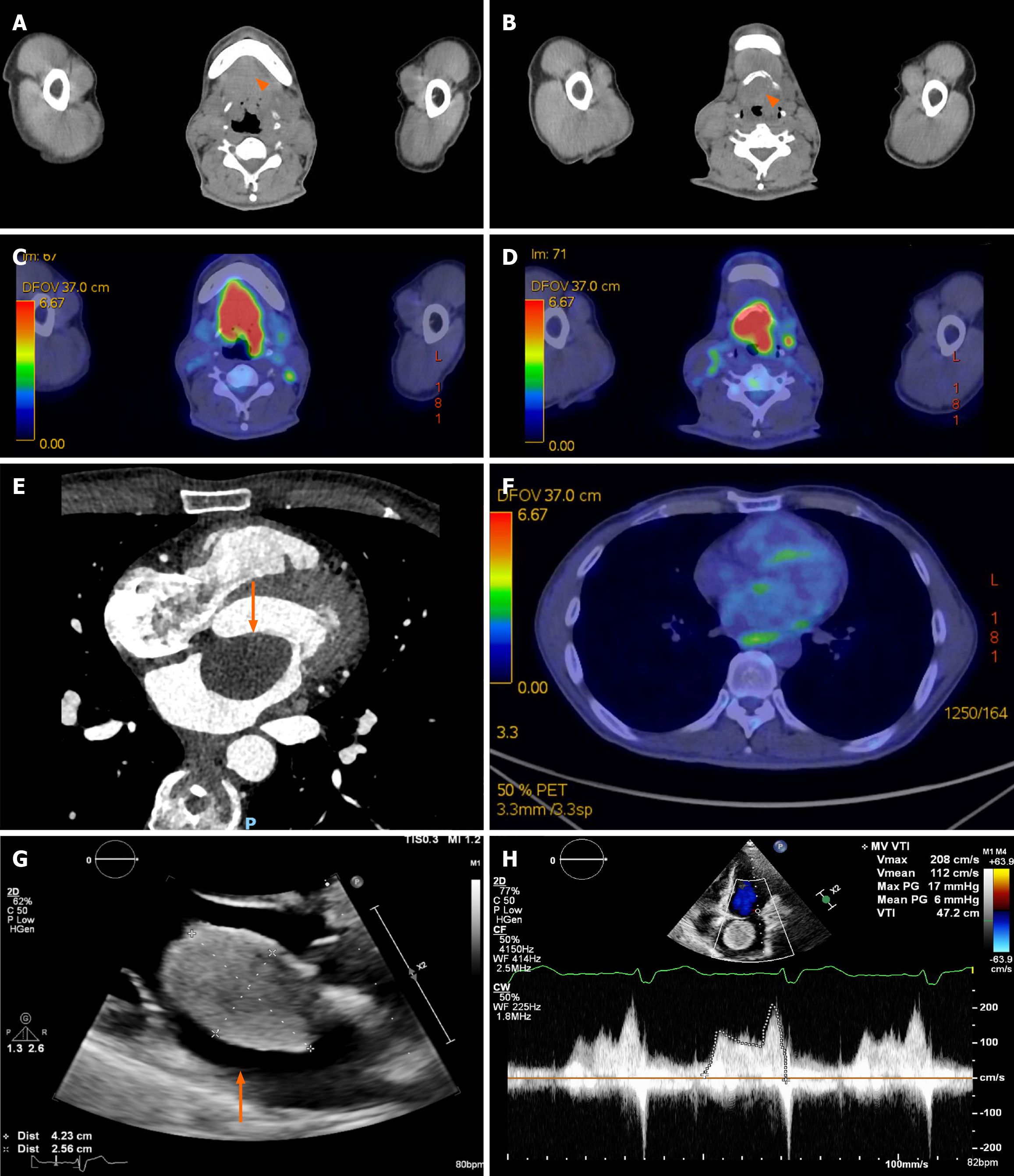
Computed tomography (CT) of the neck revealed a focal malignant tumor (4.6 cm × 3.6 cm × 2.8 cm) involving the supraglottic larynx with focal erosion of the left side of the hyoid bone, and multiple metastatic lymph nodes over the bilateral neck (Figure 1A-D). Biopsy of the lesion confirmed supraglottic squamous cell carcinoma. Staging CT of the chest and abdomen detected a 3.8 cm × 2.2 cm hypodensity in the left atrium, which showed faint (18)F-fluorodeoxyglucose uptake on a subsequent positron emission tomography (PET)/CT scan (Figure 1E and F).
A transthoracic echocardiogram (TTE) was performed and showed a large, well circumscribed heterogenous mobile echogenic lesion (4.1 cm × 2.8 cm), occupying a large extent of the left atrium, with a broad-based attachment on the left side of the interatrial septum and protruding through the mitral valve opening causing mitral inflow obstruction. The peak pressure gradient and mean pressure gradient measured 17 mmHg and 6 mmHg, respectively. The echocardiographic features were highly suggestive of atrial myxoma (Figure 1G and H). Diagnostic coronary angiogram showed 50%-70% diffuse disease proximal to the mid left anterior descending (LAD) artery, with positive functional fraction flow of 0.75 distally.
A cardiac surgeon was urgently consulted with regard to the left atrial tumor. In view of the aggressive features of the laryngeal carcinoma, it was decided that treatment involving total laryngectomy and tracheostomy should be performed as early as possible, followed by adjuvant therapy. However, due to the size of the cardiac lesion and its hemodynamic features, it was considered unsafe to proceed with any major oncological resection with the cardiac tumor in situ. Therefore, early cardiac surgery was recommended to prevent potential embolic events, heart failure or sudden cardiac death. Concerns remained that the recovery period required after a major cardiac operation may potentially delay or prevent further cancer treatment. After extensive multidisciplinary discussions among an otolaryngologist, cardiac surgeon, oncologist and the patient, a decision was made to proceed with early open heart surgery.
Diagnosis of locally advanced laryngeal squamous carcinoma with concurrent cardiac tumor was established. Cardiac metastasis could not yet be excluded, as this is much more common than primary cardiac tumors[1].
For patients with cardiac myxomas, primary resection is usually indicated with good surgical outcomes. Open heart surgery to remove the cardiac lesion would serve both diagnostic and therapeutic purposes in this case.
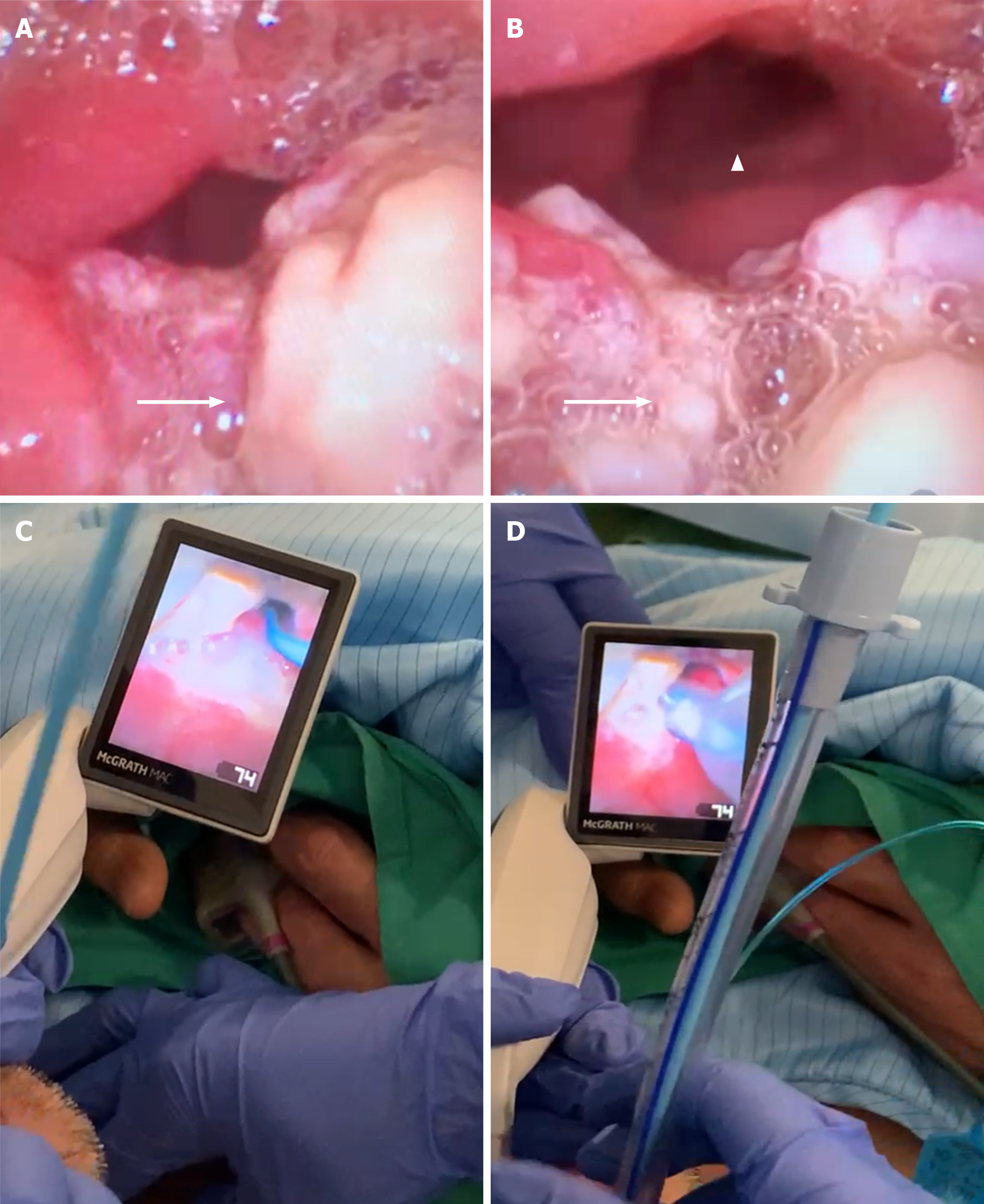
In view of the bulky laryngeal tumor in situ, the patient was prepared for general anesthesia due to a potential difficult airway, with the need for tracheostomy. Femoral arterial and venous lines were inserted before induction for mechanical circulatory support if necessary. During bedside laryngoscopy, a craggy tumor was noted at the base of the tongue, affecting bilateral vallecula, involving the entire epiglottis, while the laryngeal inlet was able to be visualized. The patient was intubated uneventfully using a video laryngoscope with bougie guidance on first pass (Figure 2). Intraoperative transesophageal echocardiogram (TEE) showed a large mass in the left atrium attached to the atrial wall at the aorto-mitral curtain, abutting the anterior mitral leaflet with obstruction of mitral inflow.
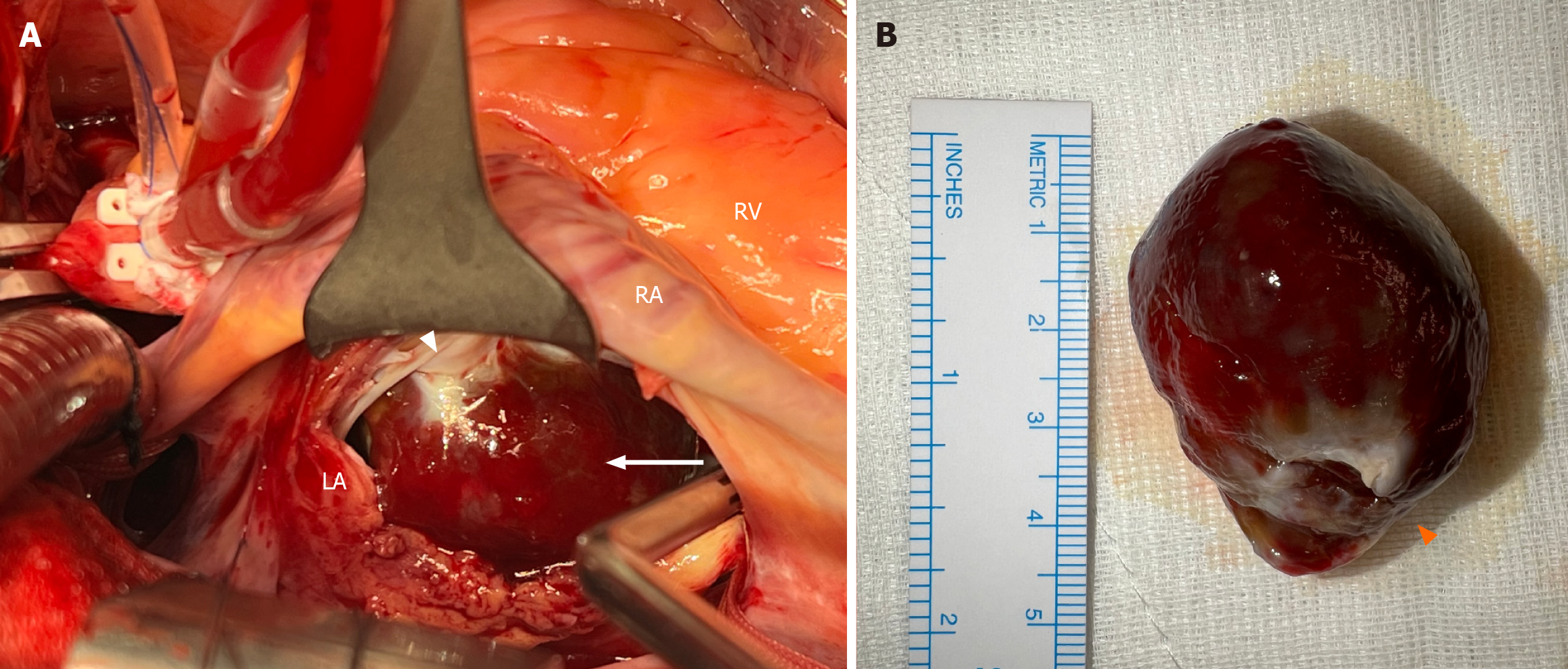
Following median sternotomy, cardiopulmonary bypass (CPB) and cardiac arrest by cardioplegia were conducted, and a left internal mammary artery to LAD bypass graft was performed. The left atrium was approached via the interatrial groove. An encapsulated solid tumor was observed in the left atrium, approximately 4 cm × 3 cm in size, and attached to the atrial wall adjacent to the left atrial appendage by a broad base, which corresponded to the TEE findings (Figure 3). The tumor was gently shaved off from the broad attachment and the endocardial base was cauterized.
The patient was weaned off CPB uneventfully with good TEE and graft flow results. He was extubated overnight in the ICU. His postoperative recovery was complicated by aspiration pneumonia requiring a course of antimicrobial treatment. He was discharged home on postoperative day 7.
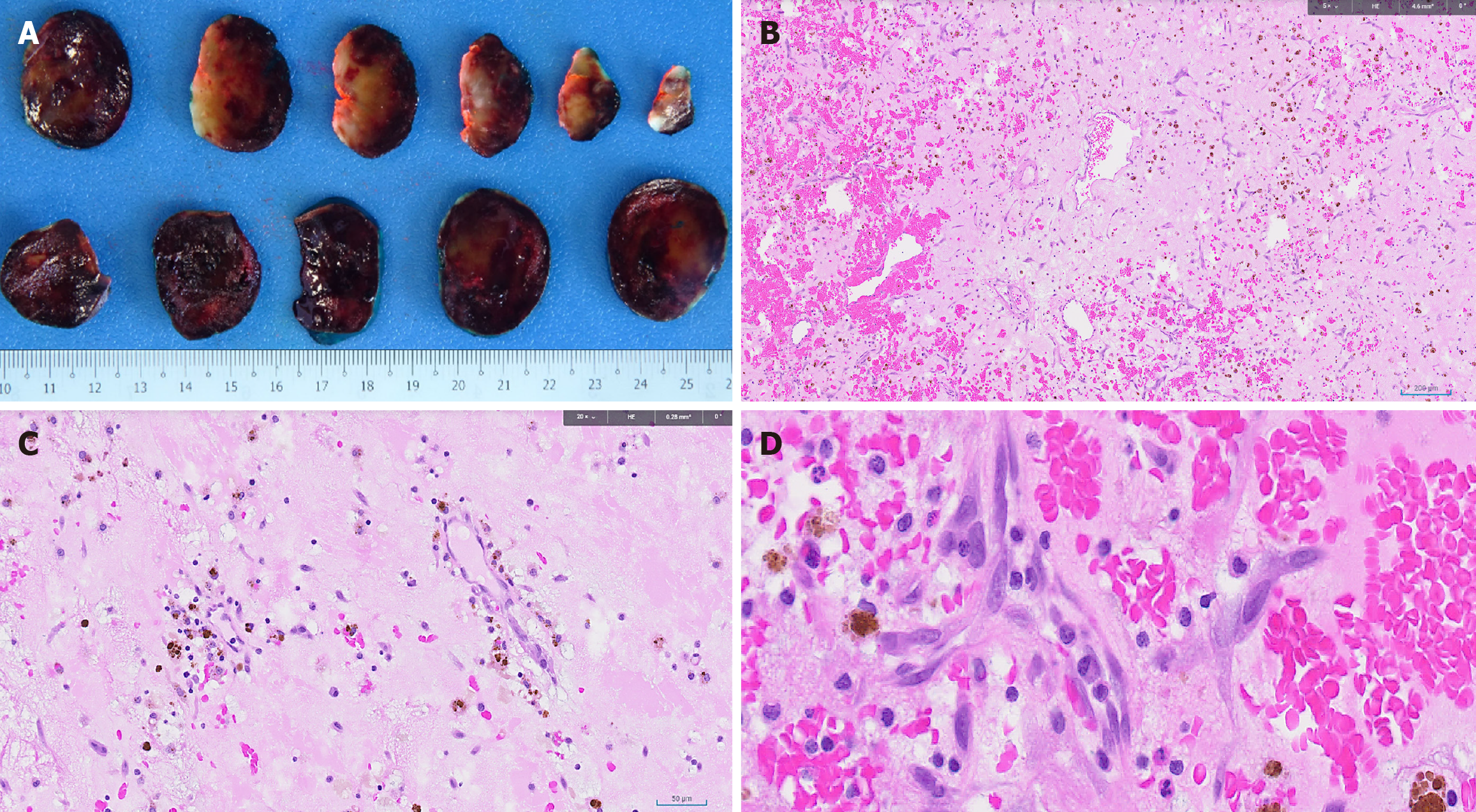
Histologic examination of the excised tumor showed bland stellate, ovoid, and plump spindle cells set in a vascularized myxoid stroma, with no high grade cytological atypia, necrosis, increased mitotic activity or metaplastic elements observed. These findings were in keeping with a cardiac myxoma (Figure 4).
The postoperative TTE showed no residual cardiac myxoma, with preserved heart function. The patient was reviewed by the oncologist and otolaryngologist 3 weeks later and induction chemotherapy was planned before surgical resection of the laryngeal cancer and reconstruction. However, the malignancy progressed rapidly, with further local invasion and peripheral metastasis. He also suffered from recurrent pulmonary infection due to aspiration. He underwent tracheostomy to relieve airway obstruction and was then continued on supportive care.
Primary cardiac neoplasms are very rare, with an incidence of 0.02% reported in meta-analyses[2]. The vast majority of them are histologically benign and cardiac myxoma is the predominant type in adult patients[3]. Myxomas are most commonly located in the left atrium, in the region of the fossa ovalis. It is postulated that they are derived from multipotent stem cells surrounding the fossa ovalis and surrounding endothelium. The St. John Sutton classification divides cardiac myxomas into solid tumors, which have a smooth surface with a compact consistency and can be removed in one piece, and papillary tumors, which are gelatinous and piecemeal removal is often necessary[4].
The classic clinical presentation of cardiac myxoma includes the triad of cardiac obstruction, systemic embolism and constitutional manifestations. Symptoms vary according to the size, shape and location of the tumor. Up to 10% of cases are asymptomatic and commonly identified incidentally on imaging studies.
Synchronous cardiac myxoma and other malignancies are extremely rare. Three to five percent of cardiac myxomas present as an autosomal dominant familial syndrome known as Carney complex, which generally occurs in younger patients. In addition to cardiac myxoma, the concomitant lesions in Carney complex include myxoid fibroadenoma of the breast, skin pigmentation abnormalities, endocrine disorders, testicular tumors, and psammomatous melanotic schwannoma[5]. Sporadic cases of cardiac myxoma with other concurrent malignancies such as lung cancer, cutaneous carcinoma, endometrial cancer, breast cancer[6], colorectal and hepatocellular carcinoma have been reported[7-11]. To our knowledge, the present case is the first report of synchronous cardiac myxoma and malignancy of laryngeal origin.
TTE remains the gold standard for the diagnosis of cardiac myxoma, with the typical appearance of a mobile mass attached by a visible stalk or broad base to the surface of the endocardium, commonly from the fossa ovalis[12]. Cardiac CT or magnetic resonance imaging (MRI) can be used as adjunctive modalities to further characterize the tumor and to distinguish it from the appearance of mural thrombi. Although there are currently no widely used clinical diagnostic indicators for cardiac myxoma, biomarkers such as the neutrophil/lymphocyte ratio has been shown to be elevated in patients with cardiac myxoma and correlates with the size of the tumor[13]. A distinctive blood-based cell phenotype has also been found with the potential to differentiate cardiac myxoma from other tumors[14]. For extracardiac malignancies, multimodal imaging studies including CT, MRI and PET/CT are usually required for differential diagnosis, to guide clinical staging and further treatment[9].
The management of synchronous tumors is challenging, as current evidence is mainly based on case series and reports with significant heterogeneity. The most life-threatening condition needs to be treated first, which may not necessarily be the myxoma. Surgical resection of cardiac myxomas results in excellent postoperative outcomes, with low complication and recurrence rates[15]. Successful cases have been reported where the extracardiac malignancy was treated while observing the cardiac myxoma[11], and where staged resection of the myxoma were conducted followed by treatment of the synchronous malignancy[16].
Simultaneous surgical resection of both tumors has also been reported with optimal results[6,17]. Compared with staged operations, concurrent operations avoid the possibility of tumor metastasis and embolic events to a large extent. A recent meta-analysis revealed that combined heart surgery and lung tumor resection had a low mortality rate and an acceptable complication rate. However, the sequence of resection during simultaneous surgery still remains controversial, with concerns regarding tumor dissemination when utilizing CPB, and CPB associated bleeding issues, fluid shifts and the systemic inflammatory response. The adverse effects of CPB on cancer prognosis have not yet been confirmed and require further investigation[18].
The removal of cardiac tumors is traditionally conducted via a median sternotomy, especially when a concomitant cardiac procedure is required, such as coronary artery bypass grafting in our case. The tumor can be excised via the bi-atrial transseptal approach, left atriotomy or right atriotomy according to its location and size. The bi-atrial transeptal approach offers optimal exposure and allows full inspection of both the left and right atrium, permitting radical resection with minimal manipulation of the cardiac tumor[19]. The minimally invasive surgical techniques are usually performed via a right thoracotomy with peripheral cannulation and have gradually been applied to resect cardiac tumors with excellent results[20,21]. As cardiac myxomas are benign neoplasm, it is not always considered necessary to completely resect the septum or the atrial wall where the tumor is attached. Experienced centers have reported a low recurrence rate with routine endocardial ablation at the base of the tumor after excision[20]. Moreover, for simultaneous resections, minimally invasive video-assisted thoracoscopic one-stage resection has been reported to remove both left atrial myxoma and concurrent right upper lobe lung cancer successfully[6], while the right thoraco-abdominal approach has been used for resection of both left atrial myxoma and esophageal carcinoma[22].
Given the lack of randomized data for this rare group of cases with simultaneous tumors, the treatment strategy needs to be tailored to individual patients, according to the stability of the patient, the aggressiveness of the malignancy, the complexity of the operation and the availability of surgical expertise. A multidisciplinary approach is imperative. The surgical results for cardiac myxoma resection are usually good. However, the long-term prognosis is mainly determined by the nature of the concurrent malignancy.
Our case is unique as the locally advanced laryngeal cancer was deemed potentially resectable at the time of diagnosis. However, the incidental finding of the left atrial myxoma with mitral inflow obstructive features, prevented the patient undergoing a complex laryngeal resection under general anesthesia. Therefore, a prompt decision was made to treat the cardiac tumor first before safely undergoing subsequent surgery. Early cardiac surgery was performed to resect the myxoma and revascularize the coronary disease. The communications in a multidisciplinary approach facilitated the workflow. Unfortunately, we were unable to contain the rapid progression of the laryngeal cancer, which prevented him from further curative oncological treatment. The patient was continued on supportive care.
Synchronous cardiac myxoma and other malignancies are extremely rare. Our case is possibly the first reported one with concurrent left atrial myxoma and laryngeal malignancy. The management of this group of patients is challenging due to limited evidence. Single resection, staged operations or simultaneous resection of both tumors have been reported with optimal results. A multidisciplinary approach is imperative to decide on the best treatment regime. The long-term prognosis mainly depends on the oncological progress of the concurrent malignancy.
We acknowledge the contribution of images by Dr. Yue Yu and Dr. An-Qi Lu from Singapore General Hospital.
| 1. | Maleszewski JJ, Bois MC, Bois JP, Young PM, Stulak JM, Klarich KW. Neoplasia and the Heart: Pathological Review of Effects With Clinical and Radiological Correlation. J Am Coll Cardiol. 2018;72:202-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 533] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 3. | Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH; ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229-4361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 1356] [Article Influence: 452.0] [Reference Citation Analysis (0)] |
| 4. | St John Sutton MG, Mercier LA, Giuliani ER, Lie JT. Atrial myxomas: a review of clinical experience in 40 patients. Mayo Clin Proc. 1980;55:371-376. [PubMed] |
| 5. | Tasoglu I, Tutun U, Lafci G, Hijaazi A, Yener U, Yalcinkaya A, Ulus T, Aksoyek A, Saritas A, Birincioglu L, Pac M, Katircioglu F. Primary cardiac myxomas: clinical experience and surgical results in 67 patients. J Card Surg. 2009;24:256-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Chen X, Li W. Minimally Invasive Video-Assisted Thoracoscopic One-Stage Resection of Left Atrial Myxoma and Right Upper Lobectomy for Lung Cancer: Case Report. Indian J Surg. 2023;85:401-404. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Nuño IN, Kang TY 4th, Arroyo H, Starnes VA. Synchronous cardiac myxoma and colorectal cancer: a case report. Tex Heart Inst J. 2001;28:215-217. [PubMed] |
| 8. | González-Cantú YM, Rodriguez-Padilla C, Tena-Suck ML, García de la Fuente A, Mejía-Bañuelos RM, Díaz Mendoza R, Quintanilla-Garza S, Batisda-Acuña Y. Synchronous Fibrolamellar Hepatocellular Carcinoma and Auricular Myxoma. Case Rep Pathol. 2015;2015:241708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Partridge AC, Sheng V, Cusimano RJ. Intracardiac Mass In A Patient With Breast Cancer. J Am Coll Cardiol. 2021;77:2685. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Iltumur K, Demir T, Ariturk Z, Toprak N, Oto O. Simultaneous occurrence of a large asymptomatic prolapsing left atrial myxoma with a cutaneous squamous cell carcinoma. Heart Surg Forum. 2015;18:E25-E27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Abaid LN, Epstein HD, Chang M, Kankus R, Goldstein BH. Endometrial Adenocarcinoma with Concomitant Left Atrial Myxoma. Case Rep Oncol. 2009;2:150-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Ma G, Wang D, He Y, Zhang R, Zhou Y, Ying K. Pulmonary embolism as the initial manifestation of right atrial myxoma: A case report and review of the literature. Medicine (Baltimore). 2019;98:e18386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Michopanou N, Schizas N, Charitos C, Rontogianni D, Saroglou G, Vatopoulos A, Eltheni R, Pavlopoulou I. Autopsy of 54 cases of surgically excised cardiac myxomas. Investigation of their impact on immune response. Heliyon. 2020;6:e04535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Donato G, Mignogna C, Santise G, Presta I, Ferrazzo T, Garo V, Maselli D, Curcio A, De Rosa S, Spaccarotella C, Mollace V, Gentile F, Indolfi C, Malara N. Distinctive phenogroup to differentiate diagnosis of cardiac myxoma vs cardiovascular disease examining blood-based circulating cell biomarkers. Sci Rep. 2023;13:20357. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Karabinis A, Samanidis G, Khoury M, Stavridis G, Perreas K. Clinical presentation and treatment of cardiac myxoma in 153 patients. Medicine (Baltimore). 2018;97:e12397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Altujjar M, Zaiem F, Sheehan E, Gan W, Mhanna M, Khokher W, Ullah I, Mujtaba MT. Rare Case of 6 cm Right Atrial Myxoma in Patient with Synchronous Endometrial Adenocarcinoma. Case Rep Cardiol. 2021;2021:4657117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | van der Merwe J, Beelen R, Martens S, Van Praet F. Single-Stage Minimally Invasive Surgery for Synchronous Primary Pulmonary Adenocarcinoma and Left Atrial Myxoma. Ann Thorac Surg. 2015;100:2352-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Braile DM, Évora PRB. Cardiopulmonary Bypass and Cancer Dissemination: A Logical But Unlikely Association. Braz J Cardiovasc Surg. 2018;33:I-II. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Jones DR, Warden HE, Murray GF, Hill RC, Graeber GM, Cruzzavala JL, Gustafson RA, Vasilakis A. Biatrial approach to cardiac myxomas: a 30-year clinical experience. Ann Thorac Surg. 1995;59:851-855; discussion 855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Jawad K, Owais T, Feder S, Lehmann S, Misfeld M, Garbade J, Borger M. Two Decades of Contemporary Surgery of Primary Cardiac Tumors. Surg J (N Y). 2018;4:e176-e181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Dang HQ, Le HT, Dinh LN. Endoscopic port access resection of left atrial myxoma: Clinical outcomes and a single surgeon's learning curve experience. JTCVS Tech. 2024;23:52-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Ni B, Lu X, Gong Q, Shao Y. Simultaneous resection of left atrial myxoma and esophageal carcinoma via right thoraco-abdominal approach. J Thorac Dis. 2016;8:E531-E534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |