Published online May 26, 2023. doi: 10.4330/wjc.v15.i5.253
Peer-review started: December 20, 2022
First decision: February 20, 2023
Revised: March 20, 2023
Accepted: April 25, 2023
Article in press: April 25, 2023
Published online: May 26, 2023
Processing time: 150 Days and 0.1 Hours
Mitral regurgitation (MR) is commonly seen in patients with severe aortic stenosis (AS) undergoing aortic valve replacement (AVR). But the long-term implications of MR in AS are unknown.
To investigate MR’s impact on survival of patients undergoing surgical AVR for severe AS.
Of the 740 consecutive patients with severe AS evaluated between 1993 and 2003, 287 underwent AVR forming the study cohort. They were followed up to death or till the end of 2019. Chart reviews were performed for clinical, echocardiographic, and therapeutic data. MR was graded on a 1-4 scale. Mortality data was obtained from chart review and the Social Security Death Index. Survival was analyzed as a function of degree of MR.
The mean age of the severe AS patients who had AVR (n = 287) was 72 ± 13 years, 46% women. Over up to 26 years of follow up, there were 201 (70%) deaths, giving deep insights into the determinants of survival of severe AS who had AVR. The 5, 10 and 20 years survival rates were 75%, 45% and 25% respectively. Presence of MR was associated with higher mortality in a graded fashion (P = 0.0003). MR was significantly associated with lower left ventricular (LV) ejection fraction and larger LV size. Impact of MR on mortality was partially mediated through lower LV ejection fraction and larger LV size. By Cox regression, MR, lower ejection fraction (EF) and larger LV end-systolic dimension were independent predictors of higher mortality (χ2 = 33.2).
Presence of greater than 2+ MR in patients with severe AS is independently associated with reduced survival in surgically managed patients, an effect incremental to reduced EF and larger LV size. We suggest that aortic valve intervention should be considered in severe AS patients when > 2+ MR occurs irrespective of EF or symptoms.
Core Tip: This study is unique in several aspects: (1) Shows that mitral regurgitation negatively impacts survival in an independent fashion, an effect incremental to left ventricular size and ejection fraction; (2) Perhaps the longest follow up of severe aortic stenosis (AS) patients undergoing aortic valve replacement (AVR) (till death or 16-26 year follow up in survivors); (3) Gives insights into potential mitral regurgitation (MR) mechanisms; and (4) Validates echocardiographic MR severity against survival in patients with severe AS undergoing AVR.
- Citation: Pai RG, Varadarajan P. Importance of concomitant functional mitral regurgitation on survival in severe aortic stenosis patients undergoing aortic valve replacement. World J Cardiol 2023; 15(5): 253-261
- URL: https://www.wjgnet.com/1949-8462/full/v15/i5/253.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i5.253
Significant mitral regurgitation (MR) is seen in about 20% of patients with severe aortic stenosis (AS) undergoing aortic valve replacement (AVR) or transcatheter aortic valve replacement (TAVR)[1-3]. Presence of MR is associated with worse symptoms, higher pulmonary artery pressures and higher short-term mortality in TAVR patients[1-5]. However, its impact on long term survival after AVR is not well characterized, especially in those with moderate or severe MR. The recent American College of Cardiology/American Heart Association (ACC/AHA) valve guidelines do not include presence of MR as an indication for AVR or TAVR[6]. We investigated the impact of different grades of MR on long term survival (to death or up to 26 years) in a cohort of patients undergoing surgical AVR for severe AS in period 1993-2003.
The research project was approved by the institutional review board of Loma Linda University, Loma Linda, California. A need for an informed consent was waived because of its retrospective, observational study design without any intervention.
The data underlying this article will be shared on reasonable request to the corresponding author. This was a retrospective observational study conducted in a large university medical center. The study was approved by the institutional review board, which waived the need for patient consent. Some of the other results of this observational study have been published previously[7,8]. This report includes 15 additional years of follow up. The echocardiographic database was searched for patients with severe AS during the period from 1993 to 2003. Severe AS was diagnosed based on the generally accepted criteria prevalent at that time[9]. The criteria used included aortic valve area ≤ 0.8 cm2 or a mean transvalvular gradient of ≥ 40 mmHg or peak gradient of ≥ 64 mmHg. This yielded a total of 740 patients. Complete clinical, echocardiographic, and pharmacological data were compiled on these patients from comprehensive chart review. Of these 287 underwent AVR and formed the study cohort.
Various clinical comorbidities were defined as follows: Hypertension was defined as a blood pressure > 130/90 mmHg, being on any antihypertensive medication, or a documented history of hypertension. Diabetes mellitus (DM) was defined as a fasting blood sugar of > 126 mg/dL or being on treatment for diabetes. Renal insufficiency was defined as a creatinine value of ≥ 2 mg/dL. Coronary artery disease (CAD) was defined as angiographic evidence of CAD with lesions > 50% as all had coronary angiograms before AVR.
All patients had standard 2-dimensional echocardiographic examinations. The left ventricular (LV) ejection fraction (EF) was assessed visually by a Level 3-trained echocardiographer and entered into a database at the time of the examination. This has been proven to be reliable and has been validated against contrast and radionuclide LV angiography[10,11]. Anatomic and Doppler examinations and measurements were performed according to the recommendations of the American Society of Echocardiography prevalent at that time and MR was graded 0-4[12,13].
Pharmacotherapy around the time of initial echo was recorded and placed into broad categories of beta blockers, calcium-channel blockers, digoxin and angiotensin receptor blockers (ARB) or angiotensin converting enzyme inhibitors (ACEI). Most of the patients continued on the medications for the length of the recorded clinical observation. Patients were on beta blockers, ACEI/ARB only if they had a concomitant indication for their use like hypertension or CAD. Beta blockers and ACEI/ARB are not recommended as primary treatment for severe AS. Hence the low treatment rate with beta blockers and ACEI/ARB.
The end point of the study was all-cause mortality. Mortality data were obtained from clinical records or the Social Security Death Index till the end of 2019.
Stat View version 5.01 (SAS Institute, Cary, North Carolina) program was used for statistical analysis. Kaplan-Meier survival curves were computed for patients with severe AS with and without MR and were compared using the log-rank statistic. Characteristics of patients with and without MR were compared using the Student t test for continuous variables and the χ2 test for categorical variables, the largest P value for differences between the groups was taken. Cox proportional hazards models was employed to adjust for clinical comorbidities and covariate imbalances. Baron and Kenny method was employed for mediation analysis[14]. P < 0.05 was considered significant.
The baseline characteristics of the severe AS patients who had AVR were: mean age 72 ± 13 years, 46% women, mean LVEF 57 ± 19%, and DM in 22%, hypertension in 55%, and CAD in 55%. The mean aortic valve area was 0.70 ± 0.17 cm2, transvalvular mean systolic gradient 44 ± 16 mmHg and peak gradient 72 ± 24 mmHg. Of the 287 patients, 42% had concomitant coronary artery bypass grafting (CABG) with AVR; 169 (59%) had no or mild (0 or1+) MR, 63 (22%) had moderate (2+) MR and 55 (19%) had moderate to severe or severe (3 or 4+) MR. Of the 55 patients with 3 or 4+ MR, 18 had mitral valve repair (n = 11) or replacement (n = 7).
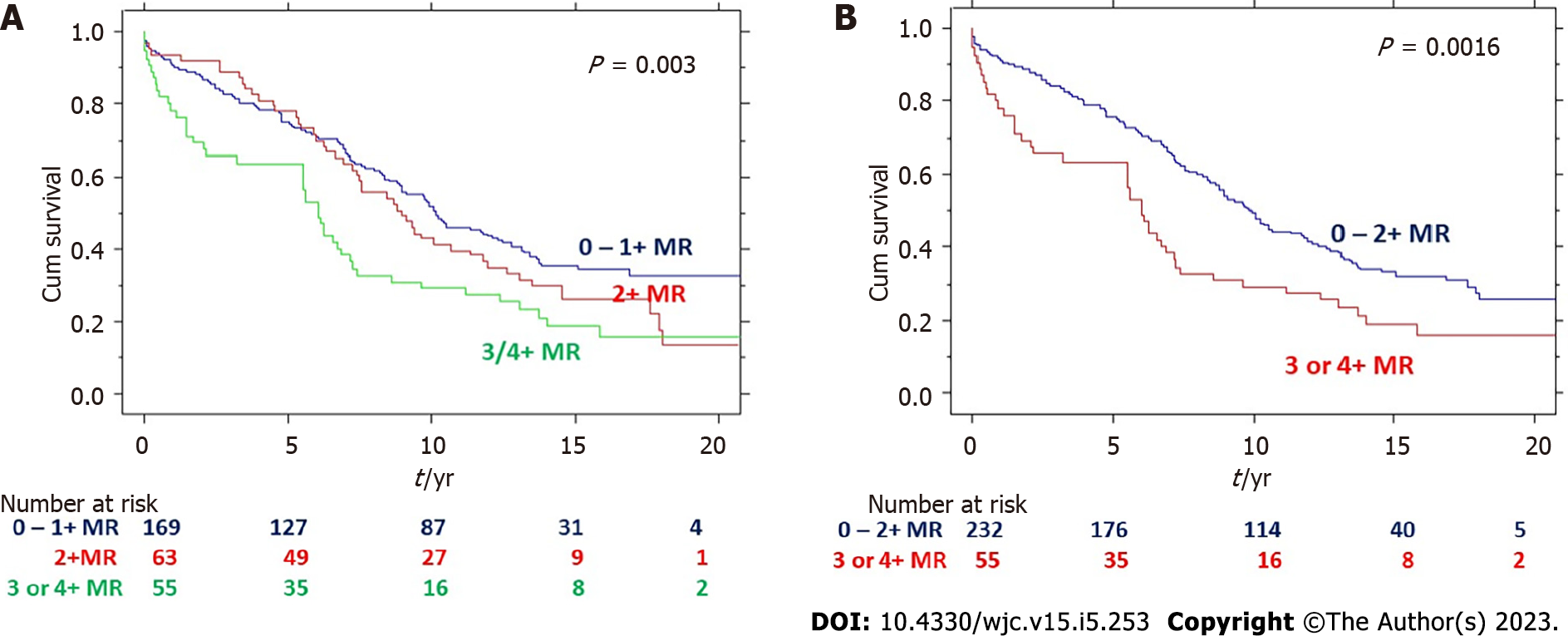
As the survival curves were similar for MR grades 0 and 1+ and very close to 2+ MR, hence these groups were combined. As shown in (Figure 1), there was a graded decrease in survival with increase in MR (P = 0.003). The 5, 10 and 20 year survivals for those with 0/1+ MR were 78%, 50% and 33% respectively compared to 78%, 43% and 14% for 2+ MR and 64%, 30% and 15% for 3 or 4+ MR respectively. Comparison between grade 0-1 and 3-4+ MR showed significant difference with P < 0.0001. Similarly, comparison between grade 2 and 3-4 +MR showed difference with P < 0.0001. Comparison between grades 0-1 and grade 2 showed slight difference with P = 0.03.
Table 1 shows the comparison of patients with different degrees of MR. As it can be seen from the table, higher degrees of MR correlated strongly with larger LV end-diastolic and end-systolic dimensions (P < 0.0001), lower LVEF (P < 0.0001) and lower LV relative wall thickness (P = 0.0002). There was no relationship with age, gender, hypertension, diabetes, CAD, renal failure or severity of AS. As expected, those with MR had higher mitral E/A velocity ratio (P < 0.0001) and shorter E wave deceleration time (P < 0.0001) indicating higher left atrial pressure, higher pulmonary artery pressure (P < 0.0001) and higher prevalence of heart failure (P = 0.0015). It is notable that 38% of patients with 3 or 4+ MR had no dyspnea or heart failure.
| 0 or 1+ MR | 2+ MR | 3 or 4+ MR | P value | |
| Age (yr) | 70 ± 12 | 74 ± 9 | 72 ± 13 | < 0.05 |
| Women (%) | 44 | 48 | 51 | 0.4 |
| Syncope (%) | 3 | 4 | 4 | 0.85 |
| Angina (%) | 38 | 49 | 47 | 0.12 |
| Dyspnea (%) | 39 | 38 | 62 | 0.002 |
| Atrial fibrillation (%) | 24 | 32 | 33 | 0.18 |
| Hypertension (%) | 58 | 57 | 44 | 0.06 |
| Diabetes mellitus (%) | 24 | 22 | 18 | 0.4 |
| Coronary artery disease (%) | 53 | 60 | 58 | 0.3 |
| Renal insufficiency (%) | 10 | 8 | 13 | 0.39 |
| LV end-diastolic diameter (mm) | 48 ± 7 | 48 ± 4 | 56 +10 | < 0.0001 |
| LV end-diastolic dimeter index (mm/m2) | 26 ± 4 | 27 ± 4 | 23 + 6 | < 0.0001 |
| Left ventricular end-systolic diameter (mm) | 31 ± 9 | 33 ± 9 | 41 + 13 | < 0.0001 |
| LV end-systolic dimeter index (mm/m2) | 16 ± 4 | 18 ± 4 | 41 + 13 | < 0.0001 |
| Ventricular septum (mm) | 14 ± 2 | 14 ± 3 | 14 + 3 | 0.2 |
| Posterior wall (mm) | 13 ± 2 | 13 ± 2 | 12 ± 2 | 0.13 |
| Relative wall thickness | 0.57 + 0.15 | 0.57 + 0.13 | 0.48 + 0.13 | 0.0006 |
| LV ejection fraction (%) | 62 ± 16 | 54 ± 17 | 45 ± 21 | < 0.0001 |
| Left atrial dimension (mm) | 41 ± 9 | 43 ± 8 | 48 ± 6 | < 0.0001 |
| Aortic valve area (cm2) | 0.72 + 0.16 | 0.68 + 0.16 | 0.70 + 0.20 | 0.18 |
| Aortic valve peak gradient (mmHg) | 73 ± 23 | 71 ± 26 | 67 +22 | 0.09 |
| Aortic valve mean gradient (mmHg) | 45 ± 15 | 43 ± 17 | 41 +15 | 0.08 |
| Aortic regurgitation grade | 0.65+0.83 | 1.16+0.91 | 1.00+1.00 | < 0.0001 |
| Mitral E/A velocity ratio | 1.00 + 0.53 | 1.09 + 0.64 | 1.60 + 0.67 | < 0.0001 |
| Mitral E wave deceleration time (ms) | 270 ± 105 | 278 ± 112 | 194 +89 | 0.0001 |
| Pulmonary artery systolic pressure (mmHg) | 40 ± 13 | 45 ± 20 | 53 +14 | < 0.0001 |
| Beta-blocker use (%) | 46 | 46 | 27 | 0.02 |
| ACEI or ARB use (%) | 25 | 33 | 36 | 0.12 |
| Digoxin use (%) | 35 | 32 | 38 | 0.47 |
| Statin use (%) | 31 | 21 | 35 | 0.09 |
| Coronary artery bypass grafting (%) | 38 | 49 | 47 | 0.12 |
| Mitral valve repair (%) | 3 | 2 | 20 | < 0.0001 |
| Mitral valve replacement (%) | 2 | 10 | 13 | 0.06 |
Though MR was strongly related to lower EF, 47% of patients with 3 or 4+ MR had an LVEF ≥ 50% (ACC/AHA guidelines use an EF threshold of < 50% as a class I indication for AVR or TAVR in patients with severe AS).
In view of strong correlations between MR grade, EF and LV size and as all these were predictive of survival, we performed mediation analysis described by Baron and Kenny by sequentially adding EF and LV end-systolic dimension to the Cox regression model that included MR severity[14]. Adding EF and LV end diastolic dimension, significantly reduced the χ2 associated with MR, but MR still remained significant (P values for MR, EF and LV end –diastolic dimension: 0.0049, < 0.0001 and 0.0013 respectively and global χ2 = 34.3). Addition of pulmonary artery pressure to the model did not improve its predictive value. This indicates that not all negative effects of MR were through increase in LV size and reduction in EF, but MR independently was related to survival as well. The results of Cox regression analysis are shown on Table 2.
| Variable | χ2 value | P value |
| LV end-diastolic dimension | 10.4 | < 0.01 |
| LV ejection fraction | 23.3 | < 0.0001 |
| Mitral regurgitation | 7.9 | < 0.01 |
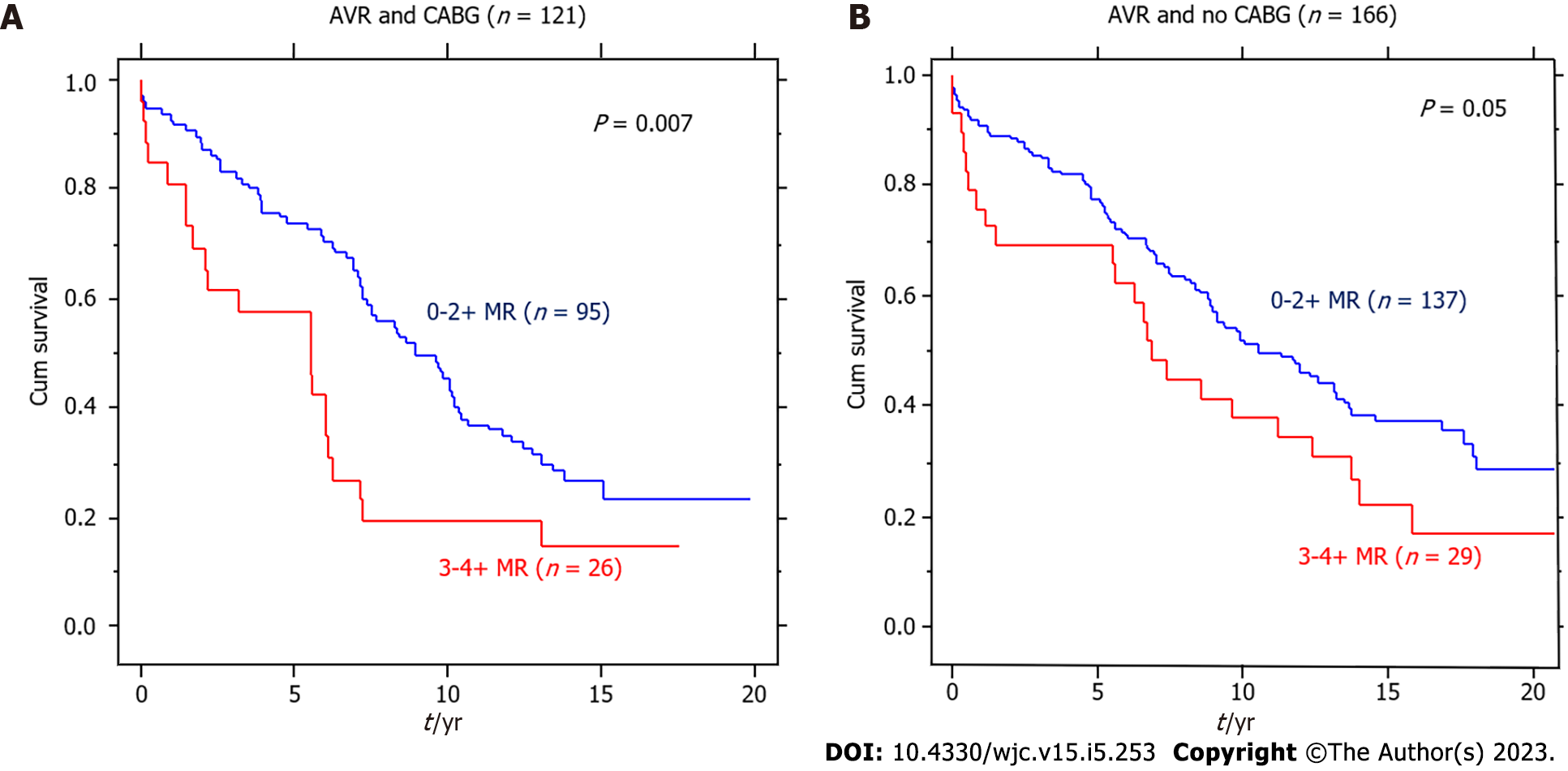
Of the 287 patients with severe AS undergoing AVR, 121 had CABG and 166 did not have CABG. As shown in Figure 2, the deleterious effect of MR was seen in both groups of these patients (P = 0.007 and P = 0.05, respectively).
Mitral valve surgery was performed in 18 of the 55 patients with 3 or 4+ MR at the time of AVR. Of these 12 patients also underwent CABG at the time of mitral valve surgery. was a trend towards better survival with mitral valve surgery with 10- and 20-year survivals being 45% and 37% respectively in those with mitral valve surgery compared to 22% and 8% respectively for those without (P = 0.08, Figure 3).
Our observational study with very long-term data suggests harm produced by MR in patients with severe AS undergoing AVR. According to ACC/AHA guidelines, MR is not considered an indication for AVR in severe AS patients in the absence of symptoms or EF < 50% or a need for concomitant cardiac surgery. About 1/3 of our patients even with 3 or 4+ MR had no symptoms and one half of them had an EF ≥ 50% without a traditional indication for AVR. These findings are novel and have major clinical implications in the management of these patients. The other strength of our study is length of follow up – to death or 16-26 years in the long-term survivors.
Presence of MR was also associated with higher left atrial and pulmonary artery pressures and some degree of LV enlargement indicative of adverse LV remodeling and functional impairment. Data from patients undergoing TAVR or AVR for severe AS indicates that MR may regress by one grade in about half the patients, but not full regression in all the patients leaving the patients with significant MR which may have negative impacts in terms of symptoms, heart failure and reduced survival or a need for another procedure to eliminate MR[4,15].
The mechanism of MR seems to be mostly functional because of an increase in LV size leading to mitral leaflet tethering in contemporary series with calcific AS, though older series included rheumatic valve disease as well[16]. There could also be an atrial mechanism due to atrial enlargement due to LV diastolic dysfunction or atrial fibrillation leading to secondary mitral annular dilatation[17]. Hence, monitoring asymptomatic severe AS patients for increases in LV or left atrial size or onset of atrial fibrillation may be important, besides evaluating for presence, degree and mechanism of MR. Performance of AVR or TAVR will reduce LV systolic pressure that drives MR. In addition, reverse remodeling of the LV and left atrium may occur as well and this will help in MR regression. One should also consider restoration of sinus rhythm to reduce the risk of genesis or progression of MR as atrial fibrillation may produce MR and even tricuspid regurgitation through atrial and annular dilation. Patients undergoing TAVR may introduce another issue of production of left bundle branch block or complete heart block needing a pacemaker. Both of these electrical disturbances may impair LV function and produce or worsen MR. These mechanistic factors should be kept in mind in patients with severe AS.
There are some limitations to this retrospective observational study. We do not have follow up echocardiographic data as the images are generally archived only for 7 years and many patients had these studies outside our healthcare system. Also, surgery for MR was at the discretion of the operating surgeon.
In summary, 3 or 4+ MR is present in about 20% of patients undergoing AVR for severe AS and negatively impacts hemodynamics, symptoms and survival. We suggest that one should be vigilant for any MR in patients with severe AS and also the potential mechanisms that may lead to MR such as progressive LV or left atrial dilation or onset of atrial fibrillation. We also suggest that presence of ≥ 3+ MR in patients severe AS should be considered as a potential indication for AVR or TAVR as appropriate.
Severe aortic stenosis (AR) and concomitant mitral regurgitation (MR) are common. But the impact of MR in those with severe AS on outcomes and management are unknown.
To study the impact of concomitant MR on outcomes in severe AS.
Does MR affect prognosis and decision making in severe AS patients.
Of the 740 consecutive patients with severe AS evaluated between 1993 and 2003, 287 underwent AVR forming the study cohort. They were followed up to death or till the end of 2019. Chart reviews were performed for clinical, echocardiographic, and therapeutic data. MR was graded on a 1-4 scale. Mortality data was obtained from chart review and the Social Security Death Index. Survival was analyzed as a function of degree of MR.
Presence of MR was associated with higher mortality in a graded fashion. MR was significantly associated with lower left ventricular (LV) ejection fraction and larger LV size. Impact of MR on mortality was partially mediated through lower LV ejection fraction and larger LV size. By Cox regression, MR, lower ejection fraction (EF) and larger LV end-systolic dimension were independent predictors of higher mortality.
Presence of greater than 2+ MR in patients with severe AS is independently associated with reduced survival in surgically managed patients, an effect incremental to reduced EF and larger LV size. We suggest that aortic valve intervention should be considered in severe AS patients when > 2+ MR occurs irrespective of EF or symptoms.
More studies are needed to study the mechanisms of MR and its prevention in severe AS patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ong H, Malaysia; Yang L, United States S-Editor: Liu XF L-Editor: A P-Editor: Yu HG
| 1. | Sethi A, Kodumuri V, Prasad V, Chaudhary A, Coromilas J, Kassotis J. Does the Presence of Significant Mitral Regurgitation prior to Transcatheter Aortic Valve Implantation for Aortic Stenosis Impact Mortality? - Meta-Analysis and Systematic Review. Cardiology. 2020;145:428-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Khawaja MZ, Williams R, Hung J, Arri S, Asrress KN, Bolter K, Wilson K, Young CP, Bapat V, Hancock J, Thomas M, Redwood S. Impact of preprocedural mitral regurgitation upon mortality after transcatheter aortic valve implantation (TAVI) for severe aortic stenosis. Heart. 2014;100:1799-1803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Sannino A, Losi MA, Schiattarella GG, Gargiulo G, Perrino C, Stabile E, Toscano E, Giugliano G, Brevetti L, Franzone A, Cirillo P, Imbriaco M, Trimarco B, Esposito G. Meta-analysis of mortality outcomes and mitral regurgitation evolution in 4,839 patients having transcatheter aortic valve implantation for severe aortic stenosis. Am J Cardiol. 2014;114:875-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Perl L, Vaturi M, Assali A, Sagie A, Weissler-Snir A, Codner P, Orvin K, Vaknin-Assa H, Shapira Y, Kornowski R. The Impact of Transcatheter Aortic Valve Implantation on Mitral Regurgitation Regression in High-Risk Patients with Aortic Stenosis. J Heart Valve Dis. 2015;24:439-444. [PubMed] |
| 5. | Boerlage-van Dijk K, Wiegerinck EM, Takama T, Koch KT, Vis MM, de Mol BA, Piek JJ, Bouma BJ, Baan J Jr. Mitral regurgitation prior to transcatheter aortic valve implantation influences survival but not symptoms. Int J Cardiol. 2016;204:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72-e227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 796] [Article Influence: 199.0] [Reference Citation Analysis (0)] |
| 7. | Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg. 2006;82:2111-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 312] [Article Influence: 16.4] [Reference Citation Analysis (1)] |
| 8. | Pai RG, Kapoor N, Bansal RC, Varadarajan P. Malignant natural history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg. 2006;82:2116-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | ACC/AHA guidelines for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association. Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). J Am Coll Cardiol. 1998;32:1486-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 542] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 10. | van Royen N, Jaffe CC, Krumholz HM, Johnson KM, Lynch PJ, Natale D, Atkinson P, Deman P, Wackers FJ. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol. 1996;77:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 249] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Amico AF, Lichtenberg GS, Reisner SA, Stone CK, Schwartz RG, Meltzer RS. Superiority of visual vs computerized echocardiographic estimation of radionuclide left ventricular ejection fraction. Am Heart J. 1989;118:1259-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 189] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5281] [Cited by in RCA: 5685] [Article Influence: 157.9] [Reference Citation Analysis (0)] |
| 13. | Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ; American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3183] [Cited by in RCA: 3086] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 14. | Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48287] [Cited by in RCA: 31723] [Article Influence: 834.8] [Reference Citation Analysis (0)] |
| 15. | Schubert SA, Yarboro LT, Madala S, Ayunipudi K, Kron IL, Kern JA, Ailawadi G, Stukenborg GJ, Ghanta RK. Natural history of coexistent mitral regurgitation after aortic valve replacement. J Thorac Cardiovasc Surg. 2016;151:1032-1039, 1042.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Palta S, Gill KS, Pai RG. Role of inadequate adaptive left ventricular hypertrophy in the genesis of mitral regurgitation in patients with severe aortic stenosis: implications for its prevention. J Heart Valve Dis. 2003;12:601-604. [PubMed] |
| 17. | Tanimoto M, Pai RG. Effect of isolated left atrial enlargement on mitral annular size and valve competence. Am J Cardiol. 1996;77:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |