Published online May 26, 2023. doi: 10.4330/wjc.v15.i5.217
Peer-review started: January 16, 2023
First decision: January 30, 2023
Revised: February 9, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: May 26, 2023
Processing time: 122 Days and 11.2 Hours
Tumor necrosis factor inhibitors (anti-TNFs) are widely used therapies for the treatment of inflammatory bowel diseases (IBD); however, their administration is not risk-free. Heart failure (HF), although rare, is a potential adverse event related to administration of these medications. However, the exact mechanism of development of HF remains obscure. TNFα is found in both healthy and damaged hearts. Its effects are concentration- and receptor-dependent, promoting either cardio-protection or cardiomyocyte apoptosis. Experimental rat models with TNFα receptor knockout showed increased survival rates, less reactive oxygen species formation, and improved diastolic left ventricle pressure. However, clinical trials employing anti-TNF therapy to treat HF had disappointing results, suggesting abolishment of the cardioprotective properties of TNFα, making cardiomyocytes susceptible to apoptosis and oxidation. Thus, patients with IBD who have risk factors should be screened for HF before initiating anti-TNF therapy. This review aims to discuss adverse events associated with the administration of anti-TNF therapy, with a focus on HF, and propose some approaches to avoid cardiac adverse events in patients with IBD.
Core Tip: Tumor necrosis factor inhibitors (anti-TNFs) are widely used for the treatment of inflammatory bowel diseases (IBD). However, heart failure, although rare, is an adverse event associated with the use of anti-TNFs in these patients. This review discusses the adverse events, especially heart failure, associated with the administration of anti-TNF therapy. We believe that our study makes a significant contribution to the literature because it discusses the current understanding in the field and proposes approaches to avoid the occurrence of adverse events due to anti-TNFs in patients with IBD.
- Citation: Grillo TG, Silveira CFDSMP, Quaglio AEV, Dutra RM, Baima JP, Bazan SGZ, Sassaki LY. Acute heart failure as an adverse event of tumor necrosis factor inhibitor therapy in inflammatory bowel disease: A review of the literature. World J Cardiol 2023; 15(5): 217-228
- URL: https://www.wjgnet.com/1949-8462/full/v15/i5/217.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i5.217
Inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), presents with chronic and progressive intestinal inflammation with periods of remission and activity, affecting mainly young people, with a peak of incidence between the third and fourth decades of life[1]. The etiopathology of IBD is poorly established, but it is believed to be related to an inappropriate inflammatory response to gut microbes in predisposed individuals[2]. There is still no curative therapy for IBD. Thus, treatment aims to alleviate symptoms, restore quality of life, and delay the progression and development of complications[3]. The choice of therapy depends on the location, activity, and severity of the disease, along with previous response to therapy and presence of complications. Evaluation of individual patient characteristics and the cost/benefit ratio of medications are also considered[4]. The therapeutic arsenal currently includes aminosalicylates (mesalazine and sulfasalazine); local and systemic corticosteroids (budesonide, prednisone, methylprednisolone, and hydrocortisone); immunosuppressants (azathioprine and 6-mercaptopurine); JAK inhibitors (tofacitinib); and biological therapies such as tumor necrosis factor inhibitors (anti-TNFs; infliximab, adalimumab, certolizumab pegol, and golimumab), anti-integrin antibody (vedolizumab), and anti-IL-12/23 antibody (ustekinumab)[4]. As anti-TNFs are widely used therapies, their adverse effects should be recognized in a timely manner to avoid patient morbidity and mortality. They are administered for the induction and maintenance of therapy in patients who fail to respond to conventional therapy. Infliximab, adali
Infliximab is a human–mouse chimeric monoclonal antibody administered intravenously. Adalimumab and golimumab are fully humanized monoclonal antibodies that are injected subcutaneously. Certolizumab pegol is a conjugated Fab antibody fragment administered subcutaneously. In CD, anti-TNFs (infliximab, adalimumab, and certolizumab pegol) are indicated for inducing remission in moderate-to-severe disease with inadequate response or intolerance to conventional therapy (steroids and/or thiopurines). They are effective in patients who are biological therapy-naive and biological therapy-refractive. Patients who achieved remission with anti-TNF agents need to continue the maintenance treatment. The effects of suspending anti-TNF therapy after long-term remission are not established; therefore, the decision must be individualized. In cases of complex perianal fistulas, the use of infliximab or adalimumab is recommended for induction and maintenance[4]. In UC, anti-TNFs are recommended in cases of moderate-to-severe active colitis, along with adalimumab, golimumab, or infliximab for inducing remission[6]. These drugs are recommended as maintenance therapy with or without thiopurines in patients who attained remission. In severe acute colitis refractory to intravenous steroids, infliximab may be a therapeutic rescue therapy[6,7]. Hypersensitivity reactions are among the most common adverse events with administration of anti-TNFs; they are either acute (during or within 24 h of the infusion) or delayed (24 h to 14 d after the infusion)[8]. The acute reactions are rapid in approximately 2% of the reactions, but < 1% lead to a severe reaction[9]. The presence of antibodies against infliximab increases the risk of infusion reactions[10], and case studies suggest that hypersensitivity to adalimumab is also due to the presence of anti-drug antibodies[11].
Neutropenia, thrombocytopenia, and anemia are also observed. Neutropenia may occur due to a blockade of TNFα (regulates proinflammatory factors involved in the differentiation and maturation of hematopoietic progenitor cells) that may mediate marrow failure due to inhibition of stem cell differentiation[12,13]. Isolated thrombocytopenia after administration of anti-TNF therapy has been reported by Salar et al[14] in 2007 and Casanova et al[15] in 2012, respectively; however, the cause/effect mechanism remains unclear. It may be related to autoimmune platelet destruction secondary to antiplatelet antibodies, immune complexes, or an idiosyncratic reaction. Anemia related to the use of anti-TNF remains debatable. Some studies have reported aplastic anemia due to administration of infliximab in rheumatoid arthritis[16] and infliximab-induced autoimmune hemolytic anemia[17].
Further, dermatological manifestations, such as eczema, psoriasis, infections, acne, dermatitis, and other erythema, are also reported. Psoriasis is a common adverse effect of anti-TNF therapy, occurring in approximately 1.5%–5% of the patients, mostly women, within an average of 2–6 mo after starting the therapy[18,19]. Autoimmune disorders, such as lupus-like syndrome, vasculitis, antiphospholipid syndrome, sarcoidosis, interstitial lung disease, optic neuritis, inflammatory eye disease, central nervous system demyelination, and peripheral neuropathies, are also reported[20]. Demyelination may occur, but it is unclear whether there is a causal relationship[21]. As anti-TNFs are immunosuppressive agents, they can increase the risk of infections of bacterial, viral, or fungal origin. Uncommon infections, such as listeriosis, have also been linked to the administration of anti-TNF therapy, with a higher risk in the first year of therapy[22,23]. Anti-TNFs can reactivate latent tuberculosis in immunocompromised individuals, emphasizing the importance of screening with clinical history, chest X-rays, and tuberculin tests before initiation of the therapy[24,25]. A high risk of infection with varicella zoster virus is observed in patients with IBD, and those receiving anti-TNF have a high risk of herpes zoster[26]. However, screening for a herpes virus infection is not required before the initiation of therapy[25]. Hepatitis B virus reactivation can also occur during anti-TNF therapy or after its withdrawal[27]. In the case of hepatitis C, biological agents do not present with a contraindication during concomitant infection and have a good safety profile; however, they are contraindicated in acute infections[28]. In patients with human immunodeficiency virus (HIV) infection, the risk/benefit of administering anti-TNFs should be weighed due to the increased risk of opportunistic infections[29]. Screening for hepatitis B and C viruses and HIV with serological tests is also recommended[25]. The possibility of cytomegalovirus infection recurrence by reactivation of latent infection is low after the use of biological therapy in most cases[8]. Screening before therapy is not required[25]. Fungal infections related to anti-TNF use are also reported, particularly in those with risk factors, such as opioid use, leukopenia, advanced age, and more severe disease[30]. The blocking of TNFα possibly alters the cytotoxic immune response to fungal infections[31]. Warris et al[32] reported a case of pulmonary aspergillosis in a patient with CD receiving infliximab. Histoplasmosis was also reported in a case series by Lee et al[33] after infliximab infusions in immunocompromised patients.
Malignancies (mainly non-melanoma skin cancer, melanoma, and lymphomas) are reported in patients with IBD due to chronic intestinal inflammation and the carcinogenic effects of immunosuppressive drugs[8,34,35]. TNF can trigger apoptosis by activating caspases, and its inhibition can lead to growth and/or metastases and tumor recurrence[36]. However, whether the use of anti-TNF monotherapy increases the overall risk of cancer in patients with IBD remains unclear[37]. Studies conducted by Biancone et al[38,39], Caspersen et al[40], and Fidder et al[41] failed to observe an increased risk of lymphoma, leukemia, or other hematologic malignancies with the use of anti-TNF monotherapy. Cardiovascular effects have also been reported, including HF, as discussed below.
HF is a clinical syndrome secondary to the inability of the heart to pump sufficient blood to supply peripheral metabolic demands or to do so under increased filling pressures. Acute HF (AHF) has been increasingly described as a condition with unique pathophysiology, distinct from that of chronic heart failure, and is among the most common causes of hospitalization in the elderly[42]. The patients can be categorized into new-onset (de novo) HF and worsening chronic HF. The latter accounts for the greater number of hospitalizations. Patients with de novo HF may have no prior risk factors, such as in myocarditis, but more commonly have preexisting conditions that favor the development of HF, or present with structural heart diseases without overt symptoms. Those with chronic HF may have precipitating factors for decompensation, including infections, poor adherence to treatment, and medications for other comorbidities[42,43]. Data on pathophysiology of AHF suggests that, apart from the architectural changes in the left ventricle due to increasing filling pressure and activation of the renin–angiotensin–aldosterone system, the inflammatory activation plays a pathogenic role in the progression of HF due to association with increasing stiffness of the vessels that leads to HF decom
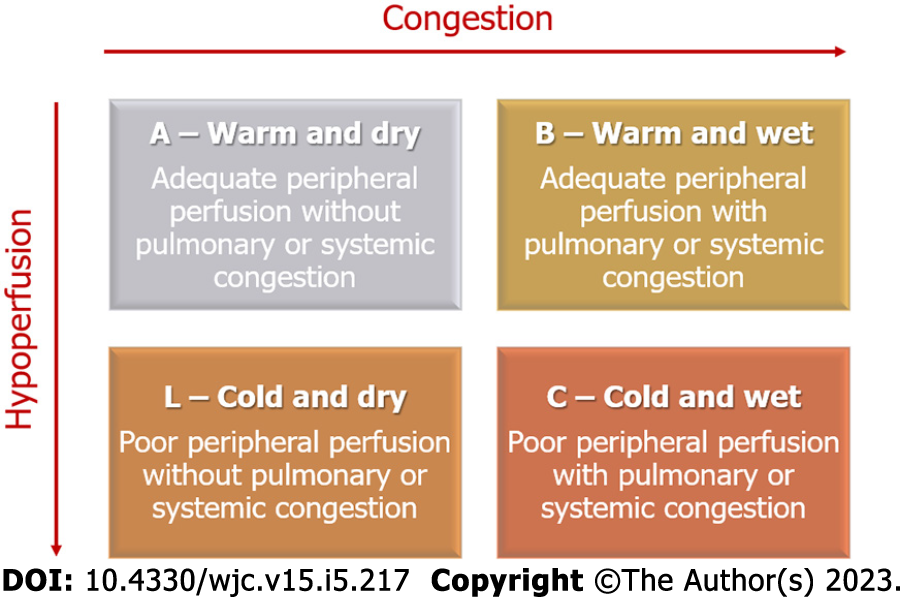
HF is diagnosed after thorough anamnesis, investigation of personal risk factors, family history, and current symptoms, such as dyspnea, edema of the lower extremities, orthopnea, paroxysmal night dyspnea, and palpitations[42]. The general clinical examination may indicate an increase in the respiratory rate and a decrease in oxygenation levels, edema, cachexia, and signs of poor perfusion, such as altered mental status. A detailed examination may indicate an increased jugular pulse, reflecting the increased left ventricle filling pressure, auscultation of the S3 and S4, along with mitral regurgitation murmur due to dilation of the left ventricle. The signs of pulmonary congestion include crackling sound on inspiration and dullness of the lung bases on percussion due to pleural effusion. The abdominal examination may show hepatomegaly due to an increase in central venous pressure, along with ascites due to right HF[50-52]. Patients should also be assessed using complementary tests, including renal function, levels of N-terminal-pro hormone B-type natriuretic peptide (NT-pro BNP), and electrolytes. The imaging examinations include electrocardiogram, chest radiography, and echocardiogram[53-56]. The treatment for AHF depends on whether the patient presents with congestion, low output, or both. Four hemodynamical profiles have been postulated for better organization of the medications employed in the early management of AHF, as demonstrated below and summarized in Figure 1[57,58].
It is also important to assess patient’s prognosis on admission to determine the requirement of advanced HF therapy, such as implantable cardiac devices or heart transplant[59]. Patients are considered to have worst prognosis if they are aged > 65 years; have a history of multiple hospitalizations; fail to adhere to treatment; present with functional classification NYHA III or IV; and have cachexia, syncope, sleep apnea, type II diabetes, or depression. Other factors include having had a reversed cardiac arrest; having pulmonary disease or cognitive dysfunction; having poor perfusion, congestion, tachycardia, persistent hypotension or low tolerance to exercise; having altered electrolyte levels, such as sodium < 130; having elevated BNP, troponin, or cytokines; having hemoglobin < 11 g/dL, creatine > 2.75 mg/dL, or urea 92 mg/dL; and showing atrial fibrillation in electrocardiogram, complete left bundle block, alternating T wave, long QT, low heart rate variability, progressive left ventricle dilatation, ejection fraction < 30%, right ventricle dysfunction, mitral or tricuspid regurgitation, restrictive pattern or decreased cardiac output, increase in pulmonary pressures, and peripheral vascular resistance[59].
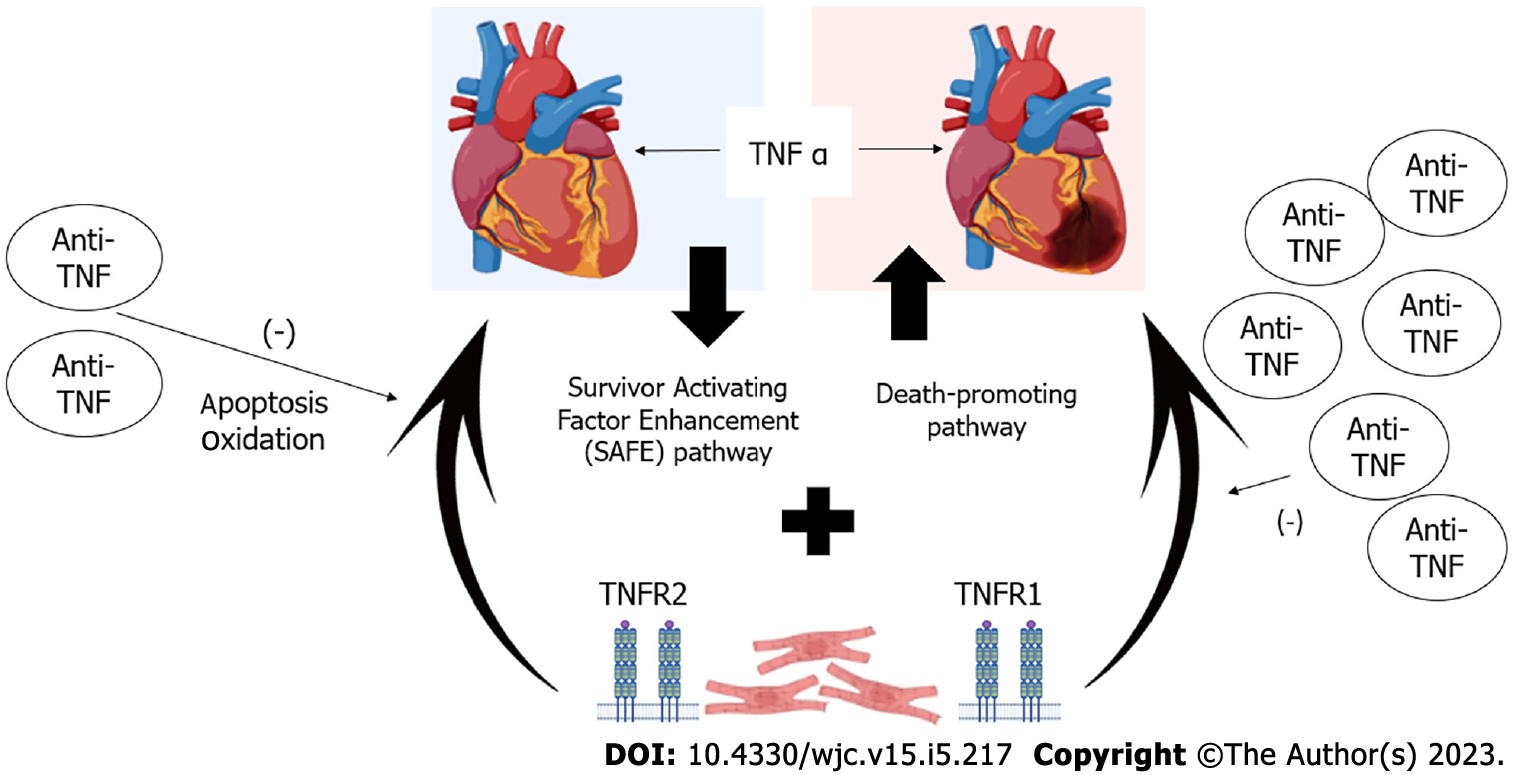
TNFα is found in both healthy and damaged hearts. Thus, it is challenging to understand its mechanisms of action. It binds to two different receptors: TNFα receptor-1 (TNFR1) and -2 (TNFR2) that are generally expressed on the heart cells[60,61]. In HF, TNFα induces β-adrenergic receptor uncoupling, increases oxidation and formation of nitric oxide, increases levels of inflammatory cytokines, and downregulates levels of contractile proteins, thus contributing to myocardial dysfunction. Long-term TNFα signaling leads to alterations in the heart geometry due to hypertrophy, apoptosis, and fibrosis[62]. The role of TNFα in HF pathophysiology is complex. Its effects are concentration-dependent and function via two different pathways: Survivor activating factor enhancement (SAFE) pathway functioning under low TNFα concentrations, and death-promoting pathway functioning in high TNFα concentrations. The SAFE pathway involves stimulation of other cytokines, such as cardiotrophin-1, that act on glycoprotein 130 receptor, leading to eccentric hypertrophy due to sarcomere organization in series. This pathway is observed in athletes with left ventricle hypertrophy[63-65]. In addition to the concentration of TNFα, its repercussion on the cardiac muscle depends on the receptor that it binds to as binding to TNFR1 may be cardio-damaging and binding to TNFR2 may be cardioprotective[60,66,67]. The mechanisms of HF caused by the use of anti-TNF are summarized in Figure 2.
Studies with animal models have suggested that TNFR1 Levels are upregulated after myocardial infarction without change in TNFR2 Levels. Other studies showed an absence of cardio-protection in acute ischemic models of TNFR1- and TNFR2-knockout rats, suggesting the involvement of both the receptors for maintaining a healthy heart[68,69]. In another knockout model study, Hamid et al[70] explored the left ventricle remodeling after myocardial infarction; the study indicated that TNFR1 knockout improved the left ventricle ejection fraction, reduced left ventricle dilatation through cardiomyocyte hypertrophy and apoptosis, and also decreased fibrosis and inflammation. In contrast, knockout of TNFR2 reversed the effects, suggesting a strong protective role of TNFR2. However, knockout of both the receptors increased survival rates, reduced reactive oxygen species formation, and improved diastolic left ventricle pressure; thus, this indicated the ambivalence of role of TNFRs on the heart health[68-70]. Further, Cacciapaglia et al[5] developed an in vitro model for TNFα preconditioning, exposing the cardiac cells to a lower dose of TNFα. The results suggested that the cells developed more resistance to subsequent TNFα toxic dose exposure and conferred more protection against oxidation and apoptosis.
From animal models to human randomized control trials, the concept of cardiac detrimental effects of TNFRs inspired the studies ATTACH, RECOVER, and RENAISSANCE (the last two are combined as RENEWAL) that were designed to understand the effects of infliximab and etanercept on HF with optimized clinical treatment. The ATTACH study failed to observe improvement and, surprisingly, indicated worsening of HF after therapy discontinuation. The RENAISSANCE study also showed an increased hazard ratio for the worsening of HF in the treatment group than that in the control group[71]. The disappointing clinical results, along with evidence from experimental models, suggest that the current rationale for the worsening of HF while administering anti-TNFα is that the dose employed in the randomized controlled trials abolishes the cardioprotective concentration of TNFα, therefore making the cardiomyocytes susceptible to apoptosis and oxidation[5]. A consensus is lacking on whether TNFα functions as a parallel phenomenon to, and not the cause for, HF, along with a possible selective cytotoxicity of anti-TNFα on cardiomyocytes in HF[62]. A study by Chung et al[72] evaluated the safety of infliximab in patients (n = 150) with moderate-to-severe HF (NYHA III or IV). The patients randomly received placebo, infliximab 5 mg/kg, and infliximab 10 mg/kg at 0, 2 and 6 wk and were followed for 28 wk. There was no improvement in the clinical status of patients who received infliximab at 14 wk, and after 28 wk, there were more hospitalizations in the infliximab 10 mg/kg group (n = 20) due to worsening of the HF condition, along with adverse clinical events persisting for up to 5 mo after discontinuation of therapy. Abedin et al[73] reported a case of acute coronary syndrome after infliximab infusion in a patient without previous heart disease. The patient was a 49-year-old Hispanic woman with rheumatoid arthritis and previously well-controlled hypertension. She presented to the emergency department 10 min after the start of infliximab infusion (20 mg had been infused). The patient had no other risk factors and no family history of coronary artery disease.
Kwon et al[74] followed patients with rheumatoid arthritis, psoriatic arthritis, and CD who were treated with an anti-TNF agent (etanercept or infliximab). A total of 47 patients developed HF; of these, 81% had no previous symptoms and 19% had worsening of preexisting symptoms. Among those who developed HF, 50% had no risk factors. The median interval between the first infusion of anti-TNF and diagnosis of HF was 3.5 mo (24 h to 24 mo). Keating et al[75] reported a case of anti-TNF-induced AHF. The 32-year-old patient had hypothyroidism and a bicuspid aortic valve and presented with Turner syndrome and CD. Biological therapy with adalimumab was initiated due to no response to budesonide. Examination indicated a transthoracic echocardiogram with normal ejection classification (> 55%). Eighteen weeks after administering adalimumab, the patient was admitted to the emergency department with edema and dyspnea. Further, we reported a case of AHF 6 mo after administering infliximab in a 50-year-old woman with CD and diabetes and a previous history of arterial hyper
Tofacitinib can be used in patients with moderate-to-severe UC who are intolerant or refractory to treatment with anti-TNFs. Despite its ease of rapid onset of action, oral administration, and low immunogenicity, it is also having risks such as venous thromboembolism and hyperlipidemia[80]. However, long-term data on adverse cardiovascular events with tofacitinib are lacking in patients with IBD, and it remains unclear whether the risk of venous thromboembolism is disease- or drug-related [79]. Ustekinumab, a monoclonal antibody, is an option in patients with CD and with moderate-to-severe UC intolerant or refractory to anti-TNFs. In IBD, the relationship of cardiovascular events in these patients is unclear[81,82]. Vedolizumab, a humanized monoclonal antibody, is also an option in patients with CD and with moderate-to-severe UC refractory or intolerant to anti-TNFs, with better outcomes in patients not treated with anti-TNFs[83,84]. Randomized and observational studies have not reported an increase in cardiovascular events in patients with IBD[85-88]; however, an increase in cerebrovascular events, such as stroke and cerebral hemorrhage, is reported[79]. The selection of the most appropriate medication in this scenario is challenging. The clinicians should carefully analyze the different medication classes available, with their safety and efficacy profiles, to define a personalized treatment strategy for each patient, considering risk factors inherent to the patient and the proposed medication, while aiming for the best outcome.
The recommendations for HF screening prior to IBD treatment with anti-TNFα drugs are lacking. Some guidelines suggest screening, while others only mention avoidance of biological drugs when a patient presents with class III/IV HF. The European Crohn's and Colitis Organization (ECCO) reports that patients with IBD have a modest increase in the risk of ischemic heart disease, especially in women; however, they have not mentioned requirement of screening tests for cardiovascular diseases before administration of biological therapy[1,4,7,89,90]. The guidelines of the American College of Gastroenterology and the Brazilian Consensus on Inflammatory Bowel Diseases have also not provided recommendations for screening these patients[6,91,92]. The guidelines of the British Society of Gastroenterology recommend that the use of anti-TNFs be contraindicated in cases of congestive HF and that screening be performed before starting treatment; however, they have no suggestions on the best strategies for screening[93]. Considering the indication of anti-TNFα drugs for other immune mediated diseases, although with a very low certainty, guidelines on rheumatoid arthritis recommend the following strategies: Inclusion of a non-TNF inhibitor in place of a TNF inhibitor for patients with NYHA class III or IV HF and also switching to a non-TNF inhibitor instead of a TNF inhibitor for patients who develop HF[94]. The stratification of patients with HF should not be challenging as a simple clinical examination is sufficient to identify patients with NYHA classes III and IV disease. However, issues arise when the patients present with severe HF and are oligosymptomatic, especially if they are not accustomed to exerting themselves on a regular basis. As patients may develop HF after pregnancy, viral infections, or alcohol abuse, yet not present with any symptoms consistent with NYHA III or IV, this makes them susceptible to underdiagnosis. Taken together, in the absence of evidence supporting heart disease screening prior to initiating anti-TNFα drugs, and considering the current availability of a low-cost, radiation-free test that can easily assess the patient’s heart function, such as echocardiogram, we recommend that an initial cardiac evaluation be a part of the patients’ routine care. We suggest increasing employment of an echocardiogram for diagnosing HF prior to initiating treatment. This strategy may prevent the incidence of adverse events in patients receiving this treatment. We hope that this review highlights this topic and would encourage future studies to clarify the benefits of using HF screening tools in patients with IBD prior to the use of anti-TNF medications to control inflammatory processes and restore quality of life, without causing further damage to the patients. It is reinforced that anti-TNF therapy has changed the course of treatment for IBD and other immune-mediated diseases in recent decades, altering its progressive and disabling course. Due to the more frequent use of these therapies, concerns about safety arise, and this article reinforces the importance of studying the subject in greater depth, including investigating the role of cardiac receptors and their relationship with the appearance of adverse events in these patients. Another point worth mentioning is the need for new algorithms and protocols, especially for populations at risk, in order to avoid the unwanted effects of the prescribed therapy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang LH, China; Wen XL, China; Wu SC, China S-Editor: Xing YX L-Editor: Wang TQ P-Editor: Yu HG
| 1. | Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F. European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1298] [Article Influence: 162.3] [Reference Citation Analysis (0)] |
| 2. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1561] [Article Influence: 111.5] [Reference Citation Analysis (1)] |
| 3. | Sairenji T, Collins KL, Evans DV. An Update on Inflammatory Bowel Disease. Prim Care. 2017;44:673-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (13)] |
| 4. | Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, Adamina M, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, El-Hussuna A, Ellul P, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gomollon F, González-Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Spinelli A, Stassen L, Uzzan M, Vavricka S, Verstockt B, Warusavitarne J, Zmora O, Fiorino G. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2020;14:4-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 905] [Article Influence: 181.0] [Reference Citation Analysis (2)] |
| 5. | Cacciapaglia F, Navarini L, Menna P, Salvatorelli E, Minotti G, Afeltra A. Cardiovascular safety of anti-TNF-alpha therapies: facts and unsettled issues. Autoimmun Rev. 2011;10:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (2)] |
| 6. | Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114:384-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 1042] [Article Influence: 173.7] [Reference Citation Analysis (0)] |
| 7. | Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnár T, Raine T, Sebastian S, de Sousa HT, Dignass A, Carbonnel F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;11:769-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 938] [Cited by in RCA: 867] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 8. | Shivaji UN, Sharratt CL, Thomas T, Smith SCL, Iacucci M, Moran GW, Ghosh S, Bhala N. Review article: managing the adverse events caused by anti-TNF therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49:664-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 9. | Cheifetz A, Smedley M, Martin S, Reiter M, Leone G, Mayer L, Plevy S. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 356] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 10. | Ricart E, Panaccione R, Loftus EV, Tremaine WJ, Sandborn WJ. Infliximab for Crohn's disease in clinical practice at the Mayo Clinic: the first 100 patients. Am J Gastroenterol. 2001;96:722-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Steenholdt C, Svenson M, Bendtzen K, Thomsen OØ, Brynskov J, Ainsworth MA. Acute and delayed hypersensitivity reactions to infliximab and adalimumab in a patient with Crohn's disease. J Crohns Colitis. 2012;6:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. |
Beutler BA.
The role of tumor necrosis factor in health and disease |
| 13. | Keystone EC. Tumor necrosis factor-alpha blockade in the treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27:427-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Salar A, Bessa X, Muñiz E, Monfort D, Besses C, Andreu M. Infliximab and adalimumab-induced thrombocytopenia in a woman with colonic Crohn's disease. Gut. 2007;56:1169-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Casanova MJ, Chaparro M, Martínez S, Vicuña I, Gisbert JP. Severe adalimumab-induced thrombocytopenia in a patient with Crohn's disease. J Crohns Colitis. 2012;6:1034-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Bessissow T, Renard M, Hoffman I, Vermeire S, Rutgeerts P, Van Assche G. Review article: non-malignant haematological complications of anti-tumour necrosis factor alpha therapy. Aliment Pharmacol Ther. 2012;36:312-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Vermeire S, Noman M, Van Assche G, Baert F, Van Steen K, Esters N, Joossens S, Bossuyt X, Rutgeerts P. Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn's disease: a prospective cohort study. Gastroenterology. 2003;125:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 236] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | Iborra M, Beltrán B, Bastida G, Aguas M, Nos P. Infliximab and adalimumab-induced psoriasis in Crohn's disease: a paradoxical side effect. J Crohns Colitis. 2011;5:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Moran GW, Lim AW, Bailey JL, Dubeau MF, Leung Y, Devlin SM, Novak K, Kaplan GG, Iacucci M, Seow C, Martin L, Panaccione R, Ghosh S. Review article: dermatological complications of immunosuppressive and anti-TNF therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;8:1002-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Prinz JC. Autoimmune-like syndromes during TNF blockade: does infection have a role? Nat Rev Rheumatol. 2011;7:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Deepak P, Stobaugh DJ, Sherid M, Sifuentes H, Ehrenpreis ED. Neurological events with tumour necrosis factor alpha inhibitors reported to the Food and Drug Administration Adverse Event Reporting System. Aliment Pharmacol Ther. 2013;38:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Dixon WG, Symmons DP, Lunt M, Watson KD, Hyrich KL; British Society for Rheumatology Biologics Register Control Centre Consortium; Silman AJ; British Society for Rheumatology Biologics Register. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum. 2007;56:2896-2904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 23. | Bodro M, Paterson DL. Listeriosis in patients receiving biologic therapies. Eur J Clin Microbiol Infect Dis. 2013;32:1225-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax. 2005;60:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 321] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 25. | Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R, Esteve M, Katsanos K, Lees CW, Macmahon E, Moreels T, Reinisch W, Tilg H, Tremblay L, Veereman-Wauters G, Viget N, Yazdanpanah Y, Eliakim R, Colombel JF. European Crohn's and Colitis Organisation (ECCO). Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 746] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 26. | Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Wendling D, Auge B, Bettinger D, Lohse A, Le Huede G, Bresson-Hadni S, Toussirot E, Miguet JP, Herbein G, Di Martino V. Reactivation of a latent precore mutant hepatitis B virus related chronic hepatitis during infliximab treatment for severe spondyloarthropathy. Ann Rheum Dis. 2005;64:788-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Nathan DM, Angus PW, Gibson PR. Hepatitis B and C virus infections and anti-tumor necrosis factor-alpha therapy: guidelines for clinical approach. J Gastroenterol Hepatol. 2006;21:1366-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Toruner M, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, Colombel JF, Egan LJ. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 748] [Article Influence: 44.0] [Reference Citation Analysis (1)] |
| 30. | Stamatiades GA, Ioannou P, Petrikkos G, Tsioutis C. Fungal infections in patients with inflammatory bowel disease: A systematic review. Mycoses. 2018;61:366-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Roilides E, Dimitriadou-Georgiadou A, Sein T, Kadiltsoglou I, Walsh TJ. Tumor necrosis factor alpha enhances antifungal activities of polymorphonuclear and mononuclear phagocytes against Aspergillus fumigatus. Infect Immun. 1998;66:5999-6003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Warris A, Bjørneklett A, Gaustad P. Invasive pulmonary aspergillosis associated with infliximab therapy. N Engl J Med. 2001;344:1099-1100. [PubMed] |
| 33. | Lee JH, Slifman NR, Gershon SK, Edwards ET, Schwieterman WD, Siegel JN, Wise RP, Brown SL, Udall JN Jr, Braun MM. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 307] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 34. | Beaugerie L. Inflammatory bowel disease therapies and cancer risk: where are we and where are we going? Gut. 2012;61:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372:1441-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 419] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 36. | Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3451] [Cited by in RCA: 3575] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 37. | Nyboe Andersen N, Pasternak B, Basit S, Andersson M, Svanström H, Caspersen S, Munkholm P, Hviid A, Jess T. Association between tumor necrosis factor-α antagonists and risk of cancer in patients with inflammatory bowel disease. JAMA. 2014;311:2406-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 38. | Biancone L, Orlando A, Kohn A, Colombo E, Sostegni R, Angelucci E, Rizzello F, Castiglione F, Benazzato L, Papi C, Meucci G, Riegler G, Petruzziello C, Mocciaro F, Geremia A, Calabrese E, Cottone M, Pallone F. Infliximab and newly diagnosed neoplasia in Crohn's disease: a multicentre matched pair study. Gut. 2006;55:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Biancone L, Petruzziello C, Orlando A, Kohn A, Ardizzone S, Daperno M, Angelucci E, Castiglione F, D'Incà R, Zorzi F, Papi C, Meucci G, Riegler G, Sica G, Rizzello F, Mocciaro F, Onali S, Calabrese E, Cottone M, Pallone F. Cancer in Crohn's Disease patients treated with infliximab: a long-term multicenter matched pair study. Inflamm Bowel Dis. 2011;17:758-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Caspersen S, Elkjaer M, Riis L, Pedersen N, Mortensen C, Jess T, Sarto P, Hansen TS, Wewer V, Bendtsen F, Moesgaard F, Munkholm P; Danish Crohn Colitis Database. Infliximab for inflammatory bowel disease in Denmark 1999-2005: clinical outcome and follow-up evaluation of malignancy and mortality. Clin Gastroenterol Hepatol. 2008;6:1212-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Fidder H, Schnitzler F, Ferrante M, Noman M, Katsanos K, Segaert S, Henckaerts L, Van Assche G, Vermeire S, Rutgeerts P. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 42. | Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1366] [Cited by in RCA: 1531] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 43. | Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC; ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 733] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 44. | Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Fail Rev. 2010;15:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Janssen SP, Gayan-Ramirez G, Van den Bergh A, Herijgers P, Maes K, Verbeken E, Decramer M. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111:996-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 46. | Pagani FD, Baker LS, Hsi C, Knox M, Fink MP, Visner MS. Left ventricular systolic and diastolic dysfunction after infusion of tumor necrosis factor-alpha in conscious dogs. J Clin Invest. 1992;90:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 196] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, Toutouza M, Stefanadis C. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112:2193-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 369] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 48. | Milo O, Cotter G, Kaluski E, Brill A, Blatt A, Krakover R, Vered Z, Hershkoviz R. Comparison of inflammatory and neurohormonal activation in cardiogenic pulmonary edema secondary to ischemic versus nonischemic causes. Am J Cardiol. 2003;92:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 1263] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 50. | Mebazaa A, Pang PS, Tavares M, Collins SP, Storrow AB, Laribi S, Andre S, Mark Courtney D, Hasa J, Spinar J, Masip J, Frank Peacock W, Sliwa K, Gayat E, Filippatos G, Cleland JG, Gheorghiade M. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J. 2010;31:832-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Aronson D, Burger AJ. Relation between pulse pressure and survival in patients with decompensated heart failure. Am J Cardiol. 2004;93:785-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Inge PJ, Mehra MR, O'Connor CM, Reynolds D, Walsh MN, Yancy CW. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122:585-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 390] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 53. | Wang NC, Maggioni AP, Konstam MA, Zannad F, Krasa HB, Burnett JC Jr, Grinfeld L, Swedberg K, Udelson JE, Cook T, Traver B, Zimmer C, Orlandi C, Gheorghiade M; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008;299:2656-2666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 54. | Collins SP, Lindsell CJ, Storrow AB, Abraham WT; ADHERE Scientific Advisory Committee, Investigators and Study Group. Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med. 2006;47:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 55. | Seghatol FF, Shah DJ, Diluzio S, Bello D, Johnson MR, Cotts WG, O'Donohue JA, Bonow RO, Gheorghiade M, Rigolin VH. Relation between contractile reserve and improvement in left ventricular function with beta-blocker therapy in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2004;93:854-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Tavazzi L, Maggioni AP, Lucci D, Cacciatore G, Ansalone G, Oliva F, Porcu M; Italian survey on Acute Heart Failure Investigators. Nationwide survey on acute heart failure in cardiology ward services in Italy. Eur Heart J. 2006;27:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 171] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 57. | Forrester JS, Diamond G, Chatterjee K, Swan HJ. Medical therapy of acute myocardial infarction by application of hemodynamic subsets (second of two parts). N Engl J Med. 1976;295:1404-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 172] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Nohria A, Lewis E, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002;287:628-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 309] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 59. | Comitê Coordenador da Diretriz de Insuficiência Cardíaca; Rohde LEP, Montera MW, Bocchi EA, Clausell NO, Albuquerque DC, Rassi S, Colafranceschi AS, Freitas AF Junior, Ferraz AS, Biolo A, Barretto ACP, Ribeiro ALP, Polanczyk CA, Gualandro DM, Almeida DR, Silva ERR, Figueiredo EL, Mesquita ET, Marcondes-Braga FG, Cruz FDD, Ramires FJA, Atik FA, Bacal F, Souza GEC, Almeida GLG Junior, Ribeiro GCA, Villacorta H Junior, Vieira JL, Souza JD Neto, Rossi JM Neto, Figueiredo JA Neto, Moura LAZ, Goldraich LA, Beck-da-Silva L, Danzmann LC, Canesin MF, Bittencourt MI, Garcia MI, Bonatto MG, Simões MV, Moreira MCV, Silva MMF, Olivera MT Junior, Silvestre OM, Schwartzmann PV, Bestetti RB, Rocha RM, Simões R, Pereira SB, Mangini S, Alves SMM, Ferreira SMA, Issa VS, Barzilai VS, Martins WA. Diretriz Brasileira de Insuficiência Cardíaca Crônica e Aguda. Arq Bras Cardiol. 2018;111:436-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 60. | Schulz R. TNFalpha in myocardial ischemia/reperfusion: damage vs. protection. J Mol Cell Cardiol. 2008;45:712-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Kadokami T, McTiernan CF, Kubota T, Frye CS, Feldman AM. Sex-related survival differences in murine cardiomyopathy are associated with differences in TNF-receptor expression. J Clin Invest. 2000;106:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 62. | Sinagra E, Perricone G, Romano C, Cottone M. Heart failure and anti tumor necrosis factor-alpha in systemic chronic inflammatory diseases. Eur J Intern Med. 2013;24:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 616] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 64. | Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 65. | Sopontammarak S, Aliharoob A, Ocampo C, Arcilla RA, Gupta MP, Gupta M. Mitogen-activated protein kinases (p38 and c-Jun NH2-terminal kinase) are differentially regulated during cardiac volume and pressure overload hypertrophy. Cell Biochem Biophys. 2005;43:61-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Monden Y, Kubota T, Inoue T, Tsutsumi T, Kawano S, Ide T, Tsutsui H, Sunagawa K. Tumor necrosis factor-alpha is toxic via receptor 1 and protective via receptor 2 in a murine model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H743-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 67. | Schulz R, Heusch G. Tumor necrosis factor-alpha and its receptors 1 and 2: Yin and Yang in myocardial infarction? Circulation. 2009;119:1355-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | Irwin MW, Mak S, Mann DL, Qu R, Penninger JM, Yan A, Dawood F, Wen WH, Shou Z, Liu P. Tissue expression and immunolocalization of tumor necrosis factor-alpha in postinfarction dysfunctional myocardium. Circulation. 1999;99:1492-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 255] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 69. | Flaherty MP, Guo Y, Tiwari S, Rezazadeh A, Hunt G, Sanganalmath SK, Tang XL, Bolli R, Dawn B. The role of TNF-alpha receptors p55 and p75 in acute myocardial ischemia/reperfusion injury and late preconditioning. J Mol Cell Cardiol. 2008;45:735-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation. 2009;119:1386-1397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 71. | Coletta AP, Clark AL, Banarjee P, Cleland JG. Clinical trials update: RENEWAL (RENAISSANCE and RECOVER) and ATTACH. Eur J Heart Fail. 2002;4:559-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133-3140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1110] [Cited by in RCA: 1176] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 73. | Abedin M, Scheurich D, Reimold SC, Reimold AM. Acute coronary syndrome after infliximab infusion. Cardiology in Review. 2006;14:50-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Kwon HJ, Coté TR, Cuffe MS, Kramer JM, Braun MM. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med. 2003;138:807-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 310] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 75. | Keating E, Kelleher TB, Lahiff C. De novo Anti-TNF-α-induced Congestive Heart Failure in a Patient With Turner Syndrome and Crohn's Disease. Inflamm Bowel Dis. 2020;26:e161-e162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Grillo TG, Almeida LR, Beraldo RF, Marcondes MB, Queiróz DAR, da Silva DL, Quera R, Baima JP, Saad-Hossne R, Sassaki LY. Heart failure as an adverse effect of infliximab for Crohn's disease: A case report and review of the literature. World J Clin Cases. 2021;9:10382-10391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 77. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8225] [Cited by in RCA: 7285] [Article Influence: 1821.3] [Reference Citation Analysis (0)] |
| 78. | Khanna D, McMahon M, Furst DE. Anti-tumor necrosis factor alpha therapy and heart failure: what have we learned and where do we go from here? Arthritis Rheum. 2004;50:1040-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 79. | Sinh P, Cross R. Cardiovascular Risk Assessment and Impact of Medications on Cardiovascular Disease in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2021;27:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 80. | FDA Drug Safety Communication. FDA Approves Boxed Warning About Increased Risk of Blood Clots and Death with Higher Dose of Arthritis and Ulcerative Colitis Medicine Tofacitinib (Xeljanz, Xeljanz XR) 2019. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and. |
| 81. | Adedokun OJ, Xu Z, Marano C, O'Brien C, Szapary P, Zhang H, Johanns J, Leong RW, Hisamatsu T, Van Assche G, Danese S, Abreu MT, Sands BE, Sandborn WJ. Ustekinumab Pharmacokinetics and Exposure Response in a Phase 3 Randomized Trial of Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2020;18:2244-2255.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 82. | Sandborn WJ, Rutgeerts P, Gasink C, Jacobstein D, Zou B, Johanns J, Sands BE, Hanauer SB, Targan S, Ghosh S, de Villiers WJS, Colombel JF, Feagan BG. Long-term efficacy and safety of ustekinumab for Crohn's disease through the second year of therapy. Aliment Pharmacol Ther. 2018;48:65-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 83. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1865] [Article Influence: 155.4] [Reference Citation Analysis (1)] |
| 84. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1568] [Article Influence: 130.7] [Reference Citation Analysis (1)] |
| 85. | Narula N, Peerani F, Meserve J, Kochhar G, Chaudrey K, Hartke J, Chilukuri P, Koliani-Pace J, Winters A, Katta L, Shmidt E, Hirten R, Faleck D, Parikh MP, Whitehead D, Boland BS, Singh S, Sagi SV, Fischer M, Chang S, Barocas M, Luo M, Lasch K, Bohm M, Lukin D, Sultan K, Swaminath A, Hudesman D, Gupta N, Shen B, Kane S, Loftus EV, Siegel CA, Sands BE, Colombel JF, Sandborn WJ, Dulai PS. Vedolizumab for Ulcerative Colitis: Treatment Outcomes from the VICTORY Consortium. Am J Gastroenterol. 2018;113:1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 86. | Chaparro M, Garre A, Ricart E, Iborra M, Mesonero F, Vera I, Riestra S, García-Sánchez V, Luisa De Castro M, Martin-Cardona A, Aldeguer X, Mínguez M, de-Acosta MB, Rivero M, Muñoz F, Andreu M, Bargalló A, González-Muñoza C, Pérez Calle JL, García-Sepulcre MF, Bermejo F, Huguet JM, Cabriada JL, Gutiérrez A, Mañosa M, Villoria A, Carbajo AY, Lorente R, García-López S, Piqueras M, Hinojosa E, Arajol C, Sicilia B, Conesa AM, Sainz E, Almela P, Llaó J, Roncero O, Camo P, Taxonera C, Domselaar MV, Pajares R, Legido J, Madrigal R, Lucendo AJ, Alcaín G, Doménech E, Gisbert JP; GETECCU study group. Short and long-term effectiveness and safety of vedolizumab in inflammatory bowel disease: results from the ENEIDA registry. Aliment Pharmacol Ther. 2018;48:839-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 87. | Loftus EV Jr, Colombel JF, Feagan BG, Vermeire S, Sandborn WJ, Sands BE, Danese S, D'Haens GR, Kaser A, Panaccione R, Rubin DT, Shafran I, McAuliffe M, Kaviya A, Sankoh S, Mody R, Abhyankar B, Smyth M. Long-term Efficacy of Vedolizumab for Ulcerative Colitis. J Crohns Colitis. 2017;11:400-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 88. | Meserve J, Aniwan S, Koliani-Pace JL, Shashi P, Weiss A, Faleck D, Winters A, Chablaney S, Kochhar G, Boland BS, Singh S, Hirten R, Shmidt E, Hartke JG, Chilukuri P, Bohm M, Sagi SV, Fischer M, Lukin D, Hudesman D, Chang S, Gao Y, Sultan K, Swaminath A, Gupta N, Kane S, Loftus EV Jr, Shen B, Sands BE, Colombel JF, Siegel CA, Sandborn WJ, Dulai PS. Retrospective Analysis of Safety of Vedolizumab in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2019;17:1533-1540.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 89. | Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1585] [Cited by in RCA: 1448] [Article Influence: 181.0] [Reference Citation Analysis (0)] |
| 90. | Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, Burisch J, De Vos M, De Vries AM, Dick AD, Juillerat P, Karlsen TH, Koutroubakis I, Lakatos PL, Orchard T, Papay P, Raine T, Reinshagen M, Thaci D, Tilg H, Carbonnel F; European Crohn’s and Colitis Organisation. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:239-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 546] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 91. | Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol. 2018;113:481-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 932] [Article Influence: 133.1] [Reference Citation Analysis (0)] |
| 92. | Brazilian Study Group of Inflammatory Bowel Diseases. Consensus guidelines for the management of inflammatory bowel disease. Arq Gastroenterol. 2010;47:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 93. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group; Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1402] [Cited by in RCA: 1567] [Article Influence: 261.2] [Reference Citation Analysis (0)] |
| 94. | Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, Deane KD, Genovese M, Huston KK, Kerr G, Kremer J, Nakamura MC, Russell LA, Singh JA, Smith BJ, Sparks JA, Venkatachalam S, Weinblatt ME, Al-Gibbawi M, Baker JF, Barbour KE, Barton JL, Cappelli L, Chamseddine F, George M, Johnson SR, Kahale L, Karam BS, Khamis AM, Navarro-Millán I, Mirza R, Schwab P, Singh N, Turgunbaev M, Turner AS, Yaacoub S, Akl EA. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2021;73:924-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 558] [Article Influence: 139.5] [Reference Citation Analysis (0)] |