Published online Mar 26, 2023. doi: 10.4330/wjc.v15.i3.95
Peer-review started: November 25, 2022
First decision: December 13, 2022
Revised: January 4, 2023
Accepted: March 1, 2023
Article in press: March 1, 2023
Published online: March 26, 2023
Processing time: 115 Days and 10.6 Hours
Atrioventricular block requiring permanent pacemaker (PPM) implantation is an important complication of transcatheter aortic valve replacement (TAVR). Application of machine learning could potentially be used to predict pre-procedural risk for PPM.
To apply machine learning to be used to predict pre-procedural risk for PPM.
A retrospective study of 1200 patients who underwent TAVR (January 2014-December 2017) was performed. 964 patients without prior PPM were included for a 30-d analysis and 657 patients without PPM requirement through 30 d were included for a 1-year analysis. After the exclusion of variables with near-zero variance or ≥ 50% missing data, 167 variables were included in the random forest gradient boosting algorithm (GBM) optimized using 5-fold cross-validations repeated 10 times. The receiver operator curve (ROC) for the GBM model and PPM risk score models were calculated to predict the risk of PPM at 30 d and 1 year.
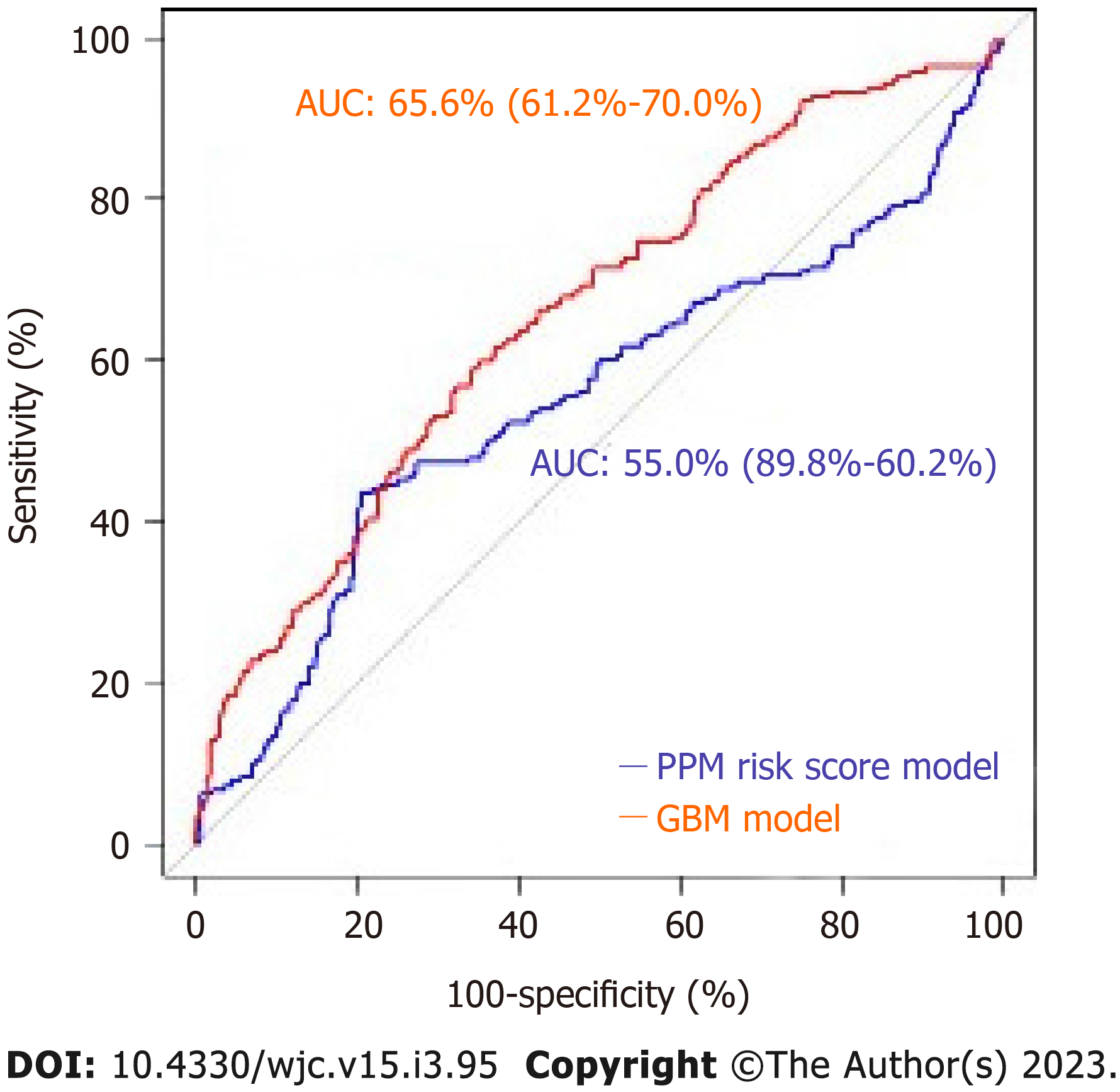
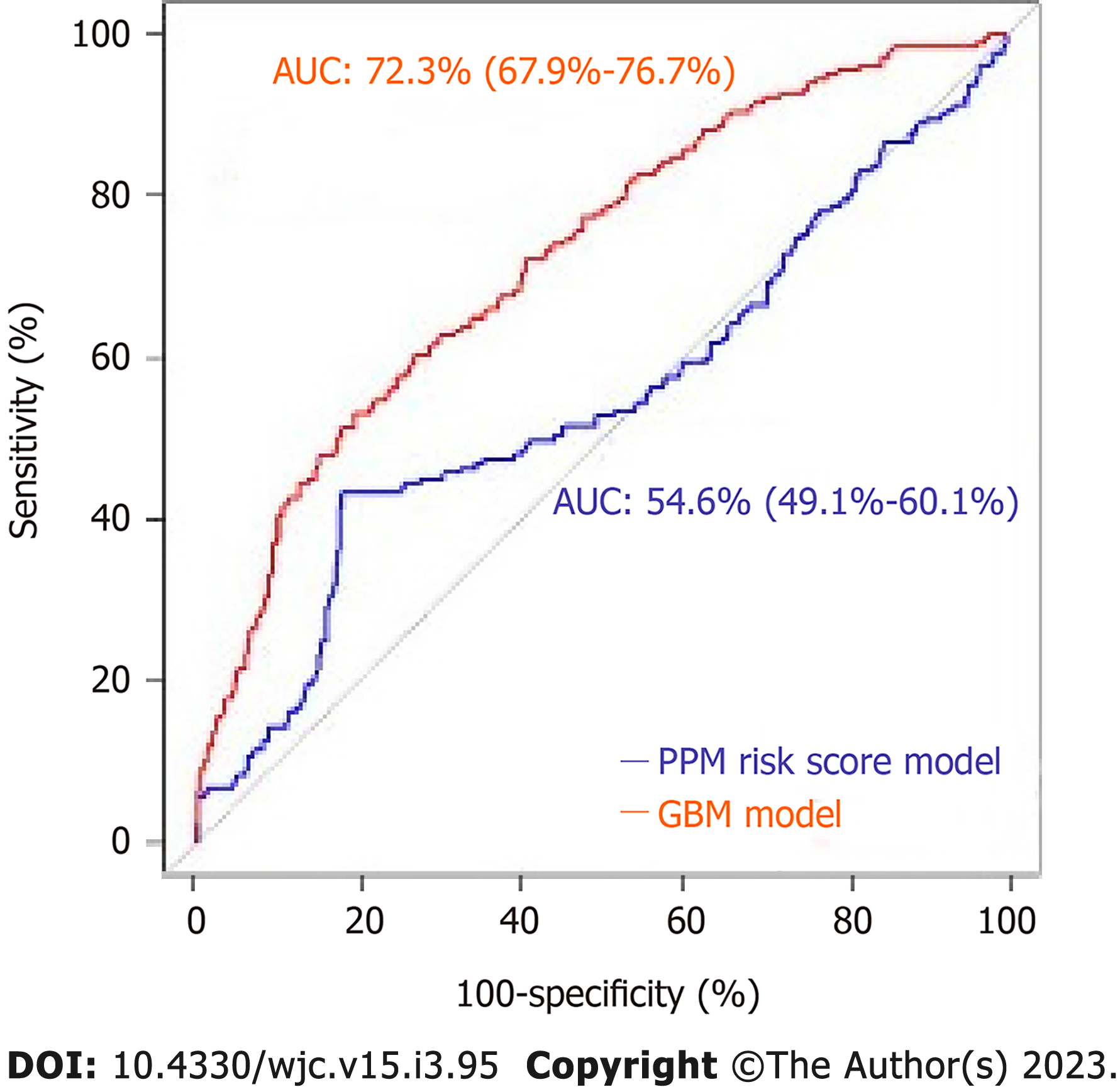
Of 964 patients included in the 30-d analysis without prior PPM, 19.6% required PPM post-TAVR. The mean age of patients was 80.9 ± 8.7 years. 42.1 % were female. Of 657 patients included in the 1-year analysis, the mean age of the patients was 80.7 ± 8.2. Of those, 42.6% of patients were female and 26.7% required PPM at 1-year post-TAVR. The area under ROC to predict 30-d and 1-year risk of PPM for the GBM model (0.66 and 0.72) was superior to that of the PPM risk score (0.55 and 0.54) with a P value < 0.001.
The GBM model has good discrimination and calibration in identifying patients at high risk of PPM post-TAVR.
Core Tip: Atrioventricular block requiring permanent pacemaker (PPM) implantation is an important complication of transcatheter aortic valve replacement. Application of machine learning could potentially be used to predict pre-procedural risk for PPM. Machine learning was used to predict patients who are at risk of developing conduction abnormalities requiring PPM at 30 d and 1 year. Our random forest machine learning model using machine learning outperforms PPM risk score model in its predictive value. Brachiocephalic to annulus distance to height ratio is the highest weighted predictor of PPM implantation at both 30-d and 1-year, which has not been previously described in the literature.
- Citation: Agasthi P, Ashraf H, Pujari SH, Girardo M, Tseng A, Mookadam F, Venepally N, Buras MR, Abraham B, Khetarpal BK, Allam M, MD SKM, Eleid MF, Greason KL, Beohar N, Sweeney J, Fortuin D, Holmes DRJ, Arsanjani R. Prediction of permanent pacemaker implantation after transcatheter aortic valve replacement: The role of machine learning. World J Cardiol 2023; 15(3): 95-105
- URL: https://www.wjgnet.com/1949-8462/full/v15/i3/95.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i3.95
Transcatheter aortic valve replacement (TAVR) is increasingly being used in preference to surgical aortic valve replacement (SAVR) in patients with aortic stenosis[1-3]. The most common complication of TAVR remains the development of atrioventricular conduction abnormalities, requiring permanent pacemaker (PPM) implantation, despite the use of improved implant performance and newer generation valves[4-12]. PPM is associated with increased length of hospital stay and mortality[13]. Additionally, advanced conduction defects requiring PPM implantation have been demonstrated to lead to worse functional capacity and clinical outcomes in patients with aortic stenosis[1-4]. The PPM requirement rate in TAVR is two to five-fold higher than in SAVR[15,16]. Certain baseline characteristics such as age, gender, pre-existing atrioventricular block, right bundle branch block, left bundle branch block[17,18], and size of the left ventricular outflow tract (LVOT), as well as procedure-related factors such as implantation depth have been shown to be associated with PPM requirement risk. Previous studies that evaluated risk factors associated with PPM requirement used data for older-generation valves and included only a limited number of variables, thus limiting their predictive potential[11,13,19,20]. Consequently, it is very important to risk stratify patients for potential need of PPM implantation post-procedure. Artificial intelligence (AI) refers broadly to analytical algorithms that iteratively learn from data, enabling machines to find hidden insights without the need for explicit programming where to look[21-24]. Machine learning (ML) is a computer science sector that uses computer algorithms to identify patterns with a multitude of variables in large datasets and thereby anticipates various data-based outcomes[25]. In this study, we used supervised ML with the gradient boosting machine learning model (GBM) to predict pre-procedural risk for PPM post-TAVR at 30 d and 1 year.
We performed a retrospective study on all patients with severe symptomatic aortic stenosis who underwent TAVR at the Mayo Clinic hospitals in Rochester, MN, Phoenix, AZ, and Jacksonville, FL between January 1, 2012, and December 30, 2017. The Mayo Clinic Institutional Review Board (IRB) approved the study protocol and research authorization to utilize medical information for clinical research was provided by the patients. A retrospective chart review of the electronic health record was used to collect baseline data, and clinical coordinators were contacted for information on follow-up visits. We identified 285 clinical variables for potential inclusion into the ML algorithm.
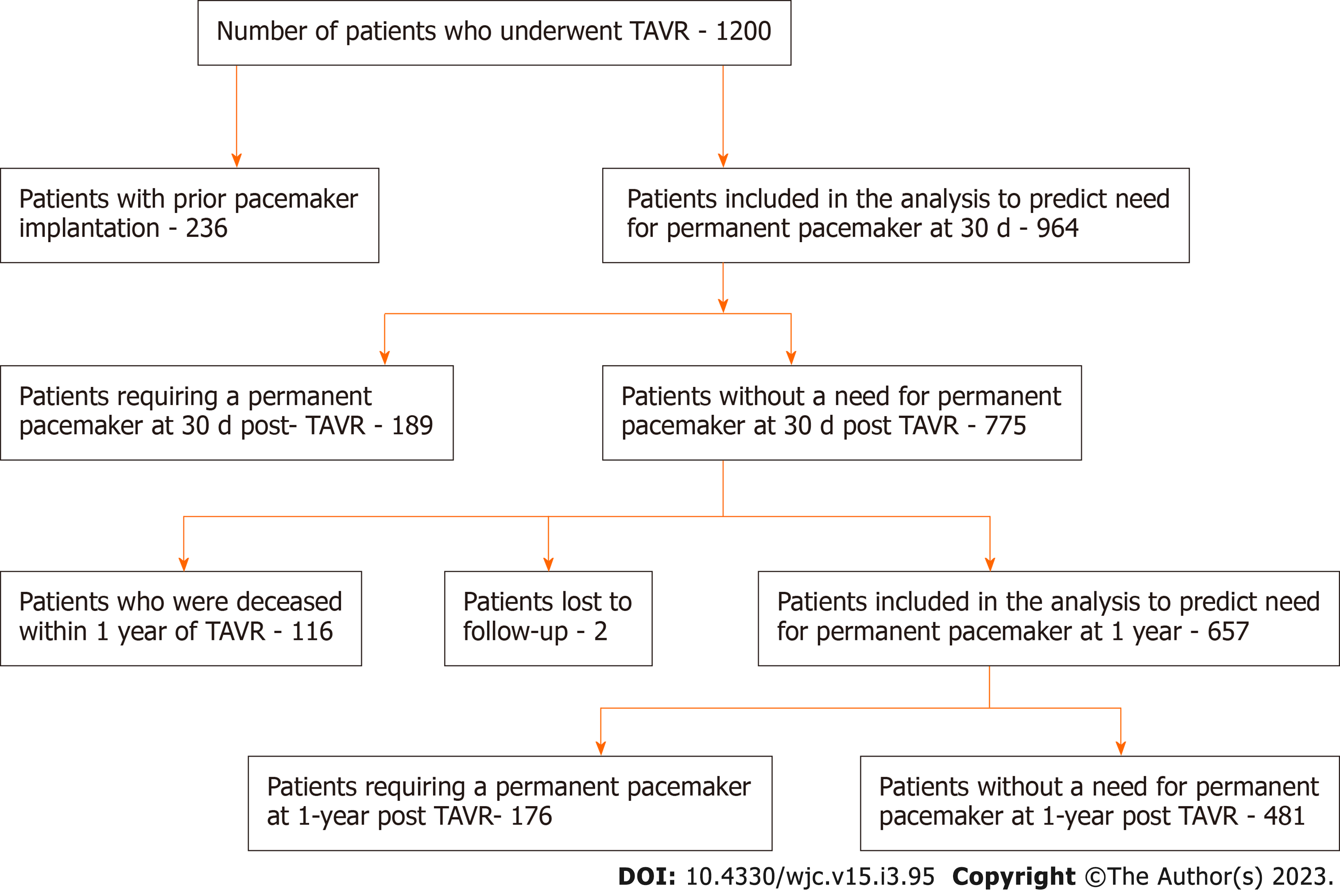
Out of 1200 patients, 236 individuals with prior pacemakers were excluded. The remaining 964 patients were included in the 30-d PPM risk prediction analysis. We first eliminated all variables with ≥ 50% missing and near-zero variance, where variables with near-zero variance have one unique value or the majority of the data is comprised within a single category. The GBM algorithm handles missing data internally by treating “missing” as its own category. This left 147 out of 285 variables to be included in the model. These variables were used to predict the risk of pacemakers 30 d post-TAVR using the GBM model. The model was optimized using 5-fold cross-validation repeated 10 times to get the highest prediction accuracy. Among the 964 patients without prior PPM who have undergone TAVR, 189 patients required PPM implantation by 30 d, 116 patients were deceased by 1 year, and 2 patients were lost to follow-up, leaving 657 patients who were included in the final analysis to predict the need for PPM at 1 year. There were 287 variables initially, but all variables with ≥50% missing or near-zero variance were eliminated leaving a total of 163 variables. Patient recruitment is summarized in Figure 1.
Clinical variables, comorbidities, and procedural factors were obtained from chart review. Definitions conformed to those provided by the Transcatheter Valve Therapy (TVT) Registry[26]. Echocardiographic variables were collected using standard ultrasound scanners. Comprehensive Doppler and 2-Dimensional Transthoracic Echocardiogram (TTE) were performed prior to the procedure. TTE images were acquired and interpreted according to the European Association of Echocardiography and American Society of Echocardiography guidelines. Multi-detector computed tomography (MDCT) was performed a month before the treatment. The size of the aortic annulus was determined pre-procedure.
The study population data set (n = 964 and n = 657) for 30 d and 1 year, respectively, had low event rates. Due to a small percentage of events, the entire data set was used in the modeling phase and was not broken into a test and train cohort. The caret R package was used to fit a GBM model from the gbm3R package using 5-fold cross-validation repeated 10 times. Model hyperparameters, specified prior to fitting the model, are tunable variables that control the chosen model’s learning process. The hyperparameters tuned were the interaction depth, number of trees, and shrinkage. The minimum number of observations required at each node was fixed at 20. Figures 2 and 4 include the top 20 variables that indicate which have the highest predictive power in classifying those with events and those without events. The study population for PPM risk was limited to those that had a trans-femoral or trans-apical approach. The PPM risk score developed by Vejpongsa et al[20] uses 6 factors. Each factor had points associated that collapsed into a three-group score (low, moderate, or high risk). Tuning of hyperparameters optimizes the target metric, that metric being the area under the receiver operating characteristic curve (AUC). AUC is a numeric metric that measures how well the model can distinguish between patients with PPM and those without PPM.
The predicted probabilities that were generated on each fold were stacked, which was repeated 10 times for each patient. The model took the average of the predicted probabilities of all 10 repeats; the average predicted probabilities for each patient were then used to compute the final AUC. The pROC R package was used to produce the ROC curves along with the 95%CI for the AUC (Figures 3 and 5). Variable importance is determined by calculating the relative influence of each variable included in the model. The variable importance plot provides a ranked list of the most significant variables in descending order.
The caret R package was used to fit a logistic regression using 5-fold cross-validation repeated 10 times. Similar to the GBM model, this process also used 5-fold cross-validation repeated 10 times, where the predicted probabilities for each fold were stacked and then averaged over all 10 repeats for each patient. The average predicted probabilities of PPM risk for each patient were used to produce the final AUC. Categorical and ordinal variables were compared either with the chi-square or Fisher exact tests and are expressed as numbers and percentages. Continuous variables were compared with the t-test and expressed as mean ± SD. Pearson’s χ2 test and Analysis of Variance were used to assess the baseline differences. A P < 0.05 was considered significant. R software version 3.4.1 (Foundation of Statistical Computing, Vienna, Austria) was used to run the analysis. Baseline characteristics, echocardiographic variables, EKG variables, and MDCT variables for 30 d and 1-year analysis are shown in the Supplementary material. Marlene Girardo and Matthew Buras are the statisticians who ran the analysis and are also authors of the paper.
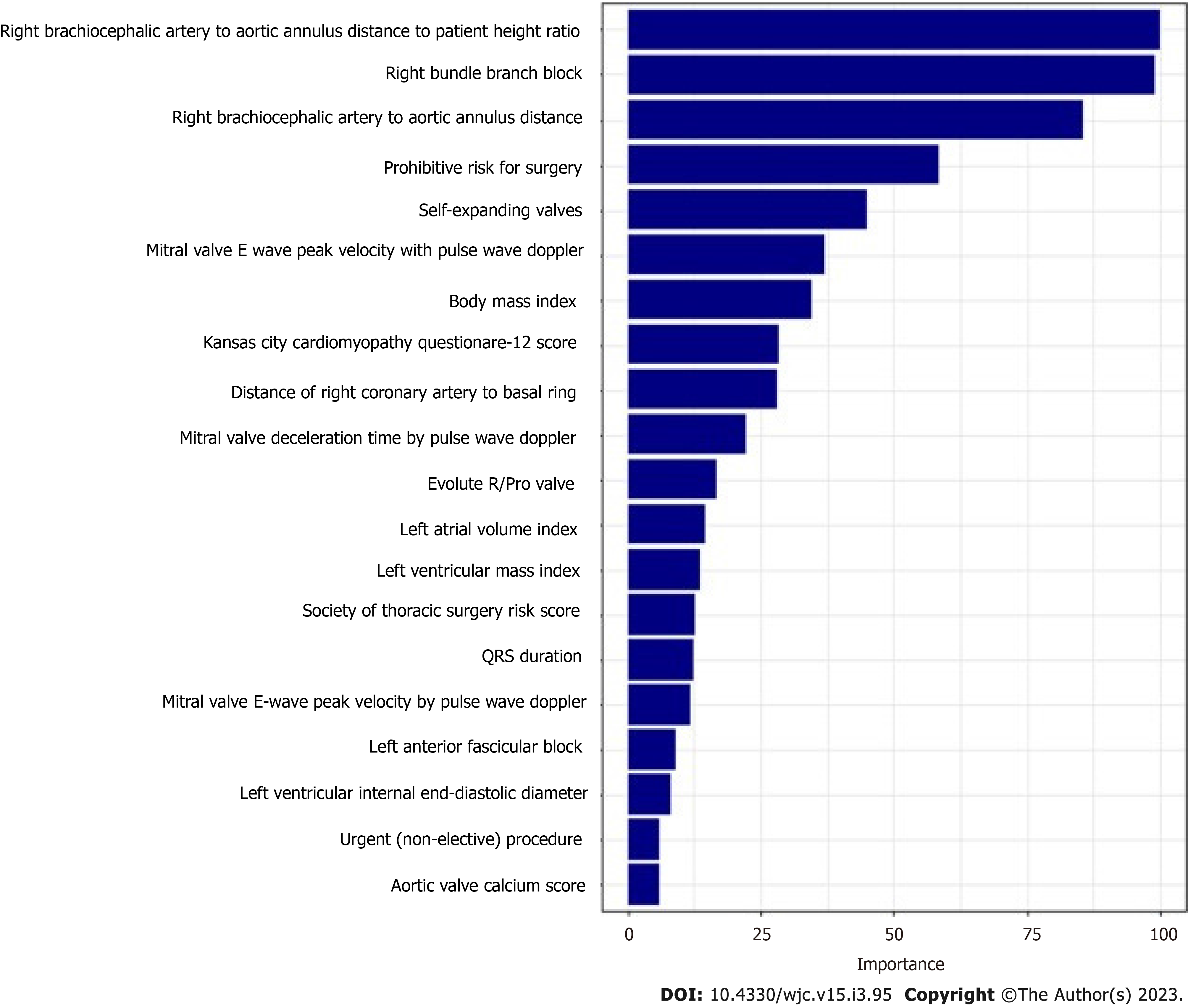
The mean age of the patients was 80.9 ± 8.7. 42.1% of patients were female and 19.6% (n = 189) required PPM at 30 d post-TAVR. 68.8% of the entire patient cohort had a balloon-expandable valve. Patients requiring PPM post-TAVR had higher proportions of prior percutaneous coronary interventions, aspirin use, trans-femoral access, self-expandable valve use, and New York Heart Association heart failure class III/IV as compared to those who did not require PPM post-TAVR. Other baseline differences between the two groups can be seen in the Supplementary material. Using our GBM machine learning algorithm, a scoring model using the 20 highest weighted predictors of PPM requirement post-TAVR was generated. The highest weighted characteristic was a higher brachiocephalic artery to annulus distance to patient height ratio, followed by right bundle branch block (RBBB), higher brachiocephalic to aortic annulus distance, high pre-operative risk, and the use of self-expanding valves (as opposed to balloon expandable valves). Figure 2 shows the full list with the relative weights of the twenty variables. The area under ROC to predict the need for PPM at 30 d for the GBM model was 0.66 (95%CI: 0.61-0.70) vs 0.55 (95%CI: 0.49-0.60) for the PPM risk score model (P < 0.001). The comparison of the ROC curves of both models is shown in Figure 3.
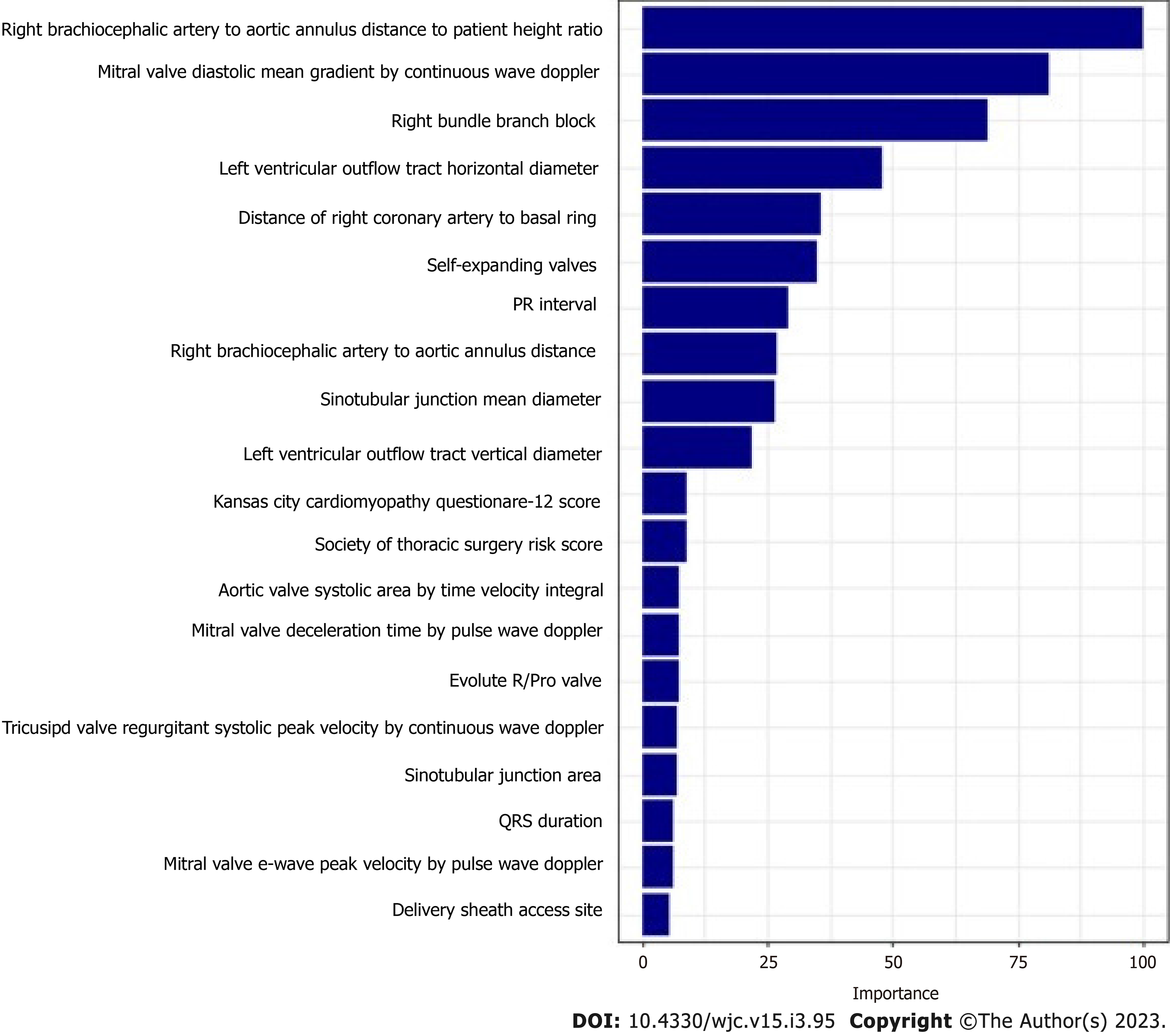
The mean age of the patients was 80.7 ± 8.2. 42.6% of patients were female and 26.7% (n = 176) required PPM at 1-year post-TAVR. 67.6% of the entire patient cohort had a balloon-expandable valve. Patients requiring PPM at 1-year post-TAVR had higher proportions of prior aortic valve intervention, aspirin use, severe mitral stenosis, elevated filling pressures, and percutaneous transfemoral access compared to those who did not require PPM at 1 year. Other baseline differences can be seen in the Supplementary material. Based on the GBM machine learning algorithm, a scoring model using the 20 highest weighted predictors of PPM dependency at 1-year post-TAVR was generated. The five highest weighted predictors were higher brachiocephalic artery to annulus distance to height ratio, higher mitral valve diastolic mean gradient, RBBB, higher LVOT diameter, and higher distance of right coronary artery to basal ring (mm). Figure 4 shows all twenty variables with the highest weightage. The area under ROC to predict the need for PPM at 1 year for the GBM model was 0.72 (95%CI: 0.67-0.76) vs 0.54 (95%CI: 0.49-0.60) for the PPM risk score model (P value < 0.001). The comparison of the ROC curves of both models is shown in Figure 5.
Given the clinical relevance of conduction abnormalities necessitating PPM, we sought to develop a risk assessment tool to predict PPM implantation in patients post-TAVR using machine learning (ML). ML seeks to mimic the thought process, learning capacity, and storage of knowledge of humans[28]. Its techniques have been in use in cardiovascular medicine, but our study is the first to predict the risk of PPM implantation in patients post-TAVR. This study demonstrates that ML could be used to accurately predict the requirement of PPM at 1-year post-TAVR with a high level of discriminatory ability. The GBM model had a modest level of discriminatory ability to predict the requirement of PPM at 30 d. Arteriovenous conduction disturbances are well-known post-TAVR. The most common conduction abnormalities post-TAVR are left bundle branch block (LBBB) and complete heart block[30,31]. Multiple mechanistic reasons for these abnormalities have been theorized, and the most popular one is that the spatial proximity of the cardiac conduction system to the calcified aortic valve[32,33], as well as the underlying conduction disease prevalence in this elderly group[34], predisposes it to damage during the TAVR procedure. Many patients require placement of PPM post-TAVR, with an incidence of 10%-15% commonly cited in the literature, with substantial variability based on the specific TAVR valve used[4]. Conduction abnormalities are clinically relevant as these patients have a higher incidence of subsequent hospitalizations, less improvement in LV function and functional status after TAVR, and possibly even higher mortality, though there is conflicting evidence regarding the latter and long-term prognosis[11,13,30,35].
The rate of PPM implantation post-TAVR in our study was 19.6% at 30 d and 26.7% at 1 year, which is similar to previous trials[8,36-39]. Pre-existing conduction abnormalities such as RBBB, LBBB, and 1st-degree AV block were significantly associated with post-TAVR PPM implantation, and these are consistent with the previous studies[12,13]. Trans-femoral access was also significantly correlated with the PPM rate, which has also been described as a risk factor in a prior registry[13]. Another variable that strongly associates with the PPM rate was self-expanding valves which are also known through prior studies[12,13]. High rates (13.3%-17%) of implantation with the Edwards Sapien 3 valve have previously been demonstrated which was also consistent with our study[19,36,40]. Brachiocephalic artery to aortic valve annulus distance to height ratio was the highest weighted predictor for PPM implantation post-TAVR at both one month and one year. As far as we are aware, we are the first to describe this variable as a predictor for PPM requirement, let alone as the highest weight predictor. It is not clear why it is associated with conduction abnormalities requiring PPM. We suspect that the longer distance of the ascending aorta proximal to the origin of the brachiocephalic artery allows for the TAVR valve to hug the outer curve of the aorta more, thus exerting more force on the right/non-cusp side where the conduction system lies. This needs to be confirmed in other studies.
Overall, the model used for the 30-d and 1-year predictors yielded a very similar set of variables. The main difference was the presence of mitral valve diastolic mean gradient on echo which was the second highest weighted predictor for PPM at 1 year but was not present in the 30-d predictive model. Whether it is the gradient itself that is associated with conduction abnormalities or the mitral annular calcification that is presumably associated with such gradients and would be expected in such populations with calcific aortic stenosis is unclear. The mitral valve and annular calcification were not one of our echocardiographic parameters that were included in the study, so further studies need to be completed. The subsequent evaluation of whether mitral valve or annular calcification is associated with conduction abnormalities independent of AS and TAVR is an obvious corollary. The comparison of our predictive model with the PPM risk score developed by Vejpongsa et al[20] which uses 6 variables for scoring, demonstrates the enhanced prognostic capability of our model (Figures 3 and 5). Other risk score models for PPM requirement post-TAVR that have been described are the Emory Risk Score developed by Kiani et al[19] and the risk score developed by Maeno et al[41]. We were unable to compare our model with these risk score models due to a lack of complete variables, including the history of syncope in the Emory risk score, and membranous septum (MS) length in the risk score. Some of the limitations of this study need to be noted. Firstly, the model is complex, and therefore its use may be limited in clinical practice. Additionally, given the large number of demographic information and clinical variables included in this model, these variables may not always be present. Nevertheless, we feel that the prognosticating ability of the model overcomes this limitation and that with the increasing use of electronic medical records, most data is available. Secondly, this was primarily a feasibility study and is retrospective in nature, which restricts our ability for defining causal associations. There is a need for prospective validation with an external cohort. Thirdly, we did not include a few variables in our model that have been included in other risk scores for PPM implantation, such as a history of syncope or distal landing zone calcium burden, as these variables were not present in enough of our cohort to include. Thus, there is a potential for change in the analysis with the inclusion of such variables. Lastly, the study included primarily referred patients in three high-volume tertiary care centers, and thus are likely higher risk and more complex than the average TAVR patient.
Machine learning was used to predict patients who are at risk of developing conduction abnormalities requiring PPM at 30 d and 1 year. Our GBM model using machine learning outperforms the PPM risk score model in its predictive value. Brachiocephalic to annulus distance to height ratio is the highest weighted predictor of PPM implantation at both 30 d and 1 year, which has not been previously described in the literature.
For aortic stenosis, it is a fact that transcatheter aortic valve replacement use has greatly increased relative to surgical replacement with the most common complications of the procedure including atrioventricular conduction abnormalities development and permanent pacemaker requirement (PPM). Hence, it is essential to risk stratify patients for potential need of PPM implantation post-procedure. We used artificial intelligence to predict pre-procedural risk for pacemaker placement post-transcatheter aortic valve replacement at 30 d and 1 year.
Previous studies that evaluated risk factors associated with permanent pacemaker requirement used data for older-generation valves and also included only a limited number of variables and hence, limiting their predictive potential. Artificial intelligence does a remarkable job of predicting variables via machine learning and the same has been used in our study.
To predict pre-procedural risk for permanent pacemaker post-transcatheter aortic valve replacement (TAVR) at 30 d and 1 year.
We performed a retrospective study on patients with severe symptomatic aortic stenosis who underwent transcatheter aortic valve replacement (TAVR). Gradient boosting machine learning model has been used for predicting probabilities.
For 30-d analysis, higher brachiocephalic artery to annulus distance to patient height ratio was the highest weighted characteristic that predicted PPM placement post- TAVR. Also for 1-year analysis, higher brachiocephalic artery to annulus distance to patient height ratio was the highest weighted characteristic that predicted PPM placement post- TAVR.
Brachiocephalic to annulus distance to height ratio is the highest weighted predictor of PPM implantation in the study both at 30 d and 1 year and it was not been previously described in the literature.
We sought to develop and have developed a risk assessment tool to predict PPM implantation post-TAVR using machine learning.
This publication was funded by Mayo Clinic Arizona Cardiovascular Clinical Research Center (MCA CV CRC). We are thankful for their generous support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Byeon H, South Korea; Morya AK, India S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5086] [Cited by in RCA: 5501] [Article Influence: 366.7] [Reference Citation Analysis (1)] |
| 2. | Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG; PARTNER 2 Investigators. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3232] [Cited by in RCA: 3815] [Article Influence: 423.9] [Reference Citation Analysis (0)] |
| 3. | Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR; PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3668] [Cited by in RCA: 3491] [Article Influence: 581.8] [Reference Citation Analysis (0)] |
| 4. | Holmes DR Jr, Nishimura RA, Grover FL, Brindis RG, Carroll JD, Edwards FH, Peterson ED, Rumsfeld JS, Shahian DM, Thourani VH, Tuzcu EM, Vemulapalli S, Hewitt K, Michaels J, Fitzgerald S, Mack MJ; STS/ACC TVT Registry. Annual Outcomes With Transcatheter Valve Therapy: From the STS/ACC TVT Registry. J Am Coll Cardiol. 2015;66:2813-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 5. | Rodés-Cabau J. Transcatheter aortic valve implantation: current and future approaches. Nat Rev Cardiol. 2011;9:15-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Généreux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, Xu K, Smith C, Serruys PW, Kappetein AP, Leon MB. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012;59:2317-2326. [RCA] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 461] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 7. | Binder RK, Webb JG, Toggweiler S, Freeman M, Barbanti M, Willson AB, Alhassan D, Hague CJ, Wood DA, Leipsic J. Impact of post-implant SAPIEN XT geometry and position on conduction disturbances, hemodynamic performance, and paravalvular regurgitation. JACC Cardiovasc Interv. 2013;6:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | De Torres-Alba F, Kaleschke G, Diller GP, Vormbrock J, Orwat S, Radke R, Reinke F, Fischer D, Reinecke H, Baumgartner H. Changes in the Pacemaker Rate After Transition From Edwards SAPIEN XT to SAPIEN 3 Transcatheter Aortic Valve Implantation: The Critical Role of Valve Implantation Height. JACC Cardiovasc Interv. 2016;9:805-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 9. | Ferreira ND, Caeiro D, Adão L, Oliveira M, Gonçalves H, Ribeiro J, Teixeira M, Albuquerque A, Primo J, Braga P, Simões L, Ribeiro VG. Incidence and predictors of permanent pacemaker requirement after transcatheter aortic valve implantation with a self-expanding bioprosthesis. Pacing Clin Electrophysiol. 2010;33:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Husser O, Pellegrini C, Kessler T, Burgdorf C, Thaller H, Mayr NP, Kasel AM, Kastrati A, Schunkert H, Hengstenberg C. Predictors of Permanent Pacemaker Implantations and New-Onset Conduction Abnormalities With the SAPIEN 3 Balloon-Expandable Transcatheter Heart Valve. JACC Cardiovasc Interv. 2016;9:244-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 11. | Nazif TM, Dizon JM, Hahn RT, Xu K, Babaliaros V, Douglas PS, El-Chami MF, Herrmann HC, Mack M, Makkar RR, Miller DC, Pichard A, Tuzcu EM, Szeto WY, Webb JG, Moses JW, Smith CR, Williams MR, Leon MB, Kodali SK; PARTNER Publications Office. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc Interv. 2015;8:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 444] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 12. | Siontis GC, Jüni P, Pilgrim T, Stortecky S, Büllesfeld L, Meier B, Wenaweser P, Windecker S. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol. 2014;64:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 510] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 13. | Fadahunsi OO, Olowoyeye A, Ukaigwe A, Li Z, Vora AN, Vemulapalli S, Elgin E, Donato A. Incidence, Predictors, and Outcomes of Permanent Pacemaker Implantation Following Transcatheter Aortic Valve Replacement: Analysis From the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2016;9:2189-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 270] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 14. | Houthuizen P, Van Garsse LA, Poels TT, de Jaegere P, van der Boon RM, Swinkels BM, Ten Berg JM, van der Kley F, Schalij MJ, Baan J Jr, Cocchieri R, Brueren GR, van Straten AH, den Heijer P, Bentala M, van Ommen V, Kluin J, Stella PR, Prins MH, Maessen JG, Prinzen FW. Left bundle-branch block induced by transcatheter aortic valve implantation increases risk of death. Circulation. 2012;126:720-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Cao C, Ang SC, Indraratna P, Manganas C, Bannon P, Black D, Tian D, Yan TD. Systematic review and meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg. 2013;2:10-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 16. | Hamm CW, Möllmann H, Holzhey D, Beckmann A, Veit C, Figulla HR, Cremer J, Kuck KH, Lange R, Zahn R, Sack S, Schuler G, Walther T, Beyersdorf F, Böhm M, Heusch G, Funkat AK, Meinertz T, Neumann T, Papoutsis K, Schneider S, Welz A, Mohr FW; GARY-Executive Board. The German Aortic Valve Registry (GARY): in-hospital outcome. Eur Heart J. 2014;35:1588-1598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 253] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 17. | Nazif TM, Williams MR, Hahn RT, Kapadia S, Babaliaros V, Rodés-Cabau J, Szeto WY, Jilaihawi H, Fearon WF, Dvir D, Dewey TM, Makkar RR, Xu K, Dizon JM, Smith CR, Leon MB, Kodali SK. Clinical implications of new-onset left bundle branch block after transcatheter aortic valve replacement: analysis of the PARTNER experience. Eur Heart J. 2014;35:1599-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 18. | Schroeter T, Linke A, Haensig M, Merk DR, Borger MA, Mohr FW, Schuler G. Predictors of permanent pacemaker implantation after Medtronic CoreValve bioprosthesis implantation. Europace. 2012;14:1759-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Kiani S, Kamioka N, Black GB, Lu MLR, Lisko JC, Rao B, Mengistu A, Gleason PT, Stewart JP, Caughron H, Dong A, Patel H, Grubb KJ, Greenbaum AB, Devireddy CM, Guyton RA, Leshnower B, Merchant FM, El-Chami M, Westerman SB, Lloyd MS, Babaliaros VC, Hoskins MH. Development of a Risk Score to Predict New Pacemaker Implantation After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2019;12:2133-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 20. | Vejpongsa P, Zhang X, Bhise V, Kitkungvan D, Shivamurthy P, Anderson HV, Balan P, Nguyen TC, Estrera AL, Dougherty AH, Smalling RW, Dhoble A. Risk Prediction Model for Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement. Structural Heart. 2018;2:328-335. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Shameer K, Johnson KW, Glicksberg BS, Dudley JT, Sengupta PP. Machine learning in cardiovascular medicine: are we there yet? Heart. 2018;104:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 253] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 22. | Shouval R, Bondi O, Mishan H, Shimoni A, Unger R, Nagler A. Application of machine learning algorithms for clinical predictive modeling: a data-mining approach in SCT. Bone Marrow Transplant. 2014;49:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Deo RC. Machine Learning in Medicine. Circulation. 2015;132:1920-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1155] [Cited by in RCA: 1957] [Article Influence: 217.4] [Reference Citation Analysis (6)] |
| 24. | Edwards FH, Cohen DJ, O'Brien SM, Peterson ED, Mack MJ, Shahian DM, Grover FL, Tuzcu EM, Thourani VH, Carroll J, Brennan JM, Brindis RG, Rumsfeld J, Holmes DR Jr; Steering Committee of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Development and Validation of a Risk Prediction Model for In-Hospital Mortality After Transcatheter Aortic Valve Replacement. JAMA Cardiol. 2016;1:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 25. | Motwani M, Dey D, Berman DS, Germano G, Achenbach S, Al-Mallah MH, Andreini D, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJ, Cury RC, Delago A, Gomez M, Gransar H, Hadamitzky M, Hausleiter J, Hindoyan N, Feuchtner G, Kaufmann PA, Kim YJ, Leipsic J, Lin FY, Maffei E, Marques H, Pontone G, Raff G, Rubinshtein R, Shaw LJ, Stehli J, Villines TC, Dunning A, Min JK, Slomka PJ. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: a 5-year multicentre prospective registry analysis. Eur Heart J. 2017;38:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 262] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 26. | Carroll JD, Edwards FH, Marinac-Dabic D, Brindis RG, Grover FL, Peterson ED, Tuzcu EM, Shahian DM, Rumsfeld JS, Shewan CM, Hewitt K, Holmes DR Jr, Mack MJ. The STS-ACC transcatheter valve therapy national registry: a new partnership and infrastructure for the introduction and surveillance of medical devices and therapies. J Am Coll Cardiol. 2013;62:1026-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 27. | Breiman L. Random Forests. Machine Learning. 2001;45: 5-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 56052] [Cited by in RCA: 34116] [Article Influence: 2843.0] [Reference Citation Analysis (0)] |
| 28. | Krittanawong C, Zhang H, Wang Z, Aydar M, Kitai T. Artificial Intelligence in Precision Cardiovascular Medicine. J Am Coll Cardiol. 2017;69:2657-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 505] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 29. | Regueiro A, Abdul-Jawad Altisent O, Del Trigo M, Campelo-Parada F, Puri R, Urena M, Philippon F, Rodés-Cabau J. Impact of New-Onset Left Bundle Branch Block and Periprocedural Permanent Pacemaker Implantation on Clinical Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Circ Cardiovasc Interv. 2016;9:e003635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 30. | Weber M, Brüggemann E, Schueler R, Momcilovic D, Sinning JM, Ghanem A, Werner N, Grube E, Schiller W, Mellert F, Welz A, Nickenig G, Hammerstingl C. Impact of left ventricular conduction defect with or without need for permanent right ventricular pacing on functional and clinical recovery after TAVR. Clin Res Cardiol. 2015;104:964-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Bleiziffer S, Ruge H, Hörer J, Hutter A, Geisbüsch S, Brockmann G, Mazzitelli D, Bauernschmitt R, Lange R. Predictors for new-onset complete heart block after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 32. | Kawashima T, Sato F. Visualizing anatomical evidences on atrioventricular conduction system for TAVI. Int J Cardiol. 2014;174:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 33. | Moreno R, Dobarro D, López de Sá E, Prieto M, Morales C, Calvo Orbe L, Moreno-Gomez I, Filgueiras D, Sanchez-Recalde A, Galeote G, Jiménez-Valero S, Lopez-Sendon JL. Cause of complete atrioventricular block after percutaneous aortic valve implantation: insights from a necropsy study. Circulation. 2009;120:e29-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Kumar P, Kusumoto FM, Goldschlager N. Bradyarrhythmias in the elderly. Clin Geriatr Med. 2012;28:703-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Urena M, Webb JG, Tamburino C, Muñoz-García AJ, Cheema A, Dager AE, Serra V, Amat-Santos IJ, Barbanti M, Immè S, Briales JH, Benitez LM, Al Lawati H, Cucalon AM, García Del Blanco B, López J, Dumont E, Delarochellière R, Ribeiro HB, Nombela-Franco L, Philippon F, Rodés-Cabau J. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation. 2014;129:1233-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 36. | Webb J, Gerosa G, Lefèvre T, Leipsic J, Spence M, Thomas M, Thielmann M, Treede H, Wendler O, Walther T. Multicenter evaluation of a next-generation balloon-expandable transcatheter aortic valve. J Am Coll Cardiol. 2014;64:2235-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 37. | Tarantini G, Mojoli M, Purita P, Napodano M, D'Onofrio A, Frigo A, Covolo E, Facchin M, Isabella G, Gerosa G, Iliceto S. Unravelling the (arte)fact of increased pacemaker rate with the Edwards SAPIEN 3 valve. EuroIntervention. 2015;11:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 38. | Herrmann HC, Thourani VH, Kodali SK, Makkar RR, Szeto WY, Anwaruddin S, Desai N, Lim S, Malaisrie SC, Kereiakes DJ, Ramee S, Greason KL, Kapadia S, Babaliaros V, Hahn RT, Pibarot P, Weissman NJ, Leipsic J, Whisenant BK, Webb JG, Mack MJ, Leon MB; PARTNER Investigators. One-Year Clinical Outcomes With SAPIEN 3 Transcatheter Aortic Valve Replacement in High-Risk and Inoperable Patients With Severe Aortic Stenosis. Circulation. 2016;134:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 39. | Nijhoff F, Abawi M, Agostoni P, Ramjankhan FZ, Doevendans PA, Stella PR. Transcatheter aortic valve implantation with the new balloon-expandable Sapien 3 versus Sapien XT valve system: a propensity score-matched single-center comparison. Circ Cardiovasc Interv. 2015;8:e002408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Binder RK, Stortecky S, Heg D, Tueller D, Jeger R, Toggweiler S, Pedrazzini G, Amann FW, Ferrari E, Noble S, Nietlispach F, Maisano F, Räber L, Roffi M, Grünenfelder J, Jüni P, Huber C, Windecker S, Wenaweser P. Procedural Results and Clinical Outcomes of Transcatheter Aortic Valve Implantation in Switzerland: An Observational Cohort Study of Sapien 3 Versus Sapien XT Transcatheter Heart Valves. Circ Cardiovasc Interv. 2015;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Maeno Y, Abramowitz Y, Kawamori H, Kazuno Y, Kubo S, Takahashi N, Mangat G, Okuyama K, Kashif M, Chakravarty T, Nakamura M, Cheng W, Friedman J, Berman D, Makkar RR, Jilaihawi H. A Highly Predictive Risk Model for Pacemaker Implantation After TAVR. JACC Cardiovasc Imaging. 2017;10:1139-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 199] [Article Influence: 24.9] [Reference Citation Analysis (0)] |