Published online May 26, 2023. doi: 10.4330/wjc.v15.i5.262
Peer-review started: December 18, 2022
First decision: March 1, 2023
Revised: March 31, 2023
Accepted: April 18, 2023
Article in press: April 18, 2023
Published online: May 26, 2023
Processing time: 152 Days and 6.5 Hours
A limited number of studies have been conducted to test the magnitudes of the association between apparent treatment resistant hypertension (aTRH) and risk of cardiovascular disease (CVD).
To investigate the association between aTRH and risk of CVD and examine whether sex and age modify this association.
We applied an observational analysis study design using data from the United States Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT recruited participants (n = 25516) from 625 primary care settings throughout the United States, Canada, Puerto Rico, and United States Virgin Islands, aged 55 and older with hypertension and at least one additional risk factor for heart disease. aTRH was assessed from the year 2 visit. CVD event was defined as one of the following from the year 2 follow-up visit: Fatal or non-fatal myocardial infarction, coronary revascularization, angina, stroke, heart failure, or peripheral artery disease. Cox proportional hazards regression was used to examine the effect of aTRH on CVD risk. Potential modifications of sex and age on this association were examined on the multi
Of the total study participants (n = 25516), 5030 experienced a CVD event during a mean of 4.7 years follow-up. aTRH was associated with a 30% increase in risk of CVD compared to non-aTRH [hazards ratio (HR) = 1.3, 95%CI: 1.19-1.42]. Sex and age modified this relationship on both multiplicative and additive scales independently. Stratified by sex, aTRH was associated with a 64% increase in risk of CVD (HR = 1.64, 95%CI: 1.43–1.88) in women, and a 13% increase in risk of CVD (HR = 1.13, 95%CI: 1.01–1.27) in men. Stratified by age, aTRH had a stronger impact on the risk of CVD in participants aged < 65 (HR = 1.53, 95%CI: 1.32–1.77) than it did in those aged ≥ 65 (HR = 1.18, 95%CI: 1.05–1.32). Significant two-way interactions of sex and aTRH, and age and aTRH on risk of CVD were observed (P < 0.05). The observed joint effect of aTRH and ages ≥ 65 years (HR = 1.85, 95%CI: 1.22–2.48) in males was less than what was expected for both additive and multiplicative models (HR = 4.10, 95%CI: 3.63–4.57 and 4.88, 95%CI: 3.66–6.31), although three-way interaction of sex, age, and aTRH on the risk of CVD and coronary heart disease did not reach a statistical significance (P > 0.05).
aTRH was significantly associated with an increased risk of CVD and this association was modified by both sex and age. Further studies are warranted to test these mechanisms.
Core Tip: Apparent treatment resistant hypertension (aTRH) increased the risk of a cardiovascular event by 30%. This association varied by sex and age, with a stronger impact in women and in younger adults. These findings highlight the importance of controlling aTRH among those with excess risk of cardiovascular disease.
- Citation: Nelson JT, Liu L. Pharmacoepidemiologic study of association between apparent treatment resistant hypertension, cardiovascular disease and interaction effect by sex and age. World J Cardiol 2023; 15(5): 262-272
- URL: https://www.wjgnet.com/1949-8462/full/v15/i5/262.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i5.262
Hypertension has long been a serious public health concern. Its impact on cardiovascular health and long-term outcomes has been well studied[1]. In the United States approximately 121.5 million (47.3%) adults suffer from hypertension leading to an added economic burden costing up to $51.1 billion per year[2]. One of the significant challenges in control of hypertension is the appearance of treatment resistant hypertension.
Treatment resistant hypertension is defined as having blood pressure (BP) that remains uncontrolled [systolic BP (SBP) ≥ 140 mmHg or diastolic BP (DBP) ≥ 90 mmHg] while a patient is on ≥ 3 different antihypertensive medications. Additionally, those who are on 4 or more different classes of antihypertensive medications, regardless of BP are also classified as treatment resistant hypertension. Individuals with diabetes or chronic kidney disease (CKD) have an altered definition, these patients with SBP/DBP ≥ 130/80 mmHg are classified as treatment resistant hypertension[3-5]. In addition to the number of antihypertensive medications and BP readings, ideally at least one of the medications should be a diuretic[6]. Apparent treatment resistant hypertension (aTRH) is used to define observed treatment resistant hypertension when factors relating to pseudoresistance (adherence to regimen, sufficient dose for therapy, etc.) are unknown[7].
It is estimated that 19.7% of patients on antihypertensive medication have aTRH, a 2% increase within the recent decade[3-5]. While it is known that treatment of hypertension can reduce the risk of cardiovascular events and mortality, little research has been done on outcomes of those with resistant hypertension[3,8]. Several cross-sectional studies have found relationships between aTRH and cardiovascular disease (CVD), however, longitudinal studies remain sparse[3,9]. What has been observed is that aTRH is associated with higher rates of cardiovascular and renal diseases, including: Coronary heart disease (CHD), peripheral artery disease (PAD), stroke, heart failure (HF), end-stage renal disease, and all-cause mortality. Of particular note is that aTRH increased the risk of CHD by 44% and the risk of death by 30% compared to non-aTRH[9,10].
Men and women experience differing rates of hypertension and CVD[11,12]. However, findings of sex-specific aTRH studies remain inconsistent[3,5,13-15]. Women experience an increase in risk of hypertension post-menopause indicating a possible impact of sex and age on the risk of aTRH and CVD outcomes[12].
Of the many risk factors for CVD, age is one of the most important factors as age is an independent risk factor for the development of atherosclerosis[5,11,16,17]. However, the degree of this effect does not impact men and women in the same way neither in the risk of incident hypertension nor in the progress of hypertension in clinical treatment[11,18]. Several studies have observed that age is significantly and independently associated with risk of aTRH[3-5,14]. However, studies of the potential modification effects of sex and age on the association between aTRH and risk of CVD are limited. In this study, we hypothesized that sex and age play an important role both independently and together in the risk of aTRH for CVD outcomes. To test this hypothesis, we examined the independent and additive effects of sex and age on aTRH and risk of CVD, and whether sex and age modify the association between aTRH and CVD risk.
We analyzed data from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). In ALLHAT, a total of 42418 participants aged 55 and older were recruited from 625 primary care settings throughout the United States, Canada, Puerto Rico, and United States Virgin Islands. Participants were randomly assigned into four groups and then received one of four antihypertensive treatment arms: Chlorthalidone, amlodipine, lisinopril, and doxazosin. Participants who received doxazosin are excluded in the analysis because this group was discontinued early. All participants had hypertension and at least one additional risk factor for CHD. Participants were followed for an average of 6 years[19-21]. The study design and process of ALLHAT have been published in detail elsewhere[21].
This study, using de-identified data from the National Heart, Lung, and Blood Institute, has been approved by Drexel University Institutional Review Board (# 1608004781).
Exposure (aTRH): In ALLHAT the recruited participants were initially randomized to one of 4 first-line antihypertensive drugs. Additional anti-hypertensive medications were added on to therapy over the course of follow-up visits, thus, aTRH was determined at the year 2 visit to allow time for additional medications to be added, consistent with follow-up studies[10,21]. aTRH was defined as BP ≥ 140/90 while on 3 antihypertensive medications, or being on 4 antihypertensive medications regardless of BP at the year 2 visit. For subjects with either type II diabetes or CKD (assessed by glomerular filtration rate, epidermal growth factor receptor (eGFR) < 60 mL/min), the cut-offs of SBP/DBP were set at ≥ 130/80 mmHg. Due to subjects being randomized to first-line anti-hypersensitive treatment, the condition that one medication be a diuretic for aTRH classification could not be applicable in the study.
Outcome (CVD): Subjects were classified as having had a CVD event if they experienced one of the following after the year 2 follow-up visit: Fatal or non-fatal myocardial infarction (MI), coronary revascularization, angina, stroke, HF, or PAD. These events were ascertained from follow-up visits and medical recording during the course of the trial[10].
Covariates: Covariates were selected based on their independent associations with both the exposure (aTRH) and outcome (CVD), existing literature, and use of a directed acyclic graph[10]. All covariates were measured at baseline of ALLHAT. They included: Race (white, African American, American Indian/Alaskan Native, Asian/Pacific Islander, other), ethnicity (Hispanic), geographic region (East, Midwest, South, West, Canada, Puerto Rico/Virgin Islands), measures of GFR, SBP and DBP at baseline, and history of CHD, history of MI or stroke, history of coronary revascularization, previous use of BP medication, smoking status (current, former, never), history of left ventricular hypertrophy, estrogen use (in women subgroup analysis only), aspirin use, and enrollment in concurrent lipid lowering trial.
Univariate analysis was conducted to describe the baseline characteristics of participants by aTRH status. Student’s t test was used to examine mean difference in continuous variables, and Chi-square tests to examine rate and proportion differences in categorical variables. We used Cox Proportional Hazards regression models to examine the association between aTRH and CVD risk with adjusting covariates. Assumptions of the proportional hazards in Cox model were tested graphically by log-log survival curves. To test potential modification effects of sex (men vs women) and age (< 65 vs ≥ 65) on the association between aTRH and CVD risk, we assessed potential multiplicative interaction (e.g., aTRH*modifier) on CVD risk. We also assessed potential additive interaction effects on CVD risk by estimating the joint effects and calculating the relative excess risk for interaction (RERI)[22]. If the RERI ≠ 0 then there is evidence that the observed additive risk of our exposures is different (more or less depending on the direction of the RERI) than what was expected. The methods of the calculations and hypothesis tests for the RERI have been discussed and published in detail elsewhere[23]. To test the overall interaction effect of sex and age and aTRH on CVD risk, we tested three-way interaction through a product interaction term (e.g., aTRH*sex*age) in Cox models. Observed joint effects were compared to expected for both additive and multiplicative models to determine presence of effect modification and subgroup analyses were performed.
Sensitivity analyses: To avoid overfitting attributable to multiple control covariates, we applied a propensity score analysis technique[24]. A propensity score for the probability of aTRH was estimated utilizing all confounders and created using the boosted CART method in the “twang” package in R software[25-27]. Extreme weights were trimmed and inverse probability weighting was used to weight observations by the propensity score[24,28]. Second, in the ALLHAT study, missing data in BP measures occurred at year 2 visit. We conducted a sensitivity analysis using Markov Chain Monte Carlo method for multiple imputation based on the randomness of the missing values[29]. Third, because of the pathophysiologic differences between hemorrhagic and ischemic stroke, we conducted a subgroup of CVD analysis by strokes and CHD (fatal CHD or nonfatal MI, coronary revascularization, or hospitalized angina). Fourth, to control the effects attributable to the conclusion of patients who had uncontrolled hypertension (BP ≥ 140/90) but with < 3 medications (n = 11223), on the estimate of hazards ratio (HR) for CVD risk, we excluded this group of patients in the final sensitivity analysis.
Data analyses were conducted using SAS software (Version 9.4, SAS Institute Inc., Cary, NC, United States). Statistical significance as set up at a two-sided P < 0.05.
From the original sample of 42418 subjects in ALLHAT, those on the doxazosin treatment arm (9061) were removed early for safety reasons. After excluding subjects with CVD events or death prior to year 2, and those missing year 2 visit information we had an analytic study sample of 25516. During the follow-up (average 4.7 years), 5030 (19.7%) participants had a CVD event. Table 1 shows baseline characteristics of the participants.
| Overall | Resistant hypertension | |||
| Yes | No | P value | ||
| Total | 25516 | 2329 (9.1) | 23187 (90.9) | |
| Treatment group | < 0.001 | |||
| Chlorthalidone | 11808 (46.3) | 853 (36.6) | 10955 (47.3) | |
| Amlodipine | 6955 (27.3) | 554 (23.8) | 6401 (27.6) | |
| Lisinopril | 6753 (26.5) | 922 (39.6) | 5831 (25.2) | |
| Race | < 0.001 | |||
| White | 15397 (60.3) | 1272 (54.6) | 14125 (60.9) | |
| African American | 8808 (34.5) | 953 (40.9) | 7855 (33.9) | |
| Am. Indian/Alaskan Native | 58 (0.2) | 3 (0.1) | 55 (0.2) | |
| Asian/Pacific Islander | 312 (1.2) | 26 (1.1) | 286 (1.2) | |
| Other | 941 (3.7) | 75 (3.2) | 866 (3.7) | |
| Hispanic | 4485 (17.6) | 204 (8.8) | 4281 (18.5) | < 0.001 |
| Sex | < 0.001 | |||
| Male | 13597 (53.3) | 1333 (57.2) | 12264 (52.9) | |
| Female | 11919 (46.7) | 996 (42.8) | 10923 (47.1) | |
| Geographic region | < 0.001 | |||
| East | 3917 (15.4) | 337 (14.5) | 3580 (15.4) | |
| Midwest | 4736 (18.6) | 449 (19.3) | 4287 (18.5) | |
| South | 10603 (41.6) | 1165 (50.0) | 9438 (40.7) | |
| West | 2635 (10.3) | 254 (10.9) | 2381 (10.3) | |
| Canada | 461 (1.8) | 33 (1.4) | 428 (1.9) | |
| PR/VI | 3164 (12.4) | 91 (3.9) | 3073 (13.3) | |
| Preventive HTN treat | 23033 (90.3) | 2242 (96.3) | 20791 (89.7) | < 0.001 |
| Obese | 10604 (41.6) | 1085 (46.6) | 9519 (41.1) | < 0.001 |
| History of MI or stroke | 5499 (21.6) | 498 (21.4) | 5001 (21.6) | 0.84 |
| History of coronary revascularization | 3016 (11.8) | 327 (14.0) | 2689 (11.6) | < 0.001 |
| Type 2 diabetes | 8975 (35.2) | 1061 (45.6) | 7914 (34.1) | < 0.001 |
| left ventricular hypertrophy | 5110 (20.0) | 551 (23.7) | 4559 (19.7) | < 0.001 |
| History of CHD | 5981 (23.6) | 534 (23.2) | 5447 (23.7) | 0.61 |
| Smoking status | < 0.001 | |||
| Current | 5491 (21.5) | 423 (18.2) | 5068 (21.9) | |
| Past | 10394 (40.7) | 992 (42.6) | 9402 (40.6) | |
| Never | 9631 (37.7) | 914 (39.2) | 8717 (37.6) | |
| Aspirin use | 9084 (35.6) | 922 (39.6) | 8162 (35.2) | < 0.001 |
| Estrogen use (in women) | 2230 (18.7) | 182 (18.3) | 2048 (18.8) | 0.84 |
| Age (years) (mean ± SD) | 66.6 ± 7.5 | 67 ± 7.5 | 67 ± 7.5 | 0.78 |
| BMI (kg/m2) (mean ± SD) | 29.7 ± 5.9 | 30 ± 5.9 | 30 ± 5.9 | < 0.001 |
| SBP (mm Hg) (mean ± SD) | 146.0 ± 15.6 | 152 ± 15.1 | 145 ± 15.5 | < 0.001 |
| DBP (mm Hg) (mean ± SD) | 84.0 ± 10.0 | 85 ± 10.6 | 84 ± 9.9 | 0.001 |
| Cholesterol (mean ± SD) | 215.7 ± 42.5 | 216 ± 44.8 | 216 ± 42.2 | 0.79 |
| GFR | 78.0 ± 19.3 | 75 ± 20.6 | 78 ± 19.2 | < 0.001 |
Overall subjects were more likely male (53.3%), from the South (41.6%), white (60.3%), and previously treated for hypertension (90.3%). Over a third (35.2%) had type 2 diabetes (T2DM), and 40.7% were former smokers. While over 40% of subjects were obese, the average body mass index (BMI) was 29.7 (SD 5.92). The average age was 66 years, and average GFR 78 mL/min. The rate of aTRH was 9.1% (n = 2329) in the total sample, 9.8% in men, 8.4%% in women, and 9.1% in those aged 65 and older. Subjects with aTRH were predominantly more likely to be African American, male, BMI ≥ 30 kg/m2, and T2DM than their corresponding counterparts.
Table 2 shows that after adjustment for covariates, aTRH remained significantly associated with risk of CVD (HR = 1.30, 95%CI: 1.19–1.42, P < 0.0001).
| Model 1 | Model 2 | Model 3 | |||||||
| HR | 95%CI | HR | 95%CI | HR | 95%CI | ||||
| aTRH | 1.51 | 1.39 | 1.64 | 1.49 | 1.37 | 1.62 | 1.3 | 1.19 | 1.42 |
| Sensitivity analyses | |||||||||
| Propensity score | 1.33 | 1.20 | 1.48 | ||||||
| Multiple imputation sensitivity analysis | 1.24 | 1.16 | 1.33 | ||||||
| CHD | 1.56 | 1.29 | 1.89 | ||||||
| Without uncontrolled on < 3 drugs | 1.3 | 1.18 | 1.44 | ||||||
Sensitivity analyses of propensity score for adjusting multi-covariates and multiple imputation for estimates of missing values produced similar results as did the analysis involving exclusion of participants with uncontrolled BP on < 3 medications. When CHD was examined as the endpoint instead of CVD, the overall risk associated with aTRH was stronger (HR = 1.56, 95%CI: 1.29–1.89).
Table 3 shows individual and joint effects. Among women, aTRH was associated with a 64% increase in risk of CVD event (95%CI: 1.43–1.88), and a 13% increase in risk of CVD (95%CI: 1.01–1.27) among men. aTRH had a stronger impact on risk of CVD among younger subjects than it did among older (HR = 1.53, 95%CI: 1.32–1.77 In those aged < 65, and HR = 1.18, 95%CI: 1.05–1.32 in those aged 65 and older). All HRs were statistically significant and the two-way multiplicative interaction terms of sex*aTRH, and age*aTRH and RERIs on risk of CVD were statistically significant (P < 0.05).
| aTRH (No) | aTRH (Yes) | |||||
| HR | (95%CI) | HR | (95%CI) | HR (95%CI) by sex | ||
| Sex | Female | Reference | 1.67 | (1.45, 1.91) | 1.64 (1.43, 1.88) | |
| Male | 1.25 | (1.16, 1.34) | 1.39 | (1.12, 1.56) | 1.13 (1.01, 1.27) | |
| HR (95%CI) (M vs F) | 1.25 | (1.16, 1.35) | 0.80 | (0.66, 0.97) | ||
| RERI (95%CI) | -0.52 | (-0.75, 0.25) | ||||
| Interaction of sex and aTRH on CVD: P < 0.0001 | ||||||
| HR (95%CI) by age | ||||||
| Age | < 65 | Reference | 1.56 | (1.42, 1.71) | 1.53 (1.32, 1.77) | |
| ≥ 65 | 1.38 | (1.31, 1.45) | 1.61 | (1.48, 1.73) | 1.18 (1.05, 1.32) | |
| ≥ 65 vs < 65 | 1.37 | (1.28, 1.47) | 1.05 | (0.87, 1.27) | ||
| RERI (95%CI) | -0.339 | (-0.61, 0.06) | ||||
| Interaction of age and aTRH on CVD: P = 0.0326 | ||||||
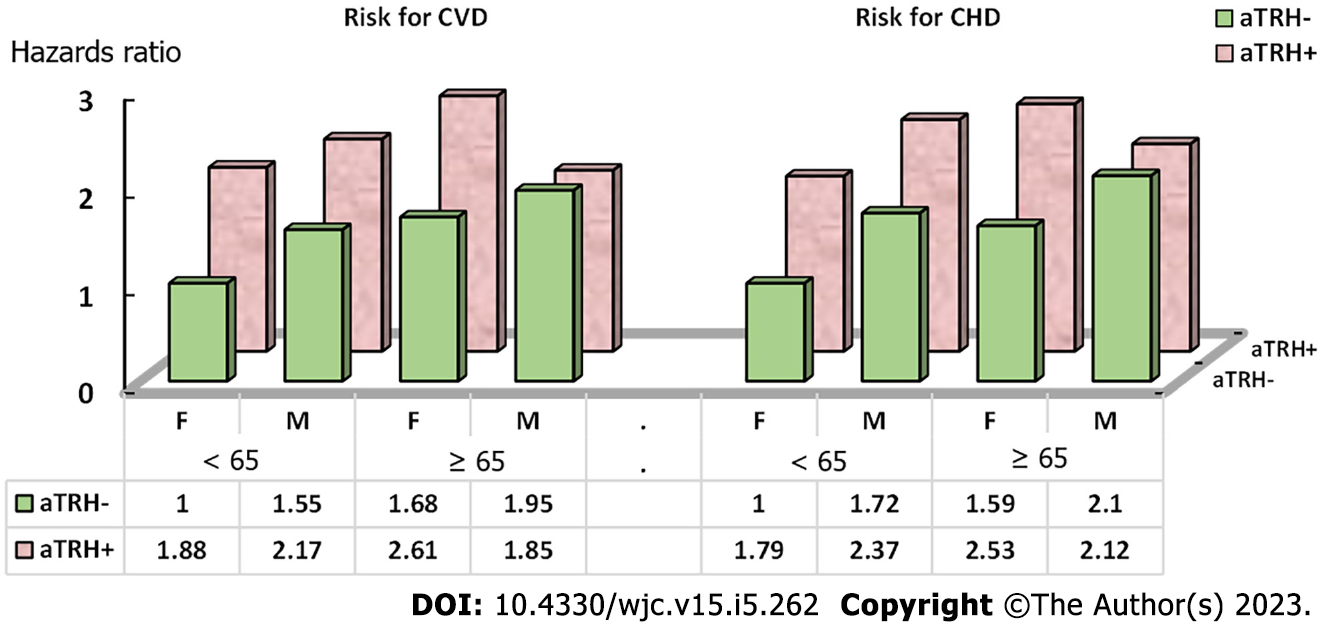
Results stratified by aTRH, age, and sex can be found in Table 4 and Figure 1. Females aged 65 and older with aTRH had the highest risk of CVD (HR = 2.61, 95%CI 2.20–3.01), followed by men < 65 years with aTRH (HR = 2.17, 95%CI: 1.76–2.58). The observed joint effect (HR = 1.85, 95%CI: 1.22–2.48) was less than expected for both additive and multiplicative models (HR = 4.10, 95%CI: 3.63–4.57 and HR = 4.88, 95%CI: 3.66–6.31 respectively). However, the three-way interaction term for sex, age and aTRH was not significant for CVD (P = 0.28), and CHD (P = 0.42).
| Sex | Age | HR | (95%CI) | |
| Risk for CVD | ||||
| aTRH | ||||
| No | Female | < 65 | Reference | |
| No | Male | < 65 | 1.55 | (1.43-1.67) |
| No | Female | 65+ | 1.68 | (1.56-1.79) |
| No | Male | 65+ | 1.95 | 1.70-2.20) |
| Yes | Male | 65+ | 1.85 | (1.22-2.48) |
| Yes | Female | < 65 | 1.88 | (1.64-2.11) |
| Yes | Male | < 65 | 2.17 | (1.76-2.58) |
| Yes | Female | 65+ | 2.61 | (2.20-3.01) |
| Three-way interaction of sex, age, and aTRH on CVD, P = 0.2841 | ||||
| Risk for CHD | ||||
| aTRH | ||||
| No | Female | < 65 | Reference | |
| No | Male | < 65 | 1.72 | (1.57-1.86) |
| No | Female | 65+ | 1.59 | (1.44-1.74) |
| No | Male | 65+ | 2.1 | (1.77-2.42) |
| Yes | Male | 65+ | 2.12 | (1.28-2.97) |
| Yes | Female | < 65 | 1.79 | (1.47-2.11) |
| Yes | Male | < 65 | 2.37 | (1.82-2.92) |
| Yes | Female | 65+ | 2.53 | (1.98-3.08) |
| Three-way interaction of sex, age, and aTRH on CHD, P = 0.4246 | ||||
The overall findings of the present study indicate that there is a positive association between aTRH and risk of CVD. Based on statistically significant two-way interactions of sex with aTRH, and age with aTRH, heterogeneous strata results, statistically significant RERIs, and differing observed and expected joint effects, this study highlights that the association between aTRH and risk of CVD is modified independently by sex and age on both the additive and multiplicative scales.
Although it appeared there was a potential three-way interaction such that sex may modify the interaction of age and aTRH, and vice versa that age may modify the interaction of sex and aTRH on CVD risk, testing for these three-way interactions were not statistically significant. The results suggest that the interaction of sex and aTRH, or of age and aTRH did not depend on the third factor. This finding adds new insights into the body of the literature, that sex and age independently modify the association between aTRH and CVD.
Treating BP becomes more difficult as a patient ages and is complicated by differing BP goals by age and a possible U-shaped relationship between BP and mortality among the elderly. Among this population also exists the risk of over-correcting and lowering BP too much which can cause other health concerns including dizziness and falls[2,30,31]. However, 2017 ACC/AHA changes to the guidelines no longer specify a different target BP for those ≥ 60 and the SPRINT study of older Americans indicated better health outcomes among those with more aggressive BP lowering targets (SBP 120 vs 140 mmHg)[32,33]. This area remains a topic of debate with more research needed on this unique population.
Research has shown that older women are more likely to adhere to medications and more likely to have their BP taken regularly, yet they appear to experience higher rates of uncontrolled hypertension[15]. Multiple mechanisms are believed to contribute to this increase in hypertension and uncontrolled hypertension in aging women including: Activation of the renin angiotensin system, obesity, activation of sympathetic nervous system, and decline in estrogen levels[15,17,34]. Stress on the body due to hypertension is believed to be more severe in women than in men[35]. Coupled with gender biases in treatment and management of CVD symptoms and events, we can envision how uncontrolled hypertension and aTRH would lead to more adverse health outcomes in women than men, especially in an aging population[36,37]. The pathophysiology of aTRH is believed to be linked to blood volume and salt, and salt-sensitive hypertension increases in women post-menopause thus we would expect aTRH to be more prevalent among post-menopausal women[18,38,39].
Very few studies have examined potential effect modifiers on the relationship between aTRH and CVD. Our findings are consistent with existing research in that aTRH is positively associated with CVD and both sex and age independently can modify the association between aTRH and CVD[3-5,9,10]. However, these studies only examined relative measures of association. Our study both confirmed presence of two-way interaction on the multiplicative scale and also identified that this modification is present on the additive scale. Epidemiologists have noted that additive interaction is most important for public health as it indicates an absolute number of cases which would be prevented if the modifier were removed[22,40].
No study to date has examined potential three-way interaction with aTRH. With health effects of sex and age linked it is important to examine how these gender disparities in cardiovascular outcomes of patients with aTRH differ between subgroups of age[11,12]. Public health programs aimed at improving awareness of aTRH should make specific effort to target women and informing them of the dangerous health consequences associated. This study suggests that physicians should take special note in their treatment considerations when female patients are experiencing uncontrolled hypertension and understand the serious cardiovascular risks associated with developing aTRH. For example, because the interaction of age and aTRH on CVD did not change by sex, we would still endorse applying these recommendations to vigorously control aTRH in women of all ages instead of focusing on the older group only.
This study has several strengths. ALLHAT provides us with one of the largest datasets to test the impact of aTRH on CVD risk. Sample size is particularly important when analyzing effect modification as it utilizes subgroup analyses. We also deployed two methods (propensity score weighting and multiple imputation) to conserve power in our analyses. Similar findings from our original analysis and both sensitivity analyses indicate our study is adequately powered. Another strength provided by use of ALLHAT is in our classification of aTRH. BP was taken twice from a seated position and averaged for each study visit, medication adherence was maintained through pill counts at each study visit, and doses of antihypertensive medications were appropriately titrated up to the maximum tolerated dose prior to adding on an additional medication. All of these factors allow for accurate ascertainment of aTRH status and reduce the likelihood of false positives due to pseudoresistance[2-5].
While a strong study, there are also several limitations. As this study is limited to its analysis of variables collected in ALLHAT only, additional data could be included in the analysis. For example, dietary salt intake was not measured in ALLHAT, thus we could not adjust for this in multivariate models as it is suggested that aTRH is influenced by blood volume and salt intake[38,39]. However, because ALLHAT is a randomized clinical trial, potential unmeasured factors are balanced among the participants. The clinical trial nature of our data source, while a strength for validity of aTRH classification, is also a limitation as subjects were selected based on pre-determined criteria best fit for the goals of ALLHAT and thus further studies are needed to confirm the results among different study populations. The study participants consisted of older adults (≥ 55) and thus findings of the study in women should also be evaluated in women aged 55 and older, instead of interpretating the results to those aged < 55. Additionally, the impact of pseudoresistance can never be completely removed and “white coat” effect is always a possibility when examining BP readings.
aTRH increases the risk of CVD in patients with hypertension and this relationship is modified by age and sex independently on both the relative (multiplicative) and absolute (additive) scales. More research is needed to shed further light on these gender differences, especially with regard to women both pre and post menopause. Future research should focus on these sex and age differences and how it might impact treatment and control of aTRH as there is still much unknown about this specific relationship. Management of aTRH should take a patient’s age and sex into consideration and more preventive interventions should be aimed at aging women as they represent the subgroup of aTRH patients with the highest combined risk of CVD.
aTRH was significantly associated with an increased risk of CVD and this association was modified by both sex and age. Further studies are warranted to test these mechanisms.
A Limited number of studies have been conducted to test the magnitudes of the association between apparent treatment resistant hypertension (aTRH) and risk of cardiovascular disease (CVD).
aTRH is significantly associated with the risk of CVD. It is important to understand whether age and sex significantly modify this association. Findings of the study could add new evidence to the body of literature, and provide new insights into further mechanism studies.
To investigate the association between aTRH and risk of CVD and examine whether sex and age modify this association.
We applied an observational analysis study design using data from the United States Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT recruited par
Of the total study participants, 5030 experienced a CVD event during a mean of 4.7 years follow-up. aTRH was associated with a 30% increase in risk of CVD compared to non-aTRH [hazards ratio (HR) = 1.3]. Sex and age modified this relationship on both multiplicative and additive scales independently. Stratified by sex, aTRH was associated with a 64% increase in risk of CVD in women, and a 13% increase in risk of CVD in men. Stratified by age, aTRH had a stronger impact on the risk of CVD in participants aged < 65 than it did in those aged ≥ 65. Significant two-way interactions of sex and aTRH, and age and aTRH on risk of CVD were observed (P < 0.05). The observed joint effect of aTRH and ages ≥ 65 years in males was less than what was expected for both additive and multiplicative models, although three-way interaction of sex, age, and aTRH on the risk of CVD and CHD did not reach a statistical significance (P > 0.05).
aTRH was significantly associated with an increased risk of CVD and this association was modified by both sex and age.
Further studies are warranted to test these mechanisms.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Awad AK, Egypt; Jain S, India S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Franklin SS, Wong ND. Hypertension and cardiovascular disease: contributions of the framingham heart study. Glob Heart. 2013;8:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K, Martin SS. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153-e639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2995] [Cited by in RCA: 3239] [Article Influence: 1079.7] [Reference Citation Analysis (0)] |
| 3. | Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB; American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Stroke Council. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension. 2018;72:e53-e90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 673] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 4. | Carey RM, Sakhuja S, Calhoun DA, Whelton PK, Muntner P. Prevalence of Apparent Treatment-Resistant Hypertension in the United States. Hypertension. 2019;73:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 5. | Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510-e526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 886] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 6. | Persell SD. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57:1076-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 577] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 7. | Egan BM. Treatment Resistant Hypertension. Ethn Dis. 2015;25:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Veterans Administration. Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effect of treatment on morbidity in hypertension: results in patients with diastolic blood pressure averaging 115-119 mmHg. JAMA. 1967;202:1038-1134. |
| 9. | Cai A, Calhoun DA. Resistant Hypertension: An Update of Experimental and Clinical Findings. Hypertension. 2017;70:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Muntner P, Davis BR, Cushman WC, Bangalore S, Calhoun DA, Pressel SL, Black HR, Kostis JB, Probstfield JL, Whelton PK, Rahman M; ALLHAT Collaborative Research Group. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension. 2014;64:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol. 2012;590:2069-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Jane F. Reckelhoff Biography. Physiologist. 2016;59:71-72. [PubMed] |
| 13. | Daugherty SL, Masoudi FA, Ellis JL, Ho PM, Schmittdiel JA, Tavel HM, Selby JV, O'Connor PJ, Margolis KL, Magid DJ. Age-dependent gender differences in hypertension management. J Hypertens. 2011;29:1005-1011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 454] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 15. | Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep. 2012;14:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Sarafidis PA. Epidemiology of resistant hypertension. J Clin Hypertens (Greenwich). 2011;13:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Pinto E. Blood pressure and ageing. Postgrad Med J. 2007;83:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 310] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 18. | Doumas M, Papademetriou V, Faselis C, Kokkinos P. Gender differences in hypertension: myths and reality. Curr Hypertens Rep. 2013;15:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, Black HR, Hamilton BP, Holland J, Nwachuku C, Papademetriou V, Probstfield J, Wright JT Jr, Alderman MH, Weiss RJ, Piller L, Bettencourt J, Walsh SM; ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4:393-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 671] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 20. | Cushman WC, Ford CE, Einhorn PT, Wright JT Jr, Preston RA, Davis BR, Basile JN, Whelton PK, Weiss RJ, Bastien A, Courtney DL, Hamilton BP, Kirchner K, Louis GT, Retta TM, Vidt DG; ALLHAT Collaborative Research Group. Blood pressure control by drug group in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich). 2008;10:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT Jr, Cushman WC, Grimm RH, LaRosa J, Whelton PK, Perry HM, Alderman MH, Ford CE, Oparil S, Francis C, Proschan M, Pressel S, Black HR, Hawkins CM. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT Research Group. Am J Hypertens. 1996;9:342-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 357] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Boston: Lippincott Williams & Wilkins, 2008. |
| 23. | Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 386] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 24. | Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6382] [Cited by in RCA: 7451] [Article Influence: 532.2] [Reference Citation Analysis (0)] |
| 25. | Lee BK, Lessler J, Stuart EA. Improving propensity score weighting using machine learning. Stat Med. 2010;29:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 480] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 26. | EREC. Economy Meets Environment: An Integrated Life Cycle Approach. Available from: https://eres.architexturez.net/doc/oai-eres-id-eres2013-258. |
| 27. | A Tutorial for the R TWANG Package. Toolkit for Weighting and Analysis of Nonequivalent Groups. R package version 1.5. Available from: https://www.rand.org/pubs/tools/TL136z1.html. |
| 28. | Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35:5642-5655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 354] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 29. | Alison Evans SW. Advanced Epidemiology Missing Data Lecture 2. 2012. https://journals.lww.com/epidem/Citation/2012/09001/S_154__Geocoding_Imprecision_and_Missing_Data_.408.aspx. |
| 30. | Delgado J, Masoli JAH, Bowman K, Strain WD, Kuchel GA, Walters K, Lafortune L, Brayne C, Melzer D, Ble A; As part of the Ageing Well Programme of the NIHR School for Public Health Research, England. Outcomes of Treated Hypertension at Age 80 and Older: Cohort Analysis of 79,376 Individuals. J Am Geriatr Soc. 2017;65:995-1003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Hyman DJ, Taffet GE. Blood pressure control in the elderly: can you have too much of a good thing? Curr Hypertens Rep. 2009;11:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential US Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation. 2018;137:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 545] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 33. | Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr, Pajewski NM; SPRINT Research Group. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. JAMA. 2016;315:2673-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 894] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 34. | Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 873] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 36. | Möller-Leimkühler AM. Gender differences in cardiovascular disease and comorbid depression. Dialogues Clin Neurosci. 2007;9:71-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 37. | Shaw LJ, Miller DD, Romeis JC, Kargl D, Younis LT, Chaitman BR. Gender differences in the noninvasive evaluation and management of patients with suspected coronary artery disease. Ann Intern Med. 1994;120:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 238] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 38. | Taler SJ, Textor SC, Augustine JE. Resistant hypertension: comparing hemodynamic management to specialist care. Hypertension. 2002;39:982-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Graves JW, Bloomfield RL, Buckalew VM Jr. Plasma volume in resistant hypertension: guide to pathophysiology and therapy. Am J Med Sci. 1989;298:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Moyses Szklo FJN. Epidemiology: beyond the basics. Burlington, MA: Jones & Bartlett Learning, 2014. |