Published online Dec 26, 2023. doi: 10.4330/wjc.v15.i12.633
Peer-review started: September 5, 2023
First decision: October 9, 2023
Revised: October 15, 2023
Accepted: November 30, 2023
Article in press: November 30, 2023
Published online: December 26, 2023
Processing time: 110 Days and 21.7 Hours
Coronary artery disease (CAD) is a leading cause of global cardiovascular morta
To investigate the therapeutic effects of combining Pericarpium Trichosanthis injection and nicorandil in elderly patients suffering from refractory angina caused by coronary heart disease.
A retrospective analysis was conducted on the data of 130 patients diagnosed with refractory coronary heart disease. Based on the different treatment regimens administered during hospitalization, the patients were divided into a control group (58 cases) and a study group (72 cases). The control group received conventional treatment, which included aspirin, statins, and nitrate vasodilators. In addition to the conventional medication, the study group received a combination treatment of Pericarpium Trichosanthis injection and nicorandil.
After treatment, the study group showed significantly higher left ventricular ejection fraction and cardiac output, and lower brain natriuretic peptide and C-reactive protein levels compared to the control group. The study group also exhibited improvements in angina, quality of life, exercise endurance, and lipid profiles. Multivariate logistic regression analysis revealed a relationship of lipid levels and heart function with the combined treatment. Some patients in the study group experienced headaches during treatment, but no significant adverse reactions were observed. Follow-up showed that the treatment was well-tolerated, with no drug-related adverse reactions detected.
Combination of Pericarpium Trichosanthis injection and nicorandil is more effective than conventional treatment in improving symptoms and heart function in elderly patients with refractory angina pectoris.
Core Tip: Combining Pericarpium Trichosanthis injection and nicorandil shows promise in improving symptoms and heart function in elderly patients with refractory angina caused by coronary disease, as demonstrated by a retrospective study. This combination resulted in significant improvements in left ventricular function, cardiac output, angina frequency, quality of life, exercise tolerance, and lipid profiles. The therapy was well-tolerated with minimal adverse reactions. These findings highlight the potential of this combined treatment as an effective therapeutic option for refractory angina in elderly patients.
- Citation: Li J, Kong MW, Xie YY, Wang ZB, Xu L, He GX. Efficacy and prognostic impact of Pericarpium Trichosanthis injection combined with nicorandil for intractable angina pectoris in elderly patients: A retrospective study. World J Cardiol 2023; 15(12): 633-641
- URL: https://www.wjgnet.com/1949-8462/full/v15/i12/633.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i12.633
Coronary artery disease (CAD) has become the leading cause of death from cardiovascular diseases worldwide, with its mortality rate only second to cancer[1]. The high prevalence of CAD is closely related to factors such as inflammation, metabolic disorders, arteriosclerosis, and thrombosis. Refractory angina pectoris is a manifestation of CAD, and refers to angina symptoms caused by coronary artery lesions that do not significantly improve or become controllable under conventional treatments (such as medication and percutaneous coronary intervention)[2]. The elderly population is a high-risk group for refractory angina, and the treatment outcomes are generally poor[2]. For refractory angina pectoris, excellent drug treatment options are crucial. Currently, drug therapy is one of the preferred methods for the treatment and prevention of cardiovascular diseases. However, many treatment options face problems such as poor efficacy and severe side effects.
Pericarpium Trichosanthis injection, a traditional Chinese medicine, is widely used in the treatment of cardio-cerebrovascular diseases[3]. Pericarpium Trichosanthis injection can improve the symptoms of cardiovascular diseases such as arteriosclerosis through its antioxidant and anti-inflammatory effects. Some studies have also found that Pericarpium Trichosanthis injection can improve blood lipids, which is beneficial for the prognosis of coronary heart disease[4]. Nicorandil relaxes coronary vascular smooth muscle by stimulating guanylyl cyclase and increasing cyclic GMP levels as well as by a second mechanism resulting in activation of K+ channels and hyperpolarizationm, that can alleviate symptoms such as coronary spasms and angina. Some studies have shown that nicorandil also has some effect in treating hypertension and heart failure[5].
In the past few decades, the combined use of Pericarpium Trichosanthis injection and nicorandil for the treatment of cardiovascular diseases has received widespread attention. However, there is currently a lack of evaluation studies for elderly patients, and it is unclear what impact this combined treatment option has on the efficacy and safety for older patients. The aim of this study was to explore the efficacy and prognostic impact of Pericarpium Trichosanthis injection combined with nicorandil in the treatment of refractory angina pectoris in elderly patients, and to provide clinical decision support for the future treatment of coronary heart disease in elderly patients.
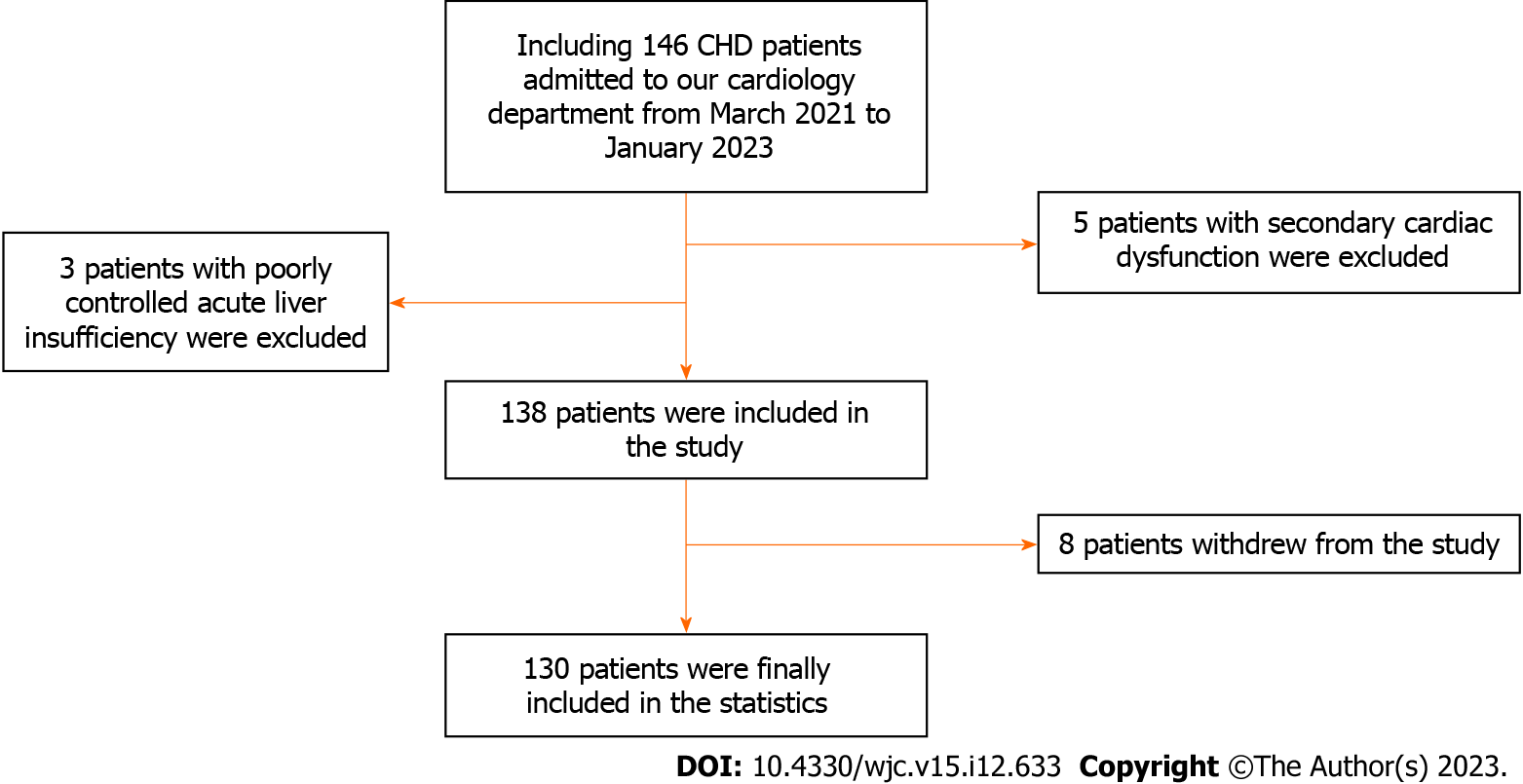
Case data: This was a multicenter study. After screening using the inclusion and exclusion criteria, 148 patients’ medical records (age 72-89 years) with coronary heart disease and refractory angina pectoris admitted to Guiqian International General Hospital and Chengdu Fifth People’s Hospital between March 2021 and January 2023 were selected. After screening and excluding cases that did not meet the criteria, a total of 130 patients were finally included in the statistical analysis, with 72 in the study group and 58 in the control group. All studies used the same method for data collection, and the screening process is shown in Figure 1. There were no significant differences in general data between the two groups (P > 0.05) (Table 1).
| Overall (n = 130) | Study group (n = 72) | Control group (n = 58) | P value | |
| Age (yr) | 73 ± 10 | 72 ± 11 | 74 ± 6 | 0.42 |
| Males | 78 (60) | 40 (56) | 38 (66) | 0.09 |
| Heart rate (bpm) | 72 ± 16 | 71 ± 16 | 72 ± 17 | 0.01a |
| Systolic pressure (mmHg) | 114 ± 21 | 114 ± 20 | 119 ± 22 | 0.27 |
| Diastolic pressure (mmHg) | 73 ± 16 | 71 ± 9 | 76 ± 7 | 0.14 |
| LVEF (%) | 77 ± 15 | 77 ± 10 | 75 ± 11 | 0.47 |
| Creatinine (mmol/L) | 100 ± 52 | 92 ± 44 | 99 ± 40 | 0.21 |
| Arterial high pressure | 10 (8) | 6 (8) | 4 (7) | 0.62 |
| Diabetes mellitus | 22 (17) | 13 (18) | 9 (16) | 0.76 |
| Dyslipidemia | 44 (34) | 25 (35) | 19(33) | 0.22 |
| Current smoking | 50 (38) | 24 (33) | 26(45) | 0.62 |
| Antithrombotics | ||||
| Aspirin | 84(65) | 50(69) | 34 (59) | 0.11 |
| Clopidogrel | 46 (35) | 26 (36) | 20 (34) | 0.50 |
| Direct oral anticoagulant | 6 (5) | 4(6) | 2(3) | 0.42 |
Diagnostic criteria: The diagnosis of coronary heart disease was based on the diagnostic criteria established by the Chinese Medical Association for Coronary Heart Disease: (1) Confirmation of at least one major coronary artery with luminal stenosis ≥ 50% through coronary angiography; (2) Auxiliary examinations such as electrocardiography, cardiac troponin, and exercise testing indicating myocardial ischemia, coronary heart disease, or acute coronary syndrome; and (3) Presence of typical angina symptoms such as chest tightness and chest pain. The diagnosis of coronary heart disease was made if was met and at the same time or was also met. The diagnostic criteria for refractory angina pectoris mainly included: (1) Pain: Persistent angina symptoms for > 3 mo, with no obvious cause or significant improvement despite ongoing treatment; and (2) Congestive heart failure.
Inclusion criteria: (1) Applicants who met the above diagnostic criteria; (2) Inclusion criteria; and (3) The medical records were complete.
Exclusion criteria: (1) Cardiac insufficiency secondary to other diseases; (2) Acute onset of severe myocardial infarction; (3) In severe liver disease, alanine aminotransferase and aspartate aminotransferase were twice the upper limit of the reference range; (4) Malignant tumors; and (5) Surgery within 2 wk.
Treatment approach: The control group was treated according to the conventional Western Medicine Guidelines for Coronary Heart Disease (2nd edition)[6] and the recommendations of the China Coronary Heart Disease Prevention Strategy 2015[7]. This systematic and individualized medication included nitrate drugs to dilate coronary arteries and increase myocardial blood flow, statins and antiplatelet drugs, as well as calcium channel blockers and β-receptor blockers to reduce myocardial contractility and heart rate, thereby reducing myocardial oxygen consumption. For patients with underlying diseases such as hypertension and diabetes, hypoglycemic and antihypertensive treatments were given. The treatment course was 1-2 wk, and after discharge, patients were advised to take long-term secondary prevention drugs for coronary heart disease and to attend follow-up visits at 2 mo, 6 mo, and 1 year.
The treatment group, in addition to the conventional treatment of the control group, was given a combination treatment of nicorandil and Pericarpium Trichosanthis injection. Pericarpium Trichosanthis injection (Shanghai Shybio Pharmaceutical Co. Ltd.) was administered at 12 mL/dose, once daily. Nicorandil (Sihuan Kebao Pharmaceutical) was administered at 5 mg/dose, three times daily. The treatment continued for 1-2 wk. After discharge, patients were advised to take long-term secondary preventive drugs for coronary heart disease, and nicorandil, and to attend follow-up visits at 2 mo, 6 mo, and 1 year. The clinical efficacy of the two groups of patients after treatment was compared.
Efficacy evaluation: The efficacy of treatment of refractory angina pectoris in coronary heart disease was evaluated using multiple indicators, including improvement in cardiac function, frequency and duration of angina, exercise tolerance, quality of life, and cardiovascular events[8]. Specific evaluation indicators included the following: (1) Cardiac function evaluation was performed using cardiac ultrasound, including left ventricular ejection fraction (LVEF), cardiac output (CO), stroke volume, and heart rate. Data on B-type natriuretic peptide (BNP) and C-reactive protein (CRP) were collected during the treatment process; (2) Changes in the frequency and duration of angina: The Canadian Cardiovascular Society (CCS) angina scale was used to evaluate the treatment effect based on improvement in subjective symptoms and signs; (3) Changes in exercise tolerance: Maximum exercise tolerance and angina attacks before and after treatment were evaluated through a treadmill test combined with a 6-min walking test (6MWT); (4) Changes in quality of life: Changes in quality of life were evaluated using the self-acceptance questionnaire (SAQ); and (5) Occurrence of cardiovascular events: Through 2 years of follow-up, the occurrence of cardiovascular endpoints was observed, including sudden cardiac death, myocardial infarction, heart failure, and fatal arrhythmia. Sudden cardiac death was defined as sudden and unexpected death, within 1 h after the onset of cardiac-related symptoms, or no evidence other than cardiac disease was found within 24 h after the onset of symptoms. This definition was jointly established by the American Heart Association and European Society of Cardiology[9,10]. Myocardial infarction was defined based on clinical manifestations, electrocardiography, myocardial enzyme studies, and imaging examinations[11]. Heart failure was defined by clinical manifestations such as dyspnea, fatigue, and generalized edema; signs including cardiac murmurs, enlarged cardiac borders, and increased heart rate; cardiac function assessment including elevated BNP or NT-proBNP in the blood, and echocardiography and/or magnetic resonance imaging showing decreased ventricular contractile function[12]. Fatal arrhythmia was defined by clinical manifestations of syncope or sudden death, and electrocardiography showed types of arrhythmia including ventricular tachycardia, ventricular fibrillation, and long QT syndrome[13].
Assessment and measurement of blood lipids: We determined the changes in lipid levels before and after treatment in patients through medical records. The diagnostic criteria for hyperlipidemia were: (1) Serum high-density lipoprotein (HDL) < 9.0 mmol/L; (2) Total cholesterol (TC) ≥ 2.1 mmol/L; and (3) Serum triglyceride (TG) ≥ 1.70 mmol/L.
We used SPSS version 20.0 statistical software for statistical analyses. Quantitative data that conformed to a normal distribution are expressed as the mean ± SD, and single-factor analysis of variance was used for comparisons among multiple groups; least significant difference t test was used for pairwise comparisons; and independent sample t test was used for comparisons between two groups. Categorical data are expressed as percentages, and the χ2 test was used for comparisons. Logistic regression analysis was performed on indicators with significant differences in single-factor analysis, and P < 0.05 was considered statistically significant.
Before treatment, there was no significant difference in LVEF, CO, BNP, CRP, or other indicators between the two groups (P > 0.05). After treatment, compared with the control group, the study group had higher LVEF and CO, and lower BNP and CRP, with significant differences (P < 0.05) (Table 2).
| Group | LVEF, % | CO, L/min | BNP, ng/L | CRP, mg/L | ||||
| Control group | Study group | Control group | Study group | Control group | Study group | Control group | Study group | |
| Pretherapy | 43.3 ± 2.2 | 47.3 ± 2.2 | 4.8 ± 0.0 | 5.2 ± 0.3 | 724.5 ± 42.5 | 405.7 ± 57.4 | 17.4 ± 3.4 | 11.2 ± 1.2 |
| Post-treatment | 44.7 ± 4.1 | 54.5 ± 4.6 | 4.9 ± 0.2 | 5.6 ± 0.4 | 711.5 ± 33.4 | 282.6 ± 44.3 | 17.6 ± 3.2 | 8.4 ± 1.2 |
| t | 0.6 | 14.2 | 0.2 | 0.8 | 31.7 | 296.5 | 4.7 | 5.1 |
| P value | 0.25 | 0.01a | 0.38 | 0.04a | 0.03a | 0.00a | 0.60 | 0.03a |
Before treatment, there was no significant difference in CCS angina classification, SQA scores, or 6MWT between the groups (P > 0.05). After treatment, compared with the control group, the study group showed improvement in chest pain symptoms, with a significant decrease in CCS angina classification (P < 0.05) and a significant improvement in 6MWT (P < 0.05). However, the improvement in SQA scores was not significant (Table 3).
| Group | CCS angina grade (score) | SAQ grade (score) | 6WMT (m) | |||
| Control group | Study group | Control group | Study group | Control group | Study group | |
| Pretherapy | 1.6 ± 0.5 | 1.0 ± 0.5 | 66.4 ± 12.5 | 60.9 ± 16.0 | 352.4 ± 100.2 | 414.2 ± 84.1 |
| Post-treatment | 1.3 ± 0.7 | 1.8 ± 0.5 | 68.3 ± 10.7 | 63.3 ± 16.3 | 385.0 ± 110.1 | 371.2 ± 85.2 |
| t | 2.8 | 13.2 | 28.5 | 33.6 | 28.7 | 225.1 |
| P value | 0.76 | 0.02a | 0.36 | 0.53 | 0.85 | 0.01a |
There was no significant difference in serum low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TC), or triglyceride (TG) between the two groups before treatment (P > 0.05). After 2 mo of treatment, the study group showed a significant decrease in TC and TG compared with the control group, while LDL and HDL did not show significant changes (P > 0.01) (Table 4).
| Group | LDL (mmol/L) | HDL (mmol/L) | TC (mmol/L) | TG (mmol/L) | ||||
| Control group | Study group | Control group | Study group | Control group | Study group | Control group | Study group | |
| Pretherapy | 3.6 ± 1.4 | 1.6 ± 3.1 | 1.3 ± 0.0 | 1.2 ± 0.3 | 9.0 ± 3.8 | 7.3 ± 1.4 | 6.2 ± 1.6 | 4.6 ± 0.6 |
| Post-treatment | 3.0 ± 2.1 | 1.4 ± 3.7 | 1.2 ± 0.2 | 1.3 ± 0.4 | 8.7 ± 2.2 | 5.2 ± 0.9 | 6.5 ± 1.4 | 3.2 ± 0.9 |
| t | 0.6 | 4.2 | 0.2 | 1.1 | 1.7 | 3.5 | 4.7 | 3.1 |
| P value | 0.26 | 0.54 | 0.18 | 0.11 | 0.32 | 0.01a | 0.60 | 0.03a |
LDL, HDL, TC, and TG (lipid indexes) as well as LVEF, CO, BNP, and CRP (cardiac function indexes) were used as independent variables, and whether to use Pericarpium Trichosanthis injection combined with nicorandil was used as the dependent variable in the multiple logistic regression. LDL, TC, TG, LVEF, CO, and BNP were significantly related to the use of the combination therapy (P < 0.05), and the combined treatment had an inhibitory effect on LDL, TC, TG, and BNP, and a promotive effect on LVEF and CO (Table 5).
| Variable | SE | Wald χ2 | P value | OR (95%CI) |
| LDL | 1.12 | 1.26 | 0.03 | 0.30 (0.08-0.49) |
| HDL | 0.58 | 0.90 | 0.47 | 1.21 (1.02-1.87) |
| TG | 1.87 | 3.60 | 0.05 | 0.57 (0.24-0.75) |
| TC | 1.79 | 0.33 | 0.04 | 0.65 (0.43-0.69) |
| LVEF | 0.05 | 0.70 | 0.01 | 1.16 (1.00-1.27) |
| CO | 0.08 | 0.65 | 0.01 | 1.83 (1.02-2-36) |
| BNP | 2.75 | 5.60 | 0.03 | 0.44 (0.10-0.64) |
| CRP | 0.85 | 3.15 | 0.54 | 0.76 (0.23-0.95) |
During the treatment period, 12 (16.7%) patients in the study group experienced headaches, with an average duration of 4 d and self-resolution. Throughout the entire treatment process, there was no impact on blood pressure, heart rate, or oxygen saturation in any of the patients. All 130 patients successfully completed the treatment, and 92 (71%) completed follow-up, with a median duration of 8.5 (3-12) mo. During follow-up, two patients in the study group and three in the control group died of respiratory failure caused by coronavirus disease 2019 at the 6-12-mo follow-up. No other related adverse reactions occurred in the patients during follow-up, and there were no deaths due to other causes.
Pericarpium Trichosanthis injection is a traditional Chinese medicine formulation that has been widely used in the treatment of cardiovascular and cerebrovascular diseases in recent years. Multiple studies have shown that Pericarpium Trichosanthis injection has significant clinical efficacy and minimal adverse reactions. A clinical study demonstrated that Pericarpium Trichosanthis injection significantly reduced the severity of angina and myocardial ischemic time, while also lowering the levels of myocardial enzymes, indicating its cardioprotective effects[14]. Another randomized controlled trial demonstrated that Pericarpium Trichosanthis injection has a significant effect in relieving symptoms and improving hemodynamic parameters in patients with angina[15]. Nicorandil has been widely used in the treatment of car
In our study, the study group showed higher levels of LVEF and CO compared with the control group, while BNP and CRP levels were lower, with significant differences. This indicates that the combination therapy has significant advantages in improving heart function and reducing inflammatory responses. In the evaluation of angina improvement, quality of life, and exercise endurance, the study group demonstrated better CCS angina classification and 6MWT scores compared with the control group. This implies that the combination therapy not only improves cardiac function but also alleviates symptoms, improves quality of life, and enhances exercise endurance. These results are consistent with previous studies. Research has shown that Pericarpium Trichosanthis has the therapeutic effects of clearing heat, promoting diuresis, activating blood circulation, and dissolving stasis. It can improve microcirculation in the cardiovascular system, and promote the normal transportation and metabolism of calcium ions in myocardial cells, thereby improving heart function and alleviating angina[18]. Nicorandil, as a calcium channel blocker, can inhibit the influx of calcium ions into the vascular smooth muscle beneath the endocardium, reducing the tension and resistance of coronary arteries, and improving cardiac hemodynamics[19]. However, there was no significant difference in SAQ scores between the two groups. This may be due to the wide individual variations among patients, as SAQ is a self-reported assessment. Additionally, the small sample size in this study may have limited the ability to detect significant differences.
The combined therapy showed a significant decrease in TC and TG levels compared with the control group, while LDL and HDL levels did not show significant changes. This suggests that the combination therapy of Pericarpium Trichosanthis injection and nicorandil can improve lipid metabolism, but has little effect on LDL and HDL levels[8]. Previous studies have found that Pericarpium Trichosanthis injection may affect lipid metabolism through various mechanisms, including reducing cholesterol synthesis, promoting cholesterol metabolism and excretion, and regulating fatty acid synthesis[20]. Current research indicates that the main active ingredients of Pericarpium Trichosanthis injection are cucurbitacin and cucurbitic acid, which have various pharmacological effects such as heat-clearing, detoxification, dampness-eliminating, phlegm-removing, lipid-lowering, antioxidant, and anti-inflammatory effects. Among them, cucurbitacin is believed to inhibit the activity of HMG-CoA reductase, thereby reducing cholesterol synthesis, while cucurbitic acid can promote cholesterol metabolism and excretion, and regulate fatty acid synthesis. This may be one of the mechanisms by which Pericarpium Trichosanthis injection reduces the incidence and mortality of chronic diseases such as cardiovascular and cerebrovascular diseases.
During the treatment period, 12 (16.7%) patients in the study group experienced headaches, which could potentially be related to nicorandil. Nicorandil is a calcium channel blocker that primarily acts on the heart and vascular smooth muscle, and is capable of dilating coronary arteries, peripheral vessels, and pulmonary arteries[21]. This results in a reduction in cardiac oxygen demand and blood ejection resistance, and a decrease in coronary artery contraction and spasm. However, during nicorandil treatment, adverse reactions such as headaches may occur, especially when first starting the medication. These headaches are usually mild and can resolve spontaneously, but they can sometimes affect the patient’s comfort and quality of life. This might also be the reason why the study group did not outperform the control group in terms of SAQ scores in this study. After discharge, a total of 92 (71%) patients completed the follow-up, with a median duration of 8.5 (3-12) mo. During follow-up, some patients in both groups died from coronavirus disease 2019, and no drug-related adverse reactions were found. This suggests that the combined use of Pericarpium Trichosanthis injection and nicorandil is safe and does not increase the incidence of adverse reactions.
While this study achieved significant clinical results, it still had some limitations. The protocol did not exclude the use of other drugs, which could potentially have influenced the results. In addition, the study sample was small, and larger studies are needed to verify the safety and effectiveness of this combination therapy. Looking back at the development of healthcare in recent years, the concept of combining traditional Chinese and Western medicine and the deepening of related research, along with the development and practical use of new drugs, have all effectively improved the incidence of coronary heart disease and its complications. In the field of medical practice, appropriately adjusting or updating existing prevention methods, improving the homeostasis of the patient’s internal environment as much as possible, and reducing or eliminating the adverse effects caused by the disease, are the focus of clinical physicians.
The combination of Pericarpium Trichosanthis injection and nicorandil is more effective than conventional treatment in improving symptoms and heart function in elderly patients with refractory angina pectoris.
Coronary artery disease (CAD) is a global health concern that often leads to severe cardiovascular mortality. Refractory angina pectoris, a consequential manifestation of CAD, necessitates competent drug treatments. Two potential treatments include a traditional Chinese medicine known as Pericarpium Trichosanthis and a medication named nicorandil.
This research was driven by the need to investigate effective therapeutic procedures for elderly patients suffering from refractory angina pectoris due to CAD.
The primary objective for this research was to scrutinize the therapeutic impacts of a combined treatment, namely, Pericarpium Trichosanthis injection and nicorandil, on elderly patients with refractory angina pectoris caused by CAD.
The study was performed as a retrospective study involving 130 patients diagnosed with refractory CAD; they were divided into control and intervention groups using digital randomization. While the control group received a routine treatment, the intervention group was subjected to the combined treatment of Pericarpium Trichosanthis injection and nicorandil.
The patients in the study group demonstrated significant enhancements in heart performance and lifestyle quality. Few patients experienced headaches, but no severe side-effects were observed. No drug-related adverse reactions were noted upon follow-up.
The combined treatment of Pericarpium Trichosanthis injection and nicorandil substantially surpasses conventional treatment methods in managing symptoms and heart functionality among elderly patients suffering from refractory angina pectoris.
The beneficial findings of the present study pave the way towards further exploring this combined treatment’s potential varying application for CAD and its consequent complications, ultimately improving patient wellness and prolonging life spans. Promisingly, it may add a significant contribution to personalized treatment approaches for CAD patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ong H, Malaysia; Lakusic, Croatia S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | GBD 2019 Chronic Respiratory Diseases Collaborators. Global burden of chronic respiratory diseases and risk factors, 1990-2019: an update from the Global Burden of Disease Study 2019. EClinicalMedicine. 2023;59:101936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 254] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 2. | Bauer D, Neuberg M, Nováčková M, Kočka V, Toušek P. Pre-hospital delay, clinical characteristics, angiographic findings, and in-hospital mortality in young and middle-aged adults with acute coronary syndrome: a single-centre registry analysis. Eur Heart J Suppl. 2023;25:E33-E39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 3. | Lin P, Wang Q, Liu Y, Jiang H, Lv W, Lan T, Qin Z, Yao X, Yao Z. Qualitative and quantitative analysis of the chemical profile for Gualou-Xiebai-Banxia decoction, a classical traditional Chinese medicine formula for the treatment of coronary heart disease, by UPLC-Q/TOF-MS combined with chemometric analysis. J Pharm Biomed Anal. 2021;197:113950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Zhu Y, Xia W, Liu W, Xu C, Gu N. Gualoupi (Pericarpium Trichosanthis) injection in combination with convention therapy for the treatment of angina pectoris: a Meta- analysis. J Tradit Chin Med. 2017;37:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Gitmez M, Lancellotti P. The effect of nicorandil on cardiac function and clinical outcomes in ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: a randomised trial. Acta Cardiol. 2023;78:975-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 6. | Expert Committee of Rational Drug Use of the National Health and Family Planning Commission, Chinese Pharmacists Association. Guidelines for rational drug use of coronary heart disease (2nd edition). Zhonghuo Yixue Qianyan. 2018;. [DOI] [Full Text] |
| 7. | He XQ, Liu ML. Prevention and Control Strategy of Coronary Heart Disease in China. Chinese General Practice. 2015;239-240. [DOI] [Full Text] |
| 8. | Scandalis L, Kitzman DW, Nicklas BJ, Lyles M, Brubaker P, Nelson MB, Gordon M, Stone J, Bergstrom J, Neufer PD, Gnaiger E, Molina AJA. Skeletal Muscle Mitochondrial Respiration and Exercise Intolerance in Patients With Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2023;8:575-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 9. | Myerburg RJ, Interian A Jr, Mitrani R, Kessler KM, Castellanos A. Frequency of sudden cardiac death and profiles of risk. Am J Cardiol. 1997;80:10F-19F. [RCA] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 274] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, Charron P, Corrado D, Dagres N, de Chillou C, Eckardt L, Friede T, Haugaa KH, Hocini M, Lambiase PD, Marijon E, Merino JL, Peichl P, Priori SG, Reichlin T, Schulz-Menger J, Sticherling C, Tzeis S, Verstrael A, Volterrani M; ESC Scientific Document Group. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43:3997-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 1416] [Article Influence: 472.0] [Reference Citation Analysis (0)] |
| 11. | Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2791] [Cited by in RCA: 4531] [Article Influence: 906.2] [Reference Citation Analysis (0)] |
| 12. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1381] [Article Influence: 172.6] [Reference Citation Analysis (0)] |
| 13. | Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:e91-e220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 812] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 14. | Liu P, Tan XY, Zhang HQ, Su KL, Shang EX, Xiao QL, Guo S, Duan JA. Optimal compatibility proportional screening of Trichosanthis Pericarpium - Trichosanthis Radix and its anti - Inflammatory components effect on experimental zebrafish and coughing mice. J Ethnopharmacol. 2023;319:117096. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Han G. Clinical observation of Gualoupi injection on hypertension. hin J Integr Med Cardio/Cerebrovasc Dis. 2008;6:80-81. |
| 16. | Yang J, Li W, Ding J, Dong Y, Xie X, Zhao F, Pan J, Qu H. A multivariate curve resolution-alternating least squares (MCR-ALS) technology assisted (1) H-NMR methodology for multi-component quantitation of Trichosanthis Pericarpium injection. Phytochem Anal. 2023;34:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Chu D, Zhang Z. Trichosanthis Pericarpium Aqueous Extract Protects H9c2 Cardiomyocytes from Hypoxia/Reoxygenation Injury by Regulating PI3K/Akt/NO Pathway. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Zhao QT, Huang Ch, Ju S. Study on the mechanism of acute myocardial infarction. Zhong Cheng Yao. 2014;1745-1747. |
| 19. | Choe JC, Oh JH, Lee HC, Lee JW, Park TS, Park JH, Kim E, Kim MS, Ahn J, Park JS, Lee HW, Choi JH, Cha KS. The effect of nicorandil on cardiac function and clinical outcomes in ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: a randomised trial. Acta Cardiol. 2023;78:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 20. | Wang P, Zhao QT, Gao ZH, Huang ZH, Zhang YQ. Protective Effect and Underlying Mechanism of Trichosanthes Pericarpium Injection on Acute Myocardial Infarction Rat Model. J Liaoning Zhongyi Zhazhi. 2014;41:39-40, after 2. |
| 21. | Ilyas M, Noor M, Khan HS, Haroon S, Farhat K, Ali S. Cardio protective effect of nicorandil in reperfusion injury among patients undergoing primary percutaneous coronary intervention. Pak J Med Sci. 2023;39:177-181. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |