Published online Oct 26, 2023. doi: 10.4330/wjc.v15.i10.479
Peer-review started: July 15, 2023
First decision: August 31, 2023
Revised: September 9, 2023
Accepted: September 28, 2023
Article in press: September 28, 2023
Published online: October 26, 2023
Processing time: 101 Days and 1.1 Hours
Despite the high prevalence of straight back syndrome (SBS), there is still limited research on this condition, posing challenges for effective diagnosis and treatment. The disease has been known for a long time, but there have been few related studies, which mostly consist of case reports. These studies have not been systematically summarized, making it difficult to meet the current needs of diagnosis and treatment. This article summarized the existing literature and comprehensively reviewed the diagnosis, pathogenesis, treatment, and research status of mitral valve prolapse related to SBS. We specifically emphasized the mechanisms and prognosis of SBS combined with mitral valve prolapse and discussed the latest research progress in this disease.
Core Tip: Straight back syndrome (SBS), a benign skeletal abnormality of the thorax, is typically accompanied by mitral valve prolapse. Despite its prevalence, there is limited research on this condition, making effective diagnosis and treatment challenging. Recent studies have revealed controversy on SBS and its related mechanisms. This review focused on the mechanisms and current research progress of SBS associated with mitral valve prolapse.
- Citation: Kong MW, Pei ZY, Zhang X, Du QJ, Tang Q, Li J, He GX. Related mechanisms and research progress in straight back syndrome. World J Cardiol 2023; 15(10): 479-486
- URL: https://www.wjgnet.com/1949-8462/full/v15/i10/479.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i10.479
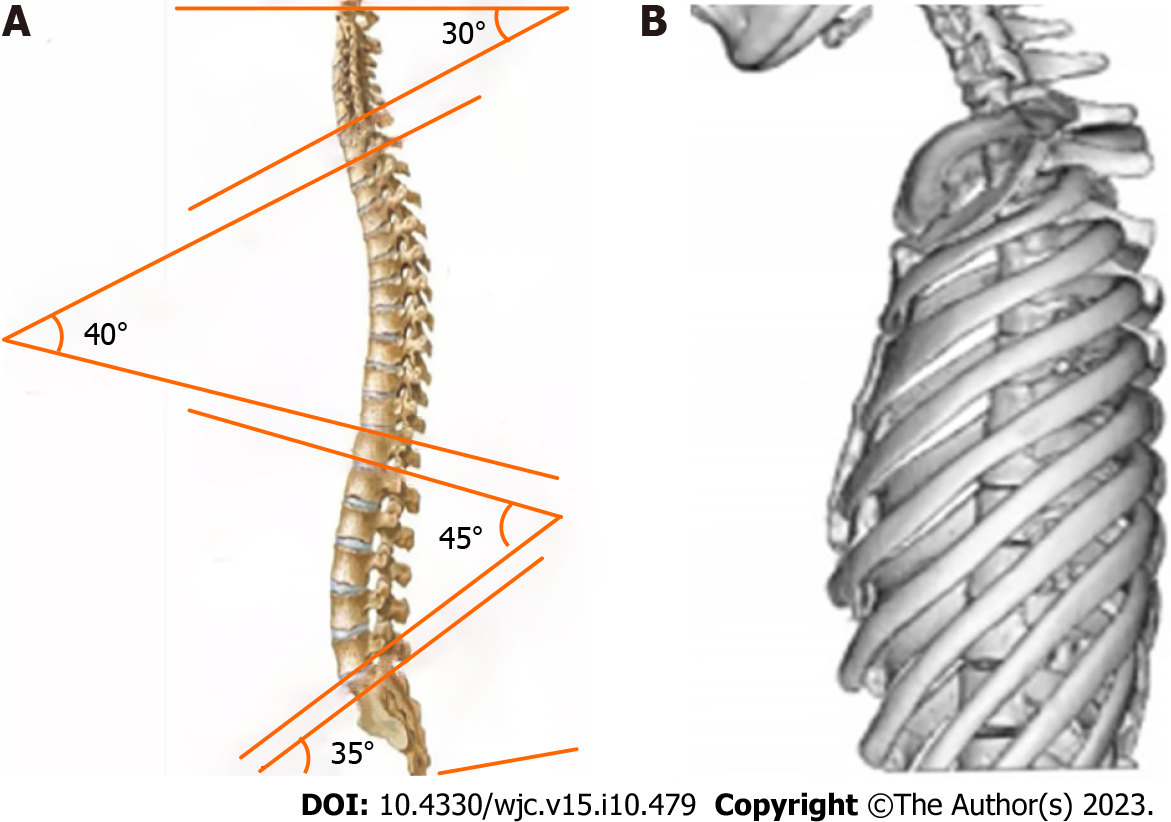
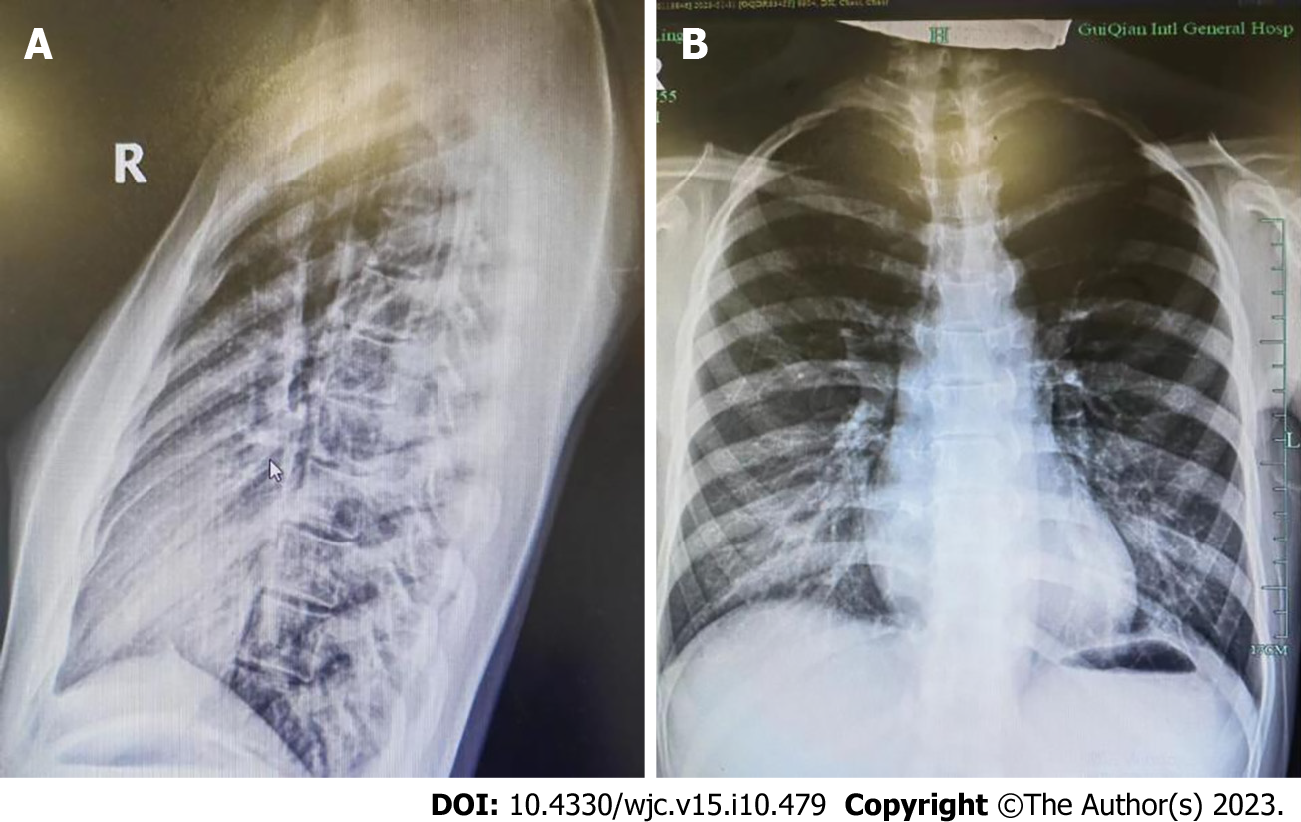
Straight back syndrome (SBS), also known as flat chest syndrome, is characterized by the disappearance of the normal kyphotic curvature of the thoracic spine, which results in a decrease in the anterior-posterior diameter of the chest and restriction of the heart (Figure 1). It was first reported by Rawlings[1] in 1960. Due to the presence of heart murmurs during cardiac examinations, patients are often referred to the cardiology department for evaluation[2]. Mitral valve prolapse (MVP) is a valvular heart disease characterized by soft texture of the mitral valve, which can billow upward and back into the left atrium during heart contraction (prolapse). Prolapse of the mitral valve can lead to mitral regurgitation. If the MVP causes significant enlargement of the atria, arrhythmias may occur. Previously, SBS-associated MVP was considered a “pseudo-heart disease,” and patients may have no symptoms or only mild clinical symptoms such as chest tightness and shortness of breath. Even when severe symptoms occur, they are mostly thought to be due to mechanical and structural changes resulting from compression of the large blood vessels in the heart[3]. However, recent studies have found that SBS-associated MVP may be related to the expression of genes, which has attracted wide attention and controversy[2].
In clinical practice, some patients with MVP are often misdiagnosed as having “senile valve disease” or “congenital heart disease” due to the lack of primary disease diagnostic criteria. In the clinic, it is not uncommon for SBS to cause changes in cardiac structure and circulatory function[4]. However, the clinical manifestations can vary widely, often leading to misdiagnosis. Unfortunately, despite being discovered over 60 years ago, research on this subject remains scarce and often centers on case reports. Presently, these studies lack systematic summarization and fail to meet the current diagnostic and treatment reference needs. Therefore, this article comprehensively reviewed the literature on the diagnosis, pathogenesis, treatment, and research status of SBS-related MVP and provided clinical assistance by summarizing the latest and most significant achievements in this field.
SBS is a benign thoracic skeletal malformation that is often misdiagnosed as heart disease as it can cause heart murmurs detectable on physical examination[5]. Patients are usually asymptomatic, and the most common symptoms are chest pain and palpitations. However, there is a lack of large-scale epidemiological investigations into the incidence of SBS in the general population. A previous study by Jiang and Li[6] reported that among 114 SBS patients, the vast majority of cases (108) occurred in individuals under 40 years of age, with approximately 60% (66 cases) occurring in those aged between 20-40 years. The prevalence in females was higher than that in males. The incidence rate significantly decreased, and symptoms were milder in patients over 40 years of age, which may be due to reduced lung tissue and chest wall elasticity with increasing age, leading to natural alleviation of the condition[7]. A study in 2022 found that thoracic vertebrae may undergo degenerative changes with increasing age. In the general population, this change may cause excessive thoracic kyphosis, but in patients with SBS, it may relieve symptoms[8].
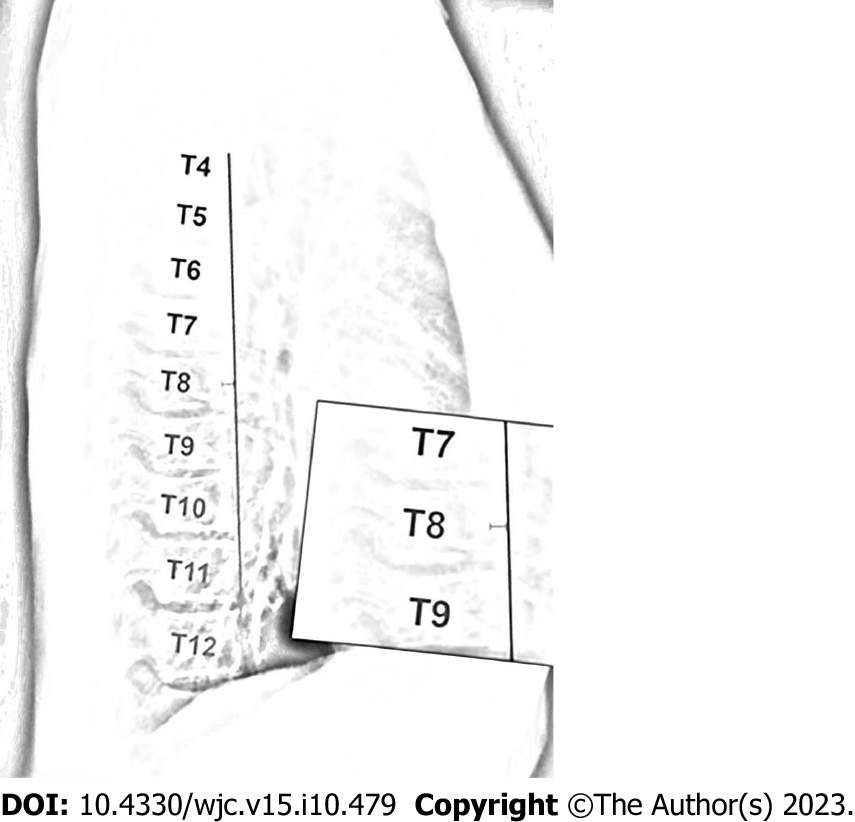
In 1956, Deleon et al[9] first proposed a diagnostic criterion for SBS, which was a ratio of anterior-posterior to transverse thoracic diameter less than 1/3 measured at the T8 level. In 1980, Davies et al[10] revised this criterion and proposed a diagnostic criterion for SBS on lateral chest radiographs, where the distance from the midpoint of the T8 vertebral body to the vertical line connecting the anterior borders of T4 and T12 was less than 1.2 cm (Figure 2). SBS typically occurs in young, lean individuals who lack normal thoracic kyphosis, leading to a decreased sagittal diameter of the thorax[11]. Auscultation may reveal splitting of the second heart sound, and a prominent murmur is generally caused by compression of the right ventricular outflow tract by the sternum, which lessens with deep inspiration. X-ray examination is the most important diagnostic method, especially left lateral images, where the spinal thoracic segments appear straight and the sagittal diameter is narrowed. Twigg et al[12] measured the ratio of thoracic anterior-posterior to transverse diameter in 24 patients with SBS and 100 normal individuals, revealing a mean ratio of 37.1%, which is lower than the normal value of 40.0%. Furthermore, X-ray features may include a heart-thorax ratio ≤ 0.5, protruding pulmonary artery segments, leftward shift of the cardiac silhouette, or signs of a pseudo-enlarged heart. In SBS patients, electrocardiography generally demonstrates normal values. In some patients, due to compression of the heart and positional changes, V1 leads may show a rsR’ pattern, avR for the Qr pattern, R/S > 1, or R/S = 1, and some individual patients may exhibit high voltage of the left ventricle, sinus bradycardia, and incomplete right bundle branch block.
In some ultrasonography results of SBS patients, there may be a concomitant finding of MVP. Previous studies have suggested that this may be related to the compression-induced deformation of the mitral valve due to narrowing of the sagittal diameter[13]. However, a study conducted in 2020 indicated that both SBS and MVP may be inherited in an autosomal dominant manner[14]. It is believed that the vertebral malformation occurs during the 8th wk of gestation before ossification of the spine, and the penetrance is incomplete, with a higher expression frequency observed in female subjects. It has been reported in the international literature that 17%-23% of MVP patients have scoliosis, while 54%-67% of scoliosis patients have concomitant MVP[15]. Based on these studies, it can be inferred that the higher incidence of MVP in SBS is not only a result of physical factors but is also related to genetic factors. National literature has not reported such a high incidence rate, which may be due to inadequate knowledge at the time.
SBS is often associated with several complications, and the underlying mechanisms are still not fully understood. One theory suggests that compression of the thorax could reduce lung capacity and cause hypoxia, leading to various cardiovascular and respiratory problems. Recent studies have shown that compression may also affect the functioning of the autonomic nervous system, leading to abnormal heart rate variability[16]. Additionally, the reduced sagittal diameter of the chest could lead to mechanical distortion of the heart and major vessels, causing abnormal blood flow[17]. In the study by Grillo et al[18], MVP was the most common associated cardiac disease, occurring in approximately 64% of SBS cases. Some studies suggest that the high incidence of MVP and scoliosis in SBS patients may be related to a common genetic background, but further research is needed to clarify this relationship.
In several previous studies, there was consistent evidence of the co-occurrence of SBS and MVP[19], but it was not until 2017 that Movahed et al[20] demonstrated a statistically significant association between these two conditions. In their study, 77% of SBS patients underwent mitral valve repair or replacement due to severe mitral regurgitation caused by MVP. There are two hypotheses regarding the mechanism of SBS co-occurring with MVP. The first hypothesis, proposed by Chen et al[3], suggests that scoliosis and MVP are both manifestations of a more common disease, which is inherited in an autosomal dominant manner with incomplete penetrance. Family studies have found a tendency towards clustering of MVP and scoliosis, indicating that these diseases may have a genetic basis.
Additionally, a number of genome-wide association studies have identified several genetic variations associated with SBS, some of which may affect the susceptibility of MVP and scoliosis. For example, one study found that the LRP2 gene located at 9q22.32 was associated with the susceptibility of scoliosis and MVP[21], whereas another study found a close relationship between the EFEMP1 gene located at 5q35.3 and both scoliosis and MVP[22]. Furthermore, recent research has suggested that the MVP comorbidity in SBS may be related to the TGF-β signaling pathway, which induces cardiomyocyte apoptosis and promotes fibroblast proliferation[23].
It has also been discovered that SBS and floppy mitral valve disease are both inherited in an autosomal dominant manner with a significant family history, and three possible gene loci (16p12.1-p11.2, 11p15.4, and 13p31.3-p32.1) have been reported[24]. Floppy mitral valve causes enlargement of the mitral valve orifice area, elongation or rupture of the chordae tendineae, and enlargement of the mitral annulus, ultimately leading to MVP. It is believed that these malformations occur during the 8th wk of gestation before ossification of the spine, with incomplete penetrance and higher expression frequency in female subjects[4]. These studies suggest that genetic variations and molecular mechanisms may play important roles in the high incidence of MVP and scoliosis in SBS patients. However, the notion of a “more common disease” proposed by Chen et al[3] has yet to be proven.
Another hypothesis suggests that physical compression is a primary factor leading to the co-occurrence of MVP in SBS. The thoracic spine of SBS patients loses its normal posterior convex curvature, resulting in a decreased distance between the sternum and spine. This leads to compression of the heart and torsion of the major vessels, ultimately leading to distorted mitral valve morphology due to pressure[25]. The study by Chen et al[3] indirectly supported this view using cardiac magnetic resonance imaging to identify a correlation between the site of cardiac compression in SBS patients and the occurrence of arrhythmia. This evidence indicated that some, if not all, of the cardiac alterations seen in SBS are influenced by physical compression factors.
The causes of cardiac morphological abnormalities in SBS may include: (1) Differing degrees of cardiac pulsation restriction due to varying degrees of front-to-back narrowing of the thoracic cavity caused by flat thoracic vertebrae; (2) Left atrial compression, which increases pulmonary circulation resistance and reduces returning blood volume; and (3) Long-term frequent rapid arrhythmia causing cardiac enlargement and decreased cardiac function. In the study by Chen et al[3] among SBS patients with cardiac morphological abnormalities, 54.2% showed a consistent location between arrhythmia origin and the cardiac compression site, suggesting that mechanical compression of the heart may lead to enhanced aberrant electrophysiological activity in the heart through the activation of self-regulatory mechanisms.
Previous understanding of SBS was limited to chest wall deformities, which may cause mild symptoms such as palpitations, chest tightness, and chest pain, while severe cases may have morphological functional changes in the heart chambers, valves, and major vessels. However, recent studies have compared cardiac magnetic resonance imaging, electrocardiogram, and electrophysiological data of 43 SBS patients and found a relationship between the cardiac compression site and arrhythmia occurrence in addition to the morphological and functional changes caused by flat chest walls[3]. This study proposed that arrhythmias are the result of cardiac compression. However, a study conducted in 2013, found that MVP comorbid with various arrhythmias is very common, with ventricular arrhythmias being the most frequent, potentially due to increased sympathetic nervous activity and stimulation of the myocardium by prolonged chordae tendineae[26]. Given that MVP frequently co-occurs with SBS, it may be one of the reasons for the frequent occurrence of arrhythmias in SBS. This view has been partially confirmed by recent research. In the study by Xia et al[27], 8.3% of MVP patients were found to have preexcitation syndrome, and follow-up results showed a generally good prognosis for SBS comorbid with MVP.
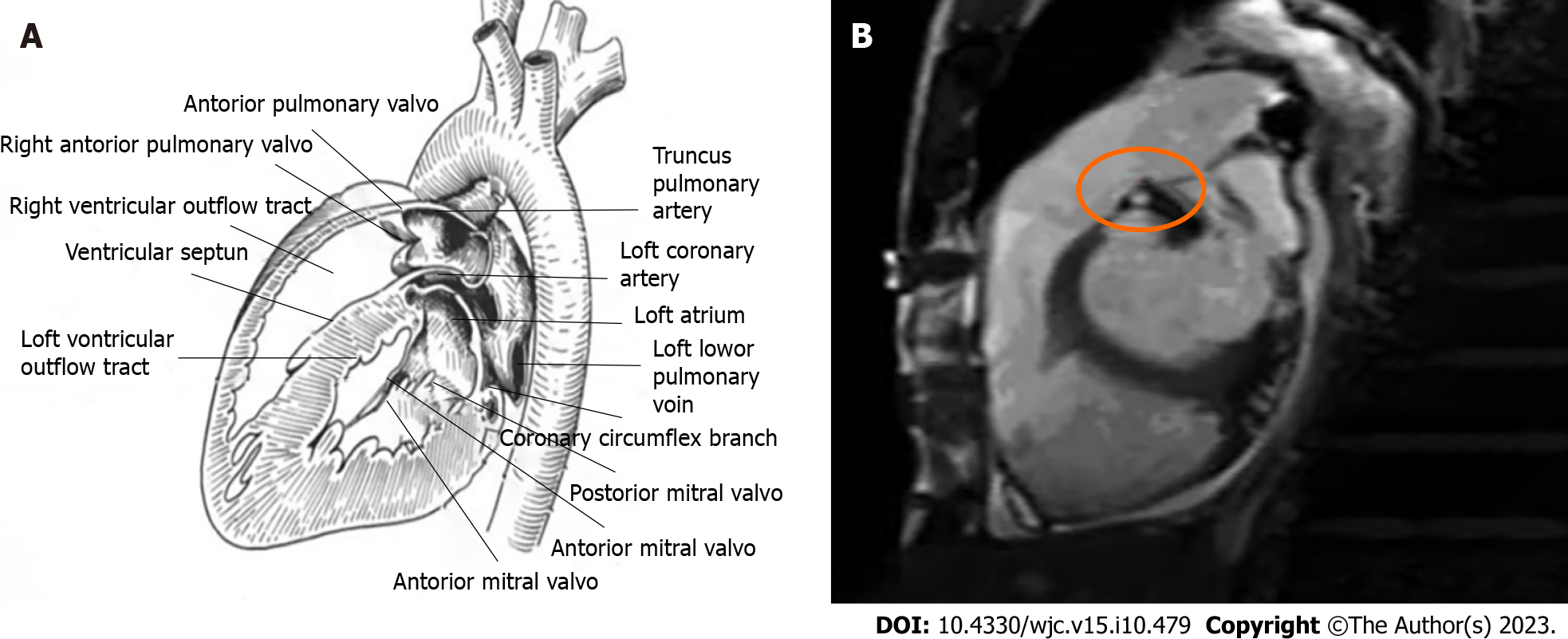
In SBS patients, cardiac murmurs may sometimes be heard on auscultation, but detailed examination often reveals no cardiac abnormalities. There have been reports of SBS being misdiagnosed as cardiac diseases with abnormal heart sounds, such as atrial septal defect, pulmonary valve stenosis, or mitral regurgitation[3]. The mechanism underlying cardiac murmurs in SBS has not been well studied, but a more credible explanation is that the loss of thoracic physiological kyphosis in SBS patients causes displacement of the heart and great vessels. This leads to the right ventricle and pulmonary artery being closer to the posterior sternum, increasing the contact area between the sternum and the posterior heart margin, resulting in a “strengthened” jet effect of blood flow, almost invariably causing grade I-III systolic murmurs in the pulmonary valve auscultation area of each patient[28]. Due to prolonged compression of the heart on the dorsal margin of the sternum, the heart is subjected to long-term overload, causing hypertrophy of cardiomyocytes characterized by increased cell volume, diameter, and length. When hypertrophy reaches the critical limit, it increases the systolic wall tension of the ventricular wall, leading to the parallel proliferation of myocardial fiber cells, followed by thickening of the myocardial fibers. As a result, the thickness of the ventricular wall increases and the cavity does not expand significantly, leading to outflow tract obstruction and the production of murmurs (Figure 3).
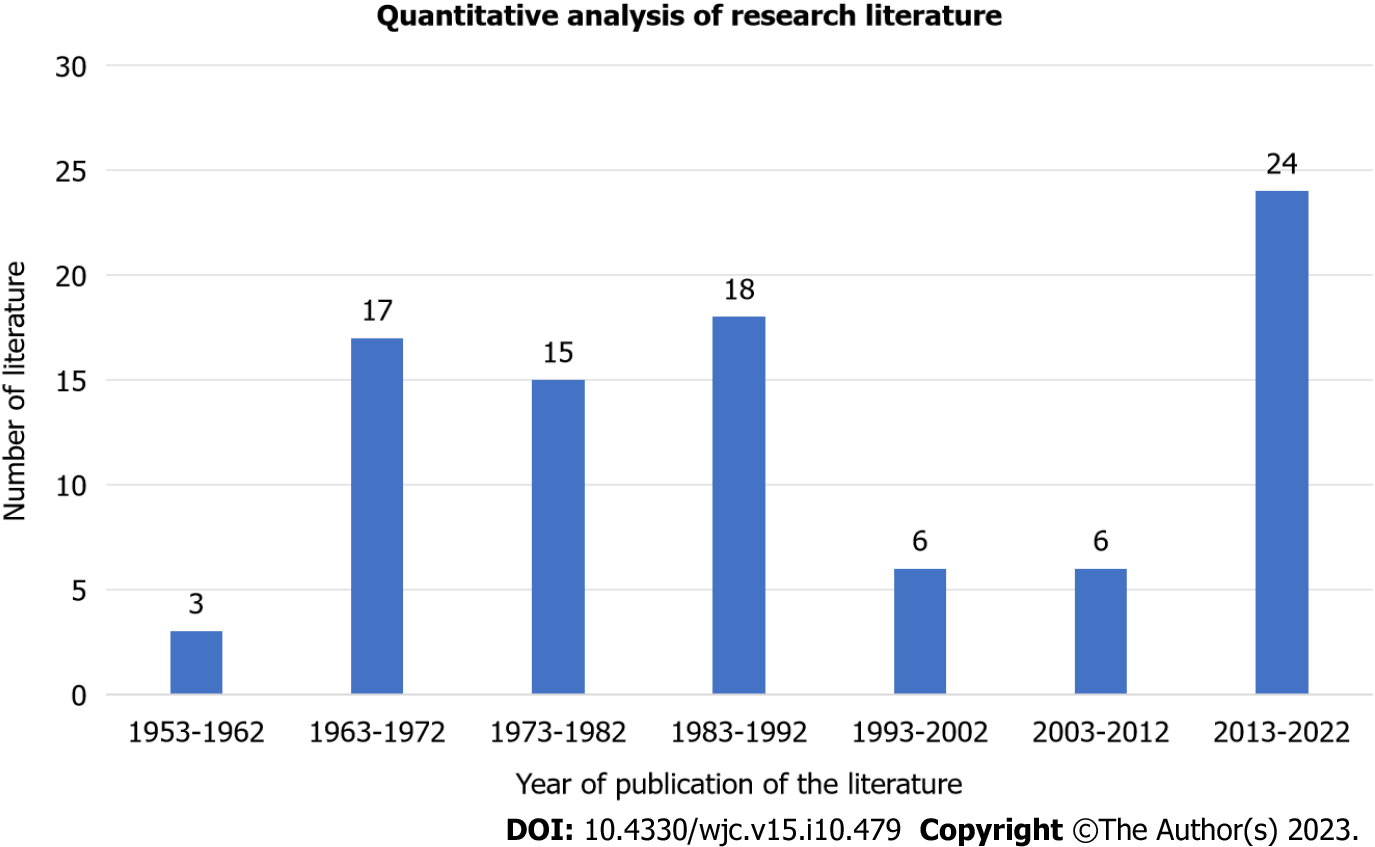
It has been over 60 years since SBS was first reported in 1960[1]. Despite the long history, the literature on this topic remains relatively scarce, with case reports dominating the available data (Figure 4). According to recent statistics, the past 10 years have seen the greatest SBS-related research activity, and significant breakthroughs have been made.
Xu et al[29] conducted a retrospective analysis of 16 cases of misdiagnosed situs inversus totalis and summarized the clinical features of SBS patients. The authors emphasized the importance of chest X-ray lateral films and concluded that echocardiography is an effective diagnostic tool for SBS. Another study conducted in the same year also highlighted the importance of chest X-ray lateral films as a key diagnostic feature of SBS (Figure 5)[30].
Hou et al[31] investigated a method for diagnosing SBS by combining the ratio of the distance from the anterior edge of the T8 vertebral body to the posterior edge of the sternum to the anterior-posterior diameter of the thorax with the ratio of the distance from the anterior edge of the T8 vertebral body to the posterior edge of the sternum to the transverse diameter of the thorax and the curvature arc height from T3 to T12. Recently, studies have also shown that this diagnostic method was more reliable for diagnosing SBS[32-35]. In a recent study of 1569 randomly selected patients who underwent 64-row chest computed tomography (CT) scans, it was found that CT could identify signs that were not visible on X-ray films, leading to a more accurate diagnosis of SBS and a better correlation of clinical symptoms with imaging findings, as has also been reported in studies overseas. Matsumoto et al[33] recently used echocardiography and right ventricular angiography to uncover the mechanism underlying the change in heart murmur with respiration, which they found to be due to variation in the diameter of the right ventricular outflow tract during respiration. It was not until 2017 that Marbella et al[34] investigated the statistical correlation between SBS and MVP and revealed that 27% of patients with severe mitral valve regurgitation caused by MVP also had SBS.
In terms of treatment, Betz et al[35] reported the successful case of a 19-year-old patient with SBS who presented with spinal pain and exertional dyspnea. The patient’s symptoms were relieved by a 12-wk course of treatment involving corrective exercises, traction, and posture adjustment. In another study, the use of 3D printing technology to simulate chest wall replacement tissue was reported to alleviate severe compression symptoms in an SBS patient[17]. Another complication of SBS is tracheomalacia caused by chronic compression of the trachea and main bronchi, resulting in decreased mediastinal diameter. In 2021, Schmid et al[36] successfully cured a 36-year-old female patient suffering from this condition by proximal aortic, brachial artery, sternoplasty, and anterior tracheal fixation surgery.
SBS is a benign thoracic skeletal abnormality that is usually associated with MVP and a heart murmur often detected during physical examination. Patients are usually asymptomatic, with chest pain and palpitations being the most common symptoms. X-ray examination is the most important diagnostic tool, showing a straightened thoracic spine and a decreased anterior-posterior diameter in the thoracic segment. Recent studies have confirmed the importance of echocardiography and CT in the diagnosis of SBS. The mechanism of SBS with MVP may be due to genetic or physical factors (compression), and the mechanism of heart murmur may also be due to physical factors or indirect causes following MVP.
Although SBS was discovered some time ago, there have been relatively few related studies. Recent studies have explored the importance of diagnostic tools in SBS and reemphasized the clinical features of SBS to avoid misdiagnosis. Some studies have also identified the mechanisms of arrhythmia in patients with SBS and the possible mechanism of heart murmur changing with respiration. Progress has been made in the treatment of SBS in some patients with severe compression symptoms through corrective exercise, traction, 3D printing technology, and surgery.
All these studies have achieved success under the development of new equipment and technologies, which may be one of the reasons for the rapid increase in related research in the past decade. Looking back on the development of medicine in recent years, adjusting or updating existing diagnostic and treatment methods to improve the quality of life of SBS patients and reduce or eliminate serious complications caused by diseases is the focus of clinical physicians and an important direction of academic research. We hope that this article will serve as a stimulus for future research and provide new ideas.
SBS is a thoracic skeletal malformation often accompanied by MVP. Diagnostic criteria include the ratio of anterior-posterior to transverse thoracic diameter and specific X-ray features. The co-occurrence of SBS and MVP may be due to genetic variation and molecular mechanisms or physical compression. Cardiac morphology and arrhythmia in SBS may be caused by restricted cardiac pulsation, left atrial compression, and sympathetic activity. Recent studies have emphasized the importance of chest X-rays, echocardiography, and CT scans for diagnosing SBS. New methods for diagnosing SBS with MVP have been proposed. Treatment options include exercise, traction, posture adjustment, 3D printing, and surgical interventions.
We would like to express our gratitude to Li-Gang Xiao and Chun-Jiao Zhao for their valuable feedback and constructive suggestions on the manuscript. We also thank Yun-Bo Lai, Wen-Qing Zhang, and Li Xu for their efforts in translating and polishing the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Emran TB, Bangladesh S-Editor: Lin C L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Rawlings MS. The "straight back" syndrome, a new cause of pseudoheart disease. Am J Cardiol. 1960;5:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Wei MD, Yang K. Straight back syndrome and frequent ventricular premature beats. Eur Heart J Cardiovasc Imaging. 2022;23:e325. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Chen L, Ma XH, Zhao L, Bai R, Li SN, Wang L, Chen H, Fan ZM, Lou MW, Niu YD. Preliminary investigation of the relationship between straight-back syndrome and idiopathic arrhythmias by cardiac magnetic resonance imaging. Zhonghua Xinxueguan Zazhi. 2017;45:948-953. [RCA] [DOI] [Full Text] [Reference Citation Analysis (4)] |
| 4. | Datey KK, Deshmukh MM, Engineer SD, Dalvi CP. Straight Back Syndrome. Br Heart J. 1964;26:614-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Shiba H, Kenzaka T. Straight Back Syndrome Presented with Chest and Back Pain: A Case Report. Int Med Case Rep J. 2022;15:611-614. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Jiang SB, Li RY. Diagnosis and treatment of straight-back syndrome. Tianjin Yiyao. 1983;7:422-424. |
| 7. | Liu JX. Straight-back syndrome. Linchuang Huicui. 1993;19:909-910. |
| 8. | Schmid S. Highly Variable Clinical Manifestations of Straight Back Syndrome in the Young and the Elderly. Ann Thorac Surg. 2022;113:2107. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Deleon AC Jr, Perloff JK, Twigg H, Majd M. The Straight Back Syndrome: Clinical Cardiovascular Manifestations. Circulation. 1965;32:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 10. | Davies MK, Mackintosh P, Cayton RM, Page AJ, Shiu MF, Littler WA. The straight back syndrome. Q J Med. 1980;49:443-460. [PubMed] |
| 11. | Shen C, Che G. Straight Back Syndrome in an Elderly Patient. Ann Thorac Surg. 2022;113:2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Twigg HL, De Leon A, Perloff JK, Maid M. The straight-back syndrome: radiographic manifestations. Radiology. 1967;88:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Soleti P, Wilson B, Vijayakumar AR, Chakravarthi PI, Reddy CG. An interesting case of straight back syndrome and review of the literature. Asian Cardiovasc Thorac Ann. 2016;24:63-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Perez-Downes JC, Leon A, Reddy P. Straight Back Syndrome. J Am Osteopath Assoc. 2020;120:485. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Udoshi MB, Shah A, Fisher VJ, Dolgin M. Incidence of mitral valve prolapse in subjects with thoracic skeletal abnormalities--a prospective study. Am Heart J. 1979;97:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Federman M, Sutton RM, Reeder RW, Ahmed T, Bell MJ, Berg RA, Bishop R, Bochkoris M, Burns C, Carcillo JA, Carpenter TC, Dean JM, Diddle JW, Fernandez R, Fink EL, Franzon D, Frazier AH, Friess SH, Graham K, Hall M, Hehir DA, Horvat CM, Huard LL, Kirkpatrick T, Maa T, Maitoza LA, Manga A, McQuillen PS, Meert KL, Morgan RW, Mourani PM, Nadkarni VM, Notterman D, Palmer CA, Pollack MM, Sapru A, Schneiter C, Sharron MP, Srivastava N, Tilford B, Viteri S, Wessel D, Wolfe HA, Yates AR, Zuppa AF, Naim MY. Survival With Favorable Neurologic Outcome and Quality of Cardiopulmonary Resuscitation Following In-Hospital Cardiac Arrest in Children With Cardiac Disease Compared With Noncardiac Disease. Pediatr Crit Care Med. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Liu Y, Wang W, Long W, Cai B, Chen C, Guan S, Luo J, Chen K. Chest wall reconstruction with digitally designed materials for straight back syndrome with tracheal stenosis: a case report. Ann Transl Med. 2021;9:1357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Grillo HC, Wright CD, Dartevelle PG, Wain JC, Murakami S. Tracheal compression caused by straight back syndrome, chest wall deformity, and anterior spinal displacement: techniques for relief. Ann Thorac Surg. 2005;80:2057-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Serratto M, Kezdi P. Absence of the physiologic dorsal kyphosis. Cardiac signs and hemodynamic manifestations. Ann Intern Med. 1963;58:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Movahed A, Majdalany D, Gillinov M, Schiavone W. Association Between Myxomatous Mitral Valve Disease and Skeletal Back Abnormalities. J Heart Valve Dis. 2017;26:564-568. [PubMed] |
| 21. | Jones G, Johnson K, Eason J, Hamilton M, Osio D, Kanani F, Baptista J, Suri M. Traboulsi syndrome caused by mutations in ASPH: An autosomal recessive disorder with overlapping features of Marfan syndrome. Eur J Med Genet. 2022;65:104572. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Xu E, Shao W, Jiang H, Lin T, Gao R, Zhou X. A Genetic Variant in GPR126 Causing a Decreased Inclusion of Exon 6 Is Associated with Cartilage Development in Adolescent Idiopathic Scoliosis Population. Biomed Res Int. 2019;2019:4678969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Kou I, Takahashi Y, Johnson TA, Takahashi A, Guo L, Dai J, Qiu X, Sharma S, Takimoto A, Ogura Y, Jiang H, Yan H, Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, Taneichi H, Hosono N, Tsuji T, Suzuki T, Sudo H, Kotani T, Yonezawa I, Londono D, Gordon D, Herring JA, Watanabe K, Chiba K, Kamatani N, Jiang Q, Hiraki Y, Kubo M, Toyama Y, Tsunoda T, Wise CA, Qiu Y, Shukunami C, Matsumoto M, Ikegawa S. Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat Genet. 2013;45:676-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 24. | Disse S, Abergel E, Berrebi A, Houot AM, Le Heuzey JY, Diebold B, Guize L, Carpentier A, Corvol P, Jeunemaitre X. Mapping of a first locus for autosomal dominant myxomatous mitral-valve prolapse to chromosome 16p11.2-p12.1. Am J Hum Genet. 1999;65:1242-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 112] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Wright CD. Straight Back Syndrome. Thorac Surg Clin. 2017;27:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Kubala M, Essayagh B, Michelena HI, Enriquez-Sarano M, Tribouilloy C. Arrhythmic mitral valve prolapse in 2023: Evidence-based update. Front Cardiovasc Med. 2023;10:1130174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Xia J, Ma XJ, Xie SR, He YF, Cheng G, Wu M. Diagnostic value of real-time three-dimensional transesophageal echocardiography for the diagnosis of mitral valve prolapse. Zhonghua Yixue Chaosheng Zazhi. 2021;18:941-947. |
| 28. | Ansari A. The "straight back" syndrome: current perspective more often associated with valvular heart disease than pseudoheart disease: a prospective clinical, electrocardiographic, roentgenographic, and echocardiographic study of 50 patients. Clin Cardiol. 1985;8:290-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Xu WJ, Wang Y, Lv LH, Ge HL. Analysis of 16 Misdiagnosed Patients with the Straight Back Syndrome. Linchuan Wuzhen Wuzhi. 2013;26:36-38. |
| 30. | Bai YG. "Straight back syndrome" X-ray diagnosis. Zhongguo Nongchun Weisheng. 2013;270-271. |
| 31. | Hou YH, Chen DQ, Zhang L. The diagnostic value of increasing the anterior distance to the posterior sternal margin/anterior and posterior thoracic diameter ratio in straight back syndrome. Shanxi Zhigong Yixueyuan Xuebao. 2016;26:9-11. |
| 32. | Yang G, Zhang MH, Yu YF, Zhang LH, Wang YX, Cao XQ. Analysis of the 64-row CT signs of straight-back syndrome. Zhejiang Linchuang Yixue. 2018;683-685. |
| 33. | Matsumoto Y, Nitta M, Nakashima R, Matsumoto K, Sugano T, Ishigami T, Ishikawa T, Tamura K, Kimura K. A mechanism of a cardiac murmur with respiratory variation in a patient with straight back syndrome. J Cardiol Cases. 2020;22:230-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Marbella Fernández D, García V, Santana AJ, Montoya-Alonso JA. The Thoracic Inlet Length as a Reference Point to Radiographically Assess Cardiac Enlargement in Dogs with Myxomatous Mitral Valve Disease. Animals (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Betz JW, Oakley PA, Harrison DE. Relief of exertional dyspnea and spinal pains by increasing the thoracic kyphosis in straight back syndrome (thoracic hypo-kyphosis) using CBP(®) methods: a case report with long-term follow-up. J Phys Ther Sci. 2018;30:185-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Schmid S, Schibilsky D, Kalbhenn J, Hassan M, Loop T, Passlick B, Beyersdorf F, Czerny M. Reconstruction of the Mediastinum and Tracheopexy for Tracheomalacia in Straight Back Syndrome. Ann Thorac Surg. 2021;112:e41-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |