Published online Jan 26, 2021. doi: 10.4330/wjc.v13.i1.11
Peer-review started: September 29, 2020
First decision: December 7, 2020
Revised: December 18, 2020
Accepted: December 28, 2020
Article in press: December 28, 2020
Published online: January 26, 2021
Processing time: 107 Days and 17.1 Hours
There is a lack of data on the clinical outcomes in patients with native valve infective endocarditis (NVIE) and diabetes mellitus (DM).
To investigate (1) trends in the prevalence of DM among patients with NVIE; and (2) the impact of DM on NVIE outcomes.
We identified 76385 with NVIE from the 2004 to 2014 National Inpatient Sample, of which 22284 (28%) had DM. We assessed trends in DM from 2004 to 2014 using the Cochrane Armitage test. We compared baseline comorbidities, microorganisms, and in-patients procedures between those with vs without DM. Propensity match analysis and multivariate logistic regression were used to investigate study outcomes in in-hospital mortality, stroke, acute heart failure, cardiogenic shock, septic shock, and atrioventricular block.
Crude rates of DM increased from in 22% in 2004 to 30% in 2014. There were significant differences in demographics, comorbidities and NVIE risk factors between the two groups. Staphylococcus aureus was the most common organism identified with higher rates in patients with DM (33.1% vs 35.6%; P < 0.0001). After propensity matching, in-hospital mortality (11.1% vs 11.9%; P < 0.0001), stroke (2.3% vs 3.0%; P < 0.0001), acute heart failure (4.6% vs 6.5%; P = 0.001), cardiogenic shock (1.5% vs 1.9%; P < 0.0001), septic shock (7.2% vs 9.6%; P < 0.0001), and atrioventricular block (1.5% vs 2.4%; P < 0.0001), were significantly higher in patients with DM. Independent predictors of mortality in NVIE patients with DM include hemodialysis, congestive heart failure, atrial fibrillation, staphylococcus aureus, and older age.
There is an increasing prevalence of DM in NVIE and it is associated with poorer outcomes. Further studies are crucial to identify the clinical, and sociodemographic contributors to this trend and develop strategies to mitigate its attendant risk.
Core Tip: In this observational study, we found increasing prevalence rates for diabetes mellitus (DM) among patients with native valve infective endocarditis (NVIE) from 2004–2014. There were significant differences in risk factors, microbiology, and in-patient procedures between patients with DM compared to those without DM. DM was associated with mortality, acute heart failure, stroke, atrioventricular block, septic shock, and cardiogenic shock. Independent predictors of in-hospital mortality in NVIE patients with DM include hemodialysis, congestive heart failure, atrial fibrillation, staphylococcus aureus, and older age.
- Citation: Abe T, Eyituoyo HO, De Allie G, Olanipekun T, Effoe VS, Olaosebikan K, Mather P. Clinical outcomes in patients with native valve infective endocarditis and diabetes mellitus. World J Cardiol 2021; 13(1): 11-20
- URL: https://www.wjgnet.com/1949-8462/full/v13/i1/11.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i1.11
Despite advancements in management strategies, infective endocarditis (IE) is associated with high mortality rates ranging from 10%-26% and an estimated five-year survival rate of 60%-70%[1-4]. Also, IE carries a significant long term morbidity risk with high rates of stroke (24%), and heart failure (49%)[1-4]. One of the challenges associated with IE management is identifying patients at increased risk of complications. While current guidelines recommend active surveillance, early stratification can help identify patients who may benefit from further intervention.
We have seen an increase in hospitalization from native valve infective endocarditis (NVIE) in the United States [from 155151 (2002-2006) to 195300 (2012-2016)][5]. This is likely related to increased risk factors such as drug abuse, advanced age, and diabetes mellitus (DM)[5-9]. There is a paucity of data on the outcomes of NVIE in DM patients. Previous studies looking at DM and IE have been limited by single-center experiences, sample size and analyses that combine NVIE and prosthetic valve infective endocarditis (PVIE)[9-16]. In this study, using a well-characterized database, we investigated trends in the prevalence of DM among patients with NVIE; and the impact of DM on in-hospital mortality, acute heart failure, stroke, septic shock, cardiogenic shock, and atrioventricular block.
Our data source was the National In-patient Sample (NIS) 2004–2014, a subset of the Healthcare Cost Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality (AHRQ)[17,18]. Briefly, NIS and HCUP are the largest all-payer inpatient database in the United States. The database contains a 20% stratified sample of all discharges from United States hospitals representing the United States population and accounts for 90% of all hospitalizations[18]. It includes information on demographics, hospital characteristics, up to 25 diagnostic and procedure codes based on the International Classification of Diseases 9th revision, Clinical Modification (ICD-9-CM), and outcomes based on patient discharge records.
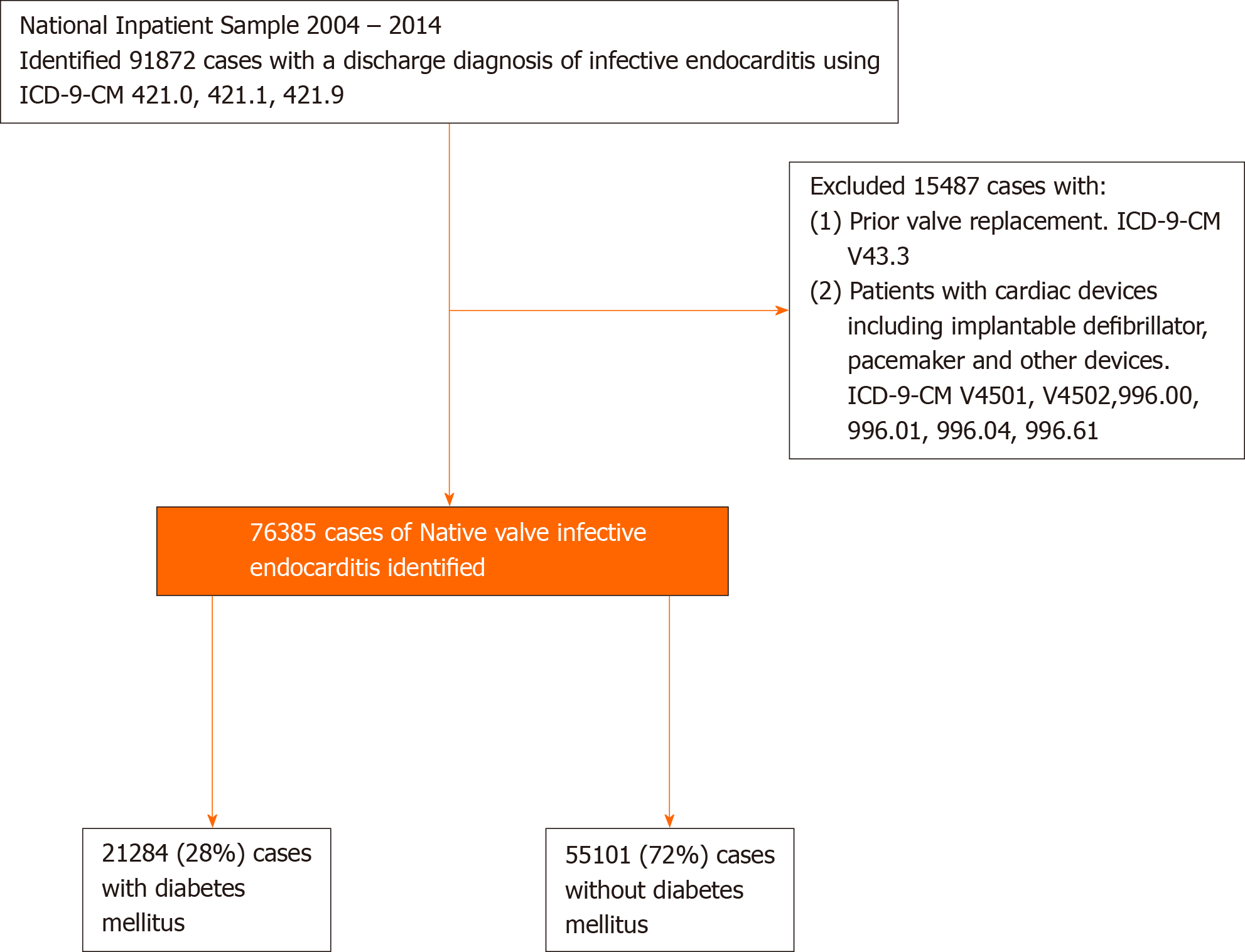
We queried NIS 2004–2014 using ICD-9-CM codes to identify patients age 18 and above, who were hospitalized with the primary diagnosis of acute or subacute IE. To limit the sample to NVIE, we excluded patients with a prior history of valve replacement and cardiac devices (Figure 1). The algorithm has been validated[5]. The cohort was then divided into those with and without DM. Demographics associated with each diagnosis were identified from NIS, and associated comorbidities were extracted from AHRQ[17,18]. This allows the identification of comorbidities that were present before admission. Other variables that could impact study outcomes, such as the type of organism and inpatient procedures, were included in the analysis. The primary endpoint was to investigate the impact of DM on in-hospital mortality, stroke, acute heart failure, cardiogenic shock, septic shock and atrioventricular block in those with NVIE. All clinical characteristics were defined using ICD-9-CM codes (Supplementary Table 1).
IBM SPSS V.25 was used for statistical analysis. Statistical significance was defined as P < 0.05. We compared baseline characteristics, organisms involved, and inpatient procedures between patients with DM compared to those without DM. Chi-square was used for categorical variables, while an independent student t-test was used for continuous variables. We performed trend analysis using the Cochrane-Armitage test to evaluate the temporal trends in the prevalence of DM in patients with NVIE.
We compared the incidence of in-hospital mortality, stroke, acute heart failure, cardiogenic shock, septic shock, and atrioventricular block. Descriptive statistics were reported in frequencies with percentages for categorical variables, while continuous variables were reported in mean, standard deviation, median, and 25th and 75th percentiles. To limit selection bias, we employed propensity score methodology to match hospitalizations with NVIE patients who had DM vs those without any DM at a 1:1 ratio. The nearest neighbor technique was adopted to match each case to control, which is closest to the calculated propensity score, with a caliper width of 0.1. The propensity score was calculated from the following 26 matching variables: Age, sex, race, atrial fibrillation, tobacco use disorder, valvular heart disease, hypothyroidism, chronic kidney disease, obesity, hypertension, congestive heart failure, chronic lung disease, hyperlipidemia, hemodialysis, chronic liver disease, peripheral artery disease, coronary artery disease, drug abuse, pulmonary hypertension, human immunodeficiency virus, congenital heart disease, history of cardiac transplant, rheumatic heart disease, staphylococcus aureus, other staphylococcus, viridians, streptococci, enterococci, group A streptococci, group B streptococci, group G streptococci, and gram-negative bacteremia. Multivariate logistic regression was then used to estimate the adjusted odds ratio of the study outcome in those with DM compared to those without DM.
Binomial regression was used to identify variables in the demographics, comorbidities, and microbiology that were associated with mortality. All significant variables were then incorporated into a multivariate logistic regression model to determine the predictors of in-hospital mortality.
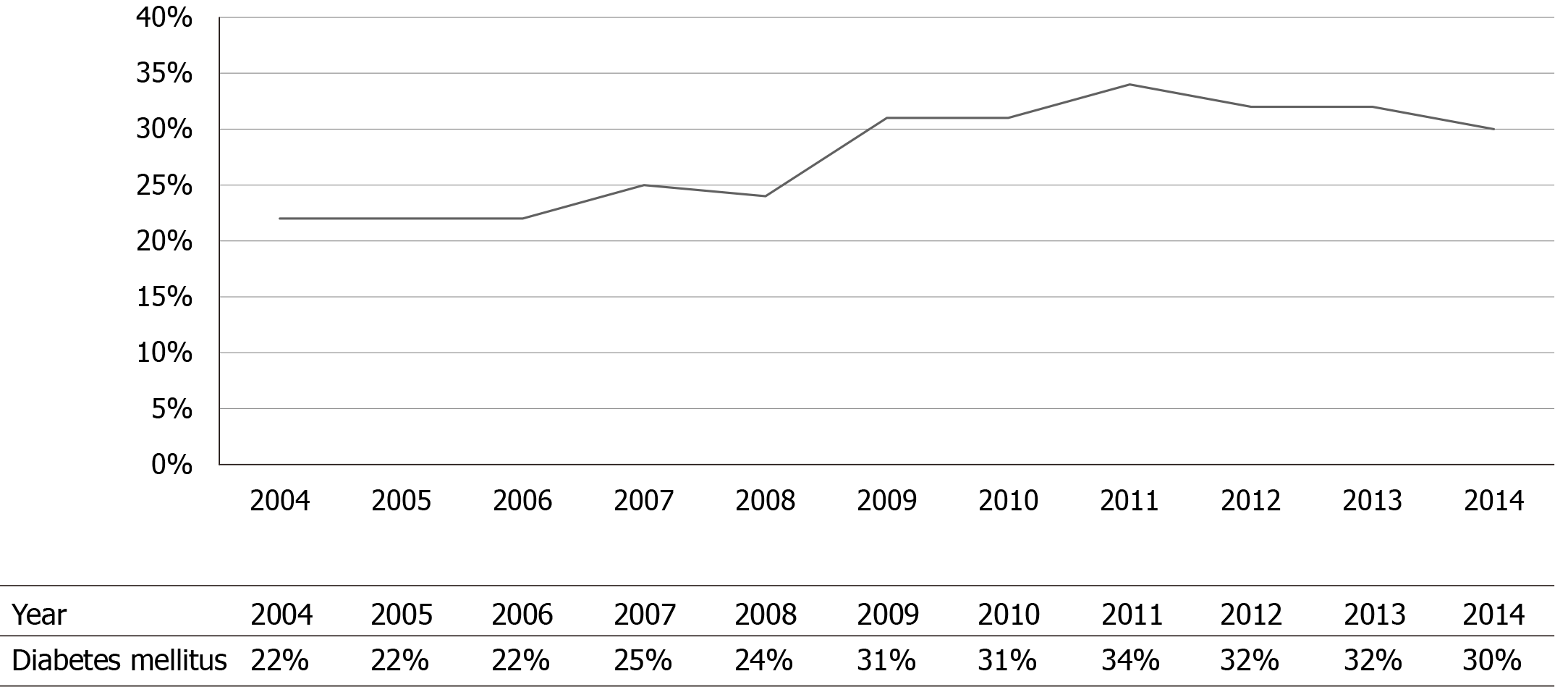
We identified 76385 patients with NVIE. Among these patients, 21284 (28%) had DM. The mean age of patients with DM was significantly higher (63.4 ± 14 vs 58.2 ± 19; P < 0.0001). The most predominant race was White American, and they were less likely to have DM compared to African, Hispanics, Asians, and Native Americans (Table 1). Patients with DM had higher rates of comorbidities, including hypertension, congestive heart failure, dyslipidemia, obesity, coronary artery disease, and pulmonary hypertension compared to those without DM (Table 1). The crude rates of DM in patients with NVIE significantly increased from 22% in 2004 to 30% in 2014; P < 0.0001 (Figure 2).
| Demographics and Co-morbidities | NVIE | ||
| No DM (%) | DM (%) | P value | |
| Age (yr), mean (SD) | 58.2 ± 19 | 63.37 ± 14 | < 0.0001 |
| Gender | < 0.0001 | ||
| Male | 58.4 | 55.6 | |
| Female | 41.6 | 44.4 | |
| Race | < 0.0001 | ||
| White | 71.9 | 62.5 | |
| Black | 15.9 | 20.0 | |
| Hispanic | 7.1 | 11.2 | |
| Asian | 1.8 | 2.4 | |
| Native Americans | 0.6 | 1.0 | |
| Other | 2.7 | 2.9 | |
| Co-morbidities | |||
| Tobacco abuse | 14.8 | 9.0 | < 0.0001 |
| Hypothyroidism | 6.2 | 9.6 | < 0.0001 |
| Hyperlipidemia | 11.5 | 25.1 | < 0.0001 |
| Valvular heart diseases | 21.6 | 19.0 | < 0.0001 |
| Chronic kidney disease | 3.8 | 7.9 | < 0.0001 |
| Obesity | 1.8 | 7.2 | < 0.0001 |
| Congestive heart failure | 26.2 | 34.2 | < 0.0001 |
| Chronic liver disease | 9.3 | 12.4 | < 0.0001 |
| Hypertension | 39.1 | 64.8 | < 0.0001 |
| HIV | 2.6 | 0.9 | < 0.0001 |
| Atrial fibrillation | 18.9 | 22.4 | < 0.0001 |
| Pulmonary hypertension | 5.8 | 6.5 | < 0.0001 |
| Coronary artery disease | 14.2 | 29.7 | < 0.0001 |
| Peripheral artery disease | 2.4 | 5.5 | < 0.0001 |
| Hemodialysis | 15.0 | 27.8 | < 0.0001 |
| History of cardiac arrest | 0.0 | 0.1 | 0.011 |
| History of drug abuse | 11.9 | 3.8 | < 0.0001 |
| Congenital heart disease | 1.8 | 0.8 | < 0.0001 |
| Rheumatic heart disease | 11.8 | 9.6 | < 0.0001 |
In terms of the infective organism involved, patients with DM had higher rates of staphylococcus aureus (35.6% vs 33.1%; P < 0.0001), other staphylococcus organisms (6.7% vs 5.4%; P < 0.0001), enterococci (7.6% vs 6.5%; P < 0.0001), group B streptococci (1.6% vs 1.3%; P < 0.0001), and gram-negative organisms (4.8 vs 3.8; P < 0.0001) (Table 2). For inpatient procedures, DM patients were less likely to undergo surgical valve replacement (8.2% vs 10.6%; P < 0.0001) (Table 3).
| Infective organisms | NVIE | ||
| No DM (%) | DM (%) | P value | |
| Staphylococcus aureus | 33.1 | 35.6 | < 0.001 |
| Other staphylococcus | 5.4 | 6.7 | < 0.001 |
| Viridans streptococci | 18.6 | 15.5 | < 0.001 |
| Enterococci | 6.5 | 7.6 | < 0.001 |
| Pneumococcus | 0.5 | 0.4 | 0.11 |
| Group A streptococci | 0.7 | 0.5 | 0.003 |
| Group B streptococci | 1.3 | 1.6 | < 0.001 |
| Group C streptococci | 0.1 | 0.1 | 0.44 |
| Group G streptococci | 0.2 | 0.2 | 0.87 |
| Gram negative | 3.8 | 4.8 | < 0.001 |
| Anaerobes | 0.4 | 0.3 | 0.82 |
| Fungemia | 0.2 | 0.2 | 0.81 |
| NVIE | |||
| Inpatient procedures | No DM (%) | DM (%) | P value |
| Surgical valve replacement | 12.3 | 9.2 | < 0.001 |
| Aortic valve replacement | 6.5 | 5.1 | < 0.001 |
| Mitral valve replacement | 5.0 | 3.7 | < 0.001 |
| Tricuspid valve replacement | 0.1 | 0.1 | 0.43 |
| Pulmonary valve replacement | 0.7 | 0.3 | < 0.001 |
After propensity matching rates of stroke (2.4% vs 1.5%; P < 0.0001), acute heart failure (6.5% vs 4.6%; P = 0.001), atrioventricular block (6.5% vs 4.6%; P < 0.0001), septic shock (9.6% vs 7.2%; P < 0.0001), cardiogenic shock (1.9% vs 1.5%; P < 0.0001), and in-hospital mortality (11.9% vs 11.1%; P < 0.0001), were significantly higher in patients with DM and NVIE, compared to those with NVIE alone. The multivariate logistic regression followed similar trends (Table 4). Predictors of in-hospital mortality in patients with NVIE and DM included hemodialysis, congestive heart failure, atrial fibrillation, staphylococcus aureus, and older age (Table 5).
| NVIE | ||||||
| No DM (%) | DM (%) | aOR | Lower CI | Upper CI | P value | |
| In-hospital mortality | 11.1 | 11.9 | 1.2 | 1.1 | 1.4 | < 0.0001 |
| Acute heart failure | 4.6 | 6.5 | 1.2 | 1.1 | 1.3 | 0.001 |
| Stroke | 2.3 | 3.0 | 1.3 | 1.1 | 1.5 | < 0.0001 |
| Atrioventricular block | 1.5 | 2.4 | 1.5 | 1.3 | 1.5 | < 0.0001 |
| Septic shock | 7.2 | 9.6 | 1.2 | 1.1 | 1.3 | < 0.0001 |
| Cardiogenic shock | 1.5 | 1.9 | 1.4 | 1.2 | 1.6 | < 0.0001 |
| 95%CI | ||||
| OR | Lower | Upper | P value | |
| Age (yr) | ||||
| 18-65 | REF | REF | REF | REF |
| > 65 | 1.02 | 1.02 | 1.02 | < 0.001 |
| Hypertension | 0.56 | 0.50 | 0.62 | < 0.0001 |
| Atrial fibrillation | 1.17 | 1.05 | 1.31 | 0.006 |
| Tobacco abuse | 0.74 | 0.61 | 0.90 | 0.003 |
| Hyperlipidemia | 0.69 | 0.61 | 0.78 | < 0.0001 |
| Congestive heart failure | 1.34 | 1.22 | 1.48 | < 0.0001 |
| Chronic liver disease | 1.51 | 1.19 | 1.92 | 0.001 |
| Staphylococcus aureus | 1.25 | 1.13 | 1.39 | < 0.0001 |
| Hemodialysis | 2.04 | 1.83 | 2.27 | < 0.0001 |
In this observational study, we found increasing prevalence rates for DM among patients with NVIE from 2004–2014. There were significant differences in risk factors, microbiology, and in-patient procedures between patients with DM compared to those without DM. DM was associated with mortality, acute heart failure, stroke, atrioventricular block, septic shock, and cardiogenic shock. Independent predictors of in-hospital mortality in NVIE patients with DM include hemodialysis, congestive heart failure, atrial fibrillation, staphylococcus aureus, and older age. Compared to other studies that have investigated the clinical outcomes of DM in IE patients[9-16]. We had a robust sample size. Also, the analyses in these studies combined NVIE and PVIE. It is essential to stratify because the clinical course, management, and outcomes significantly differ[19-21]. Lastly, we identified the independent predictors of mortality.
The overall prevalence of DM was 28%, and the crude rates of DM significantly increased from 2004–2014. It should be mentioned that there has been a parallel increase in the prevalence of DM in the United States, which had been attributed to increased risk factors such as obesity, sedentary lifestyle, enhanced detection, and increased longevity[22,23]. A study demonstrated an increased prevalence from 7.7% in 1999-2000 to 13.3% in 2015-2016 among United States adults[24]. In another study using the national health and nutrition examination survey, the prevalence increased from 9.8% in 1988–1994 to 12.4% in 2011–2012, across all age, sex, and racial groups[25].
The clinical profile of NVIE patients with DM was different compared to those without DM. DM patients had higher rates of comorbidities, and IE risk factors such as older age, and hemodialysis. They were less likely to have structural heart disease (valvular heart disease and congenital heart disease) and intravenous drug abuse (Table 1). Several other studies have reported similar findings[8,10,12-14]. We also found significant differences in the organisms involved. DM patients had higher rates of staphylococcus species, enterococci, and gram-negative microorganisms. This is also consistent with prior studies and likely due to increased health care utilization in DM patients, exposing them to nosocomial infections and immune dysfunctions, rendering them more susceptible to skin and soft tissue infections[16,26].
There are a couple of explanations for the poor outcomes among NVIE patients with DM. First, they are more likely to have staphylococcus aureus infections. Staphylococcus aureus tends to stick and multiply on heart valves, promoting vegetation, abscess formation, mechanical complications, and mortality[27-29]. Secondly, IE is characterized by an immunologic response that leads to immune complex formation; the exaggerated immunologic response in DM patients likely contributed to poor outcomes noted in this study[30]. Finally, lower rates of life-saving procedures such a surgical valve replacement, as demonstrated in this study, and higher rates of comorbidities in DM patients are other explanations.
Current IE management strategies center around presumed patient mortality risk. Generally, low-risk patients can be safely managed with antibiotics. At the same time, aggressive intervention such as valve replacement is recommended for those at high risk of mortality, suffering from, acute heart failure, large vegetation, and mechanical complications such as valvular dysfunction, and perivalvular abscess[31-34]. These recommendations stem from observational studies demonstrating mortality benefit[31-34]. In this study, odds for in-hospital mortality was 20% higher in DM patients compared to those without DM. Staphylococcus aureus was highly prevalent among patients with DM, and it was significantly associated with mortality. Several other studies have linked staphylococcus aureus to death, likely due to severe valvular damage and complications[4,27,28,34]. Early surgery should be considered in NVIE secondary to staphylococcus aureus, especially in DM patients, due to severe valve destruction and increased mortality.
Other predictors of mortality among DM patients in this study include congestive heart failure, hemodialysis, and atrial fibrillation. This suggests that prevention and aggressive management of comorbid conditions in DM patients could potentially decrease associated NVIE mortality. NVIE is characterized by bacteremia, bacteria colonization, adhesion on cardiac valves and vegetation formation[35]. Immune dysfunction, micro- and macro-angiopathies, and decreased bactericidal activity of the gastrointestinal and genitourinary system make DM patients more susceptible to infections[36]. Tight glycemic control, vaccination, and adequate skin care will help reduce bacteremia and NVIE in DM patients.
Some limitations of our study should also be noted. First, data on glycemic control and management of DM were not available. It is well known that strict glycemic control can improve the clinical outcome in patients with DM[4]. Secondly, the study lacked information on clinical variables such as echocardiographic features, vegetation size, and antimicrobials therapy, all of which might impact clinical outcomes[4].
Among patients with NVIE, DM is associated with increased mortality and complications. This is likely due to higher rates of staphylococcus bacteremia, underlying comorbidities, and immune dysfunction. Further studies should focus on prevention and management strategies among DM patients with NVIE.
There is a lack of data on the clinical outcomes in patients with native valve infective endocarditis (NVIE) and diabetes mellitus (DM).
Previous studies looking at DM and infective endocarditis (IE) have included analyses that combine NVIE and prosthetic valve IE.
In this study, aim to investigate the temporal trends in the prevalence of DM in NVIE and investigate the impact of DM on NVIE outcomes.
The National Inpatient Sample 2004–2014 was queried. Cochrane Armitage test was used for trend analysis. Propensity match scoring and multivariate logistic regression were used to investigate study outcomes (Supplementary Table 2).
We identified 76385 patients with NVIE, of which 21284 (28%) had DM. Patients with DM had more comorbidities, were more likely to have staphylococcus infection, and less likely to undergo surgical valve replacement. In-hospital mortality, and IE related complications such as stroke, acute heart failure, cardiogenic shock, septic shock, and atrioventricular block, were significantly higher in patients with DM. Independent predictors of mortality in NVIE patients with DM include hemodialysis, congestive heart failure, atrial fibrillation, staphylococcus aureus, and older age.
There is an increasing prevalence of DM in NVIE and it is associated with poorer outcomes.
Further studies are crucial to identify the clinical, and sociodemographic contributors to this trend and develop strategies to mitigate its attendant risk.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Heart Association, No. 000258201449; American College of Cardiology, No. 3332058; American College of Physicians, No. 0363287; and American Society of Preventive Cardiology.
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: MD HS S-Editor: Fan JR L-Editor: A P-Editor: Li JH
| 1. | Shih CJ, Chu H, Chao PW, Lee YJ, Kuo SC, Li SY, Tarng DC, Yang CY, Yang WC, Ou SM, Chen YT. Long-term clinical outcome of major adverse cardiac events in survivors of infective endocarditis: a nationwide population-based study. Circulation. 2014;130:1684-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Cahill TJ, Baddour LM, Habib G, Hoen B, Salaun E, Pettersson GB, Schäfers HJ, Prendergast BD. Challenges in Infective Endocarditis. J Am Coll Cardiol. 2017;69:325-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 428] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 3. | Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falcó V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1676] [Cited by in RCA: 1640] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 4. | Chu VH, Cabell CH, Benjamin DK Jr, Kuniholm EF, Fowler VG Jr, Engemann J, Sexton DJ, Corey GR, Wang A. Early predictors of in-hospital death in infective endocarditis. Circulation. 2004;109:1745-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 288] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 5. | Khan MZ, Munir MB, Khan MU, Khan SU, Benjamin MM, Balla S. Contemporary Trends in Native Valve Infective Endocarditis in United States (from the National Inpatient Sample Database). Am J Cardiol. 2020;125:1678-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3709] [Cited by in RCA: 4383] [Article Influence: 626.1] [Reference Citation Analysis (0)] |
| 7. | Critchley JA, Carey IM, Harris T, DeWilde S, Hosking FJ, Cook DG. Glycemic Control and Risk of Infections Among People With Type 1 or Type 2 Diabetes in a Large Primary Care Cohort Study. Diabetes Care. 2018;41:2127-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 8. | Lin CJ, Chua S, Chung SY, Hang CL, Tsai TH. Diabetes Mellitus: An Independent Risk Factor of In-Hospital Mortality in Patients with Infective Endocarditis in a New Era of Clinical Practice. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Wei XB, Liu YH, Huang JL, Chen XL, Yu DQ, Tan N, Chen JY, He PC. Prediabetes and diabetes are both risk factors for adverse outcomes in infective endocarditis. Diabet Med. 2018;35:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Benvenga RM, De Rosa R, Silverio A, Matturro R, Zambrano C, Masullo A, Mastrogiovanni G, Soriente L, Ascoli R, Citro R, Piscione F, Galasso G. Infective endocarditis and diabetes mellitus: Results from a single-center study from 1994 to 2017. PLoS One. 2019;14:e0223710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Bishara J, Peled N, Samra Z, Sagie A, Leibovici L, Pitlik S. Infective endocarditis in diabetic and non-diabetic patients. Scand J Infect Dis. 2004;36:795-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Kourany WM, Miro JM, Moreno A, Corey GR, Pappas PA, Abrutyn E, Hoen B, Habib G, Fowler VG Jr, Sexton DJ, Olaison L, Cabell CH; ICE MD Investigators. Influence of diabetes mellitus on the clinical manifestations and prognosis of infective endocarditis: a report from the International Collaboration on Endocarditis-Merged Database. Scand J Infect Dis. 2006;38:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Chirillo F, Bacchion F, Pedrocco A, Scotton P, De Leo A, Rocco F, Valfrè C, Olivari Z. Infective endocarditis in patients with diabetes mellitus. J Heart Valve Dis. 2010;19:312-320. [PubMed] |
| 14. | Olmos C, Vilacosta I, Pozo E, Fernández C, Sarriá C, López J, Ferrera C, Maroto L, González I, Vivas D, Palacios J, San Román JA. Prognostic implications of diabetes in patients with left-sided endocarditis: findings from a large cohort study. Medicine (Baltimore). 2014;93:114-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Moreno R, Zamorano J, Almería C, Villate A, Rodrigo JL, Herrera D, Alvarez L, Morán J, Aubele A, Mataix L, De Marco E, Sánchez-Harguindey L. Influence of diabetes mellitus on short- and long-term outcome in patients with active infective endocarditis. J Heart Valve Dis. 2002;11:651-659. [PubMed] |
| 16. | Duval X, Alla F, Doco-Lecompte T, Le Moing V, Delahaye F, Mainardi JL, Plesiat P, Célard M, Hoen B, Leport C; Association pour l'Etude et la Prévention de l'Endocardite Infectieuse (AEPEI). Diabetes mellitus and infective endocarditis: the insulin factor in patient morbidity and mortality. Eur Heart J. 2007;28:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Houchens R, Ross D, Elixhauser A, Jiang J. Nationwide Inpatient Sample (NIS) Redesign Final Report. 2014. HCUP Methods Series Report # 2014-04 ONLINE. April 4, 2014. [cited 2020, Dec 12]. U.S. Agency for Healthcare Research and Quality. Available from: http://www.hcupus.ahrq.gov/reports/methods/methods.jsp. |
| 18. | Nationwide Inpatient Sample (NIS) Healthcare cost and utilization project (HCUP). 2000-2011. [cited 2020, Dec 12]. Agency for Healthcare Research and Quality, Rockville, MD2013. Available from: www.hcup-us.ahrq.gov/nisoverview.jsp. |
| 19. | Tornos P, Iung B, Permanyer-Miralda G, Baron G, Delahaye F, Gohlke-Bärwolf Ch, Butchart EG, Ravaud P, Vahanian A. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart. 2005;91:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 285] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 20. | Que YA, Moreillon P. Infective endocarditis. Nat Rev Cardiol. 2011;8:322-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 21. | Romano G, Carozza A, Della Corte A, De Santo LS, Amarelli C, Torella M, De Feo M, Cerasuolo F, Cotrufo M. Native versus primary prosthetic valve endocarditis: comparison of clinical features and long-term outcome in 353 patients. J Heart Valve Dis. 2004;13:200-8; discussion 208. [PubMed] |
| 22. | Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: Insights from Yesterday, Today, and Future Trends. Popul Health Manag. 2017;20:6-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 443] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 23. | Benoit SR, Hora I, Albright AL, Gregg EW. New directions in incidence and prevalence of diagnosed diabetes in the USA. BMJ Open Diabetes Res Care. 2019;7:e000657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 24. | Fang M. Trends in Diabetes Management Among US Adults: 1999-2016. J Gen Intern Med. 2020;35:1427-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988-2012. JAMA. 2015;314:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1462] [Cited by in RCA: 1460] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 26. | Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, Rutten GE. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 687] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 27. | Salvador VB, Chapagain B, Joshi A, Brennessel DJ. Clinical Risk Factors for Infective Endocarditis in Staphylococcus aureus Bacteremia. Tex Heart Inst J. 2017;44:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Kanafani ZA, Kourany WM, Fowler VG Jr, Levine DP, Vigliani GA, Campion M, Katz DE, Corey GR, Boucher HW. Clinical characteristics and outcomes of diabetic patients with Staphylococcus aureus bacteremia and endocarditis. Eur J Clin Microbiol Infect Dis. 2009;28:1477-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3118] [Cited by in RCA: 3131] [Article Influence: 313.1] [Reference Citation Analysis (0)] |
| 30. | Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, Fowler VG Jr. Infective endocarditis. Nat Rev Dis Primers. 2016;2:16059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 271] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 31. | Jamil M, Sultan I, Gleason TG, Navid F, Fallert MA, Suffoletto MS, Kilic A. Infective endocarditis: trends, surgical outcomes, and controversies. J Thorac Dis. 2019;11:4875-4885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB; Coselli JS. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J Thorac Cardiovasc Surg 2017; 153: 1241-1258. e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 283] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 33. | Hubers SA, DeSimone DC, Gersh BJ, Anavekar NS. Infective Endocarditis: A Contemporary Review. Mayo Clin Proc. 2020;95:982-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 34. | Miro JM, Anguera I, Cabell CH, Chen AY, Stafford JA, Corey GR, Olaison L, Eykyn S, Hoen B, Abrutyn E, Raoult D, Bayer A, Fowler VG Jr; International Collaboration on Endocarditis Merged Database Study Group. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis. 2005;41:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 35. | Sullam PM, Drake TA, Sande MA. Pathogenesis of endocarditis. Am J Med. 1985;78:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16 Suppl 1:S27-S36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 515] [Article Influence: 39.6] [Reference Citation Analysis (0)] |