Published online Jan 26, 2019. doi: 10.4330/wjc.v11.i1.24
Peer-review started: September 19, 2018
First decision: October 26, 2018
Revised: December 16, 2018
Accepted: December 24, 2018
Article in press: December 24, 2018
Published online: January 26, 2019
Processing time: 129 Days and 8.6 Hours
Obesity is a major health problem due to its high prevalence. The relationship between obesity and cardiovascular disease is unclear. Some studies agree that certain conditions associated with obesity, such as physical inactivity or cardiovascular risk factors, are responsible for cardiovascular risk excess among obese people. Carotid intima-media thickness and carotid plaques (CP) have been associated with cardiovascular adverse events in healthy populations, and recent data suggest a higher prevalence of subclinical carotid atherosclerosis in obese and metabolically unhealthy patients. However, there are no studies correlating subclinical atherosclerosis and adverse events (AE) in obese subjects.
To determine the association between carotid disease and AE in obese patients with negative exercise echocardiography (EE).
From January 1, 2006 to December 31, 2010, 2000 consecutive patients with a suspicion of coronary artery disease were submitted for EE and carotid ultrasonography. Exclusion criteria included previous vascular disease, left ventricular ejection fraction < 50%, positive EE, significant valvular heart disease and inferior to submaximal EE. An AE was defined as all-cause mortality, myocardial infarction and cerebrovascular accident. Subclinical atherosclerosis was defined as CP presence according to Manheim and the American Society of Echocardiography Consensus.
Of the 652 patients who fulfilled the inclusion criteria, 226 (34.7%) had body mass indexes ≥ 30 kg/m2, and 76 of them (33.6%) had CP. During a mean follow-up time of 8.2 (2.1) years, 27 AE were found (11.9%). Mean event-free survival at 1, 5 and 10 years was 99.1% (0.6), 95.1% (1.4) and 86.5% (2.7), respectively. In univariate analysis, CP predicted AE [hazard ratio (HR) 2.52, 95% confidence interval (CI) 1.17-5.46; P = 0.019]. In multivariable analysis, the presence of CP remained a predictor of AE (HR 2.26, 95%CI 1.04-4.95, P = 0.041). Other predictors identified were glomerular filtration rate (HR 0.98, 95%CI 0.96-0.99; P = 0.023), peak metabolic equivalents (HR 0.83, 95%CI 0.70–0.99, P = 0.034) and moderate mitral regurgitation (HR 5.02, 95%CI 1.42–17.75, P = 0.012).
Subclinical atherosclerosis defined by CP predicts AE in obese patients with negative EE. These patients could benefit from aggressive prevention measures.
Core tip: There is a controversy about obesity and coronary artery disease prognosis. Several studies suggest a greater influence of physical inactivity than that of body mass index on mortality, but there are no data addressing the influence of subclinical atherosclerosis in patients with suspected coronary artery disease submitted to a non-invasive treadmill test. Our study shows that clinical atherosclerosis in other vascular beds, such as carotid plaque presence, is a greater predictor than functional capacity. These patients could benefit from aggressive prevention measures.
- Citation: Vidal-Perez R, Franco-Gutiérrez R, Pérez-Pérez AJ, Franco-Gutiérrez V, Gascón-Vázquez A, López-López A, Testa-Fernández AM, González-Juanatey C. Subclinical carotid atherosclerosis predicts all-cause mortality and cardiovascular events in obese patients with negative exercise echocardiography. World J Cardiol 2019; 11(1): 24-37
- URL: https://www.wjgnet.com/1949-8462/full/v11/i1/24.htm
- DOI: https://dx.doi.org/10.4330/wjc.v11.i1.24
Obesity and body mass index (BMI) have increased in every nation in the last years, associating with a concomitant augmentation in the prevalence of traditional cardiovascular risk factors[1]. Obesity is independently associated with mortality and cardiovascular disease[2,3], likely through adverse remodelling of the arteries and a higher prevalence of subclinical vascular disease[4,5]. However, once cardiovascular disease (CVD) is established, the studies published so far show contradictory results. Some investigations suggest a protective effect of obesity[6,7]. Other researchers suggest that it is not the obesity itself, but certain associated characteristics, such as physical inactivity or metabolic risk factors, that explain the increased risk attributed to obese people. This suggestion gives rise to concepts such as metabolically healthy obesity[8,9] or fit obese patients[10,11].
Several epidemiological studies have demonstrated an independent association of carotid disease, defined as carotid plaques (CP) or carotid intima media thickness (CIMT), with overall mortality and cardiovascular events[12-16]. Although a negative treadmill exercise stress echocardiography is associated with good prognosis, according to European guidelines on stable coronary artery disease (CAD)[17], the annualized event rates defined as overall mortality and adverse cardiac events are nearly 1% in contemporary series[18]. It therefore seems necessary to define other tools to decrease adverse events (AE) in these patients. As we previously described, carotid disease has been associated with adverse cardiovascular events[12-15], and one advantage of carotid ultrasonography is that it is not invasive and can be performed immediately after the exercise echocardiography (EE) using the same equipment. Moreover, ultrasound assessment of carotid arteries in patients with suspected CAD without known atherosclerotic disease is a class IIa C recommendation in the aforementioned European guidelines[17].
There are no studies addressing the value of subclinical atherosclerosis, defined as carotid disease, and AE in obese patients with or without CVD. The Multi-Ethnic Study of Atherosclerosis found significantly higher CIMT values in obese patients after adjustment for traditional CVD risk factors[4] or high-sensitivity C-reactive protein values[5]. Recent publications have found a higher percentage of subclinical carotid disease among metabolically unhealthy subjects compared to those with metabolic disease absence in obese people either with[19] or without CAD[20]. These studies emphasize the concept of obesity and associated phenotypes as predictors of AE. The aim of the current study is to determine if carotid disease is a predictor of AE in obese patients with CAD suspicion and negative treadmill stress echocardiography.
We performed a retrospective cohort study of patients without significant heart or vascular disease, with a BMI ≥ 30 kg/m2 and coronary artery disease suspicion with negative EE who were submitted for carotid ultrasonography.
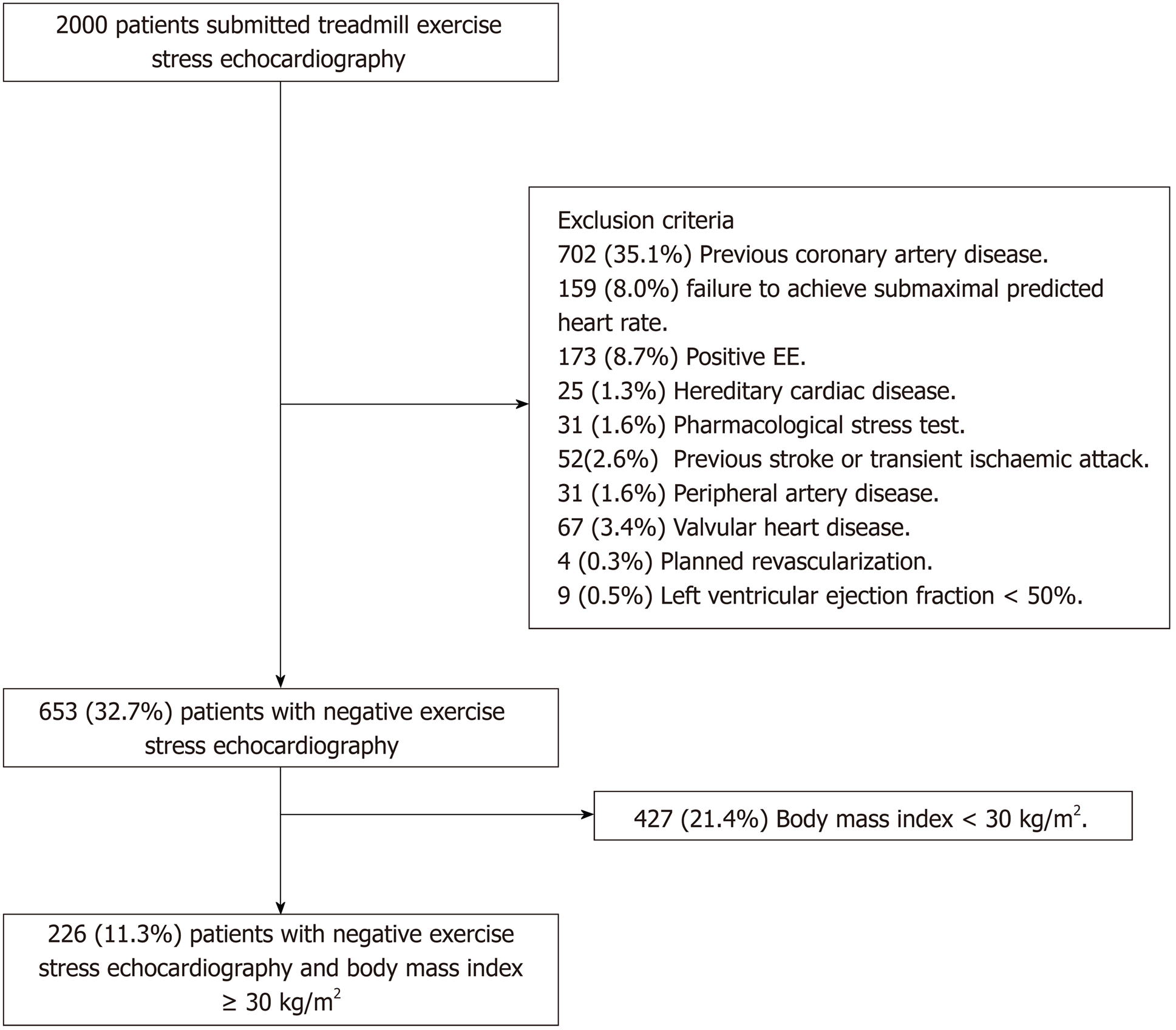
Between January 2006 and December 2010, 2000 patients were submitted for stress echocardiography and carotid ultrasonography in our centre. Of them, 226 (11.3%) were included. Exclusion criteria included previous CAD [n = 702 (35.1%)], failure to achieve submaximal predicted heart rate [n = 159 (8.0%)], positive EE [n = 173 (8.7%)], hereditary cardiac disease (e.g., Brugada syndrome, hypertrophic cardiomyopathy) [n = 25 (1.3%)], pharmacological stress test [n = 31 (1.6%)], previous stroke or transient ischaemic attack [n = 52 (2.6%)], peripheral artery disease [n = 31 (1.6%)], valvular heart disease, defined as aortic stenosis of any aetiology, mitral rheumatic stenosis or more than moderate valve regurgitation [n = 67 (3.4%)], planned revascularization [n = 4 (0.2%)], left ventricular ejection fraction less than 50% [n = 9 (0.5%)], loss during follow-up [n = 21 (1.1%)], technical problems accessing the stored images [n = 73 (3.7%)] and BMI < 30 kg/m2 [n = 426 (21.3%)]. All patients signed the informed consent before performing the test. The study was approved by the Regional Ethics Committee. Figure 1 summarizes the selection criteria.
Demographic and clinical characteristics as well as CAD pre-test probabilities (PTP) were collected from available medical records at the time of the first clinical visit when EE was requested. Baseline echocardiography, carotid ultrasonography and stress testing data were collected from digitally stored images and medical records at the time of EE performance. CAD PTP and Systematic Coronary Risk Evaluation (SCORE) were assessed according to current European Society of Cardiology guidelines[1,17]. Treatment data were collected from medical records obtained at the first visit after EE performance. Of the 226 patients, 172 (76.1%) were evaluated the same day after EE performance. For the 54 patients that were not evaluated the same day, the median time between EE and first medical was 13.5 d (interquartile range 47.3).
Physiological parameters such as blood pressure, heart rate, and a 12-lead ECG were registered at baseline and at each stage of the treadmill stress protocol. The Bruce treadmill protocol was the preferred method of exercise, but Naughton was employed in a minority of subjects. A submaximal test was defined as an achievement of 85% of the age-predicted heart rate. EE was prematurely stopped in case of physical exhaustion, significant arrhythmia, severe hypertension or hypotensive response. Electrocardiographic changes suggestive of myocardial ischaemia during testing were defined as a new ST-segment deviation of 1 mm or more, measured at 80 ms after the J point.
Echocardiographic views were attained at rest, peak and immediately after exercise, and digitally stored for later comparisons. Assessment of regional wall motion was done in a 17-segment model of the left ventricle by using a motility score that ranged from 1 to 4, depending on its motion. Baseline and exercise wall motion score index were calculated as average scores of the 17 segments at rest and peak exercise, respectively. With the exception of isolated hypokinesia of the inferobasal segment and worsening from akinesia to dyskinesia, exercise-induced echocardiography ischaemia was defined as new or worsening wall motion abnormalities developed during the stress test. When ischaemic changes affected three or more myocardial segments, the exercise test was considered as extensive ischemia, while multivessel ischaemia was defined when wall motion abnormalities were detected in two or more different coronary territories[21].
The patients were submitted to carotid ultrasonography immediately after the EE performance using the same ultrasound equipment (Philips iE33; Philips Medical Systems, Best, Holland). The ARIC protocol study[12] and expert consensus[22-24] were followed for the CIMT measurement. CP was defined as focal structures invading 0.5 mm or more into the arterial lumen, presenting an increase of at least 50% in its thickness compared to the neighbouring CIMT value, or a CIMT greater than 1.5 mm as measured from the intima-lumen limit to the media adventitia limit[22-24]. Semi-automated edge recognition software was used (QLAB; Philips 110 Medical Systems, Andover, MA, United States).
Subclinical atherosclerosis was defined as a binary variable as CP presence/absence. Both carotid ultrasonography and EE stored images were examined by two cardiologists with broad experience in cardiac and carotid imaging who were blinded to the AE. A third expert reviewed the images in case of any doubt or disagreement.
Follow-up data were obtained from the hospital database, medical records and death certificates. In the case of doubt, the Regional Mortality Registry was consulted. AE was defined as a combined endpoint of all-cause mortality, myocardial infarction and cerebrovascular accident. Myocardial infarction was defined as specified by the third universal definition of the myocardial infarction expert consensus document[25]. Stroke was defined as a loss of neurological function caused by an ischaemic event that lasted for more than 24 h and left residual signs.
No statistical sample-size calculation was done in our study. On the one hand, this was an innovative unicentric study in terms of using carotid ultrasonography in obese patients with an EE with good prognosis. On the other hand, no previously published studies were found for statistical determination of sample size calculations.
Continuous variables were reported as the mean (standard deviation) or median (interquartile range) depending on Shapiro-Wilk normality test results, whereas categorical variables were reported as percentages. Cumulative death, myocardial infarction and cerebrovascular accident curves were calculated by the Kaplan-Meier method and compared using the log rank-test. Cox proportional-hazards regression was used for both univariate and multivariate analyses. All variables with P values less or equal to 0.2 were included in the multivariable analysis, and a retention set of 0.1 was applied. A P value of 0.05 or less was considered to be statistically significant.
Of the 226 patients, 76 (33.6%) had subclinical atherosclerosis defined by CP presence. Patients with CP were older (P < 0.01), with a higher prevalence of hypertension (P = 0.002) and dyslipidaemia (P = 0.027), higher SCORE (P < 0.001), lower glomerular filtration rate (P < 0.001), lower high-density lipoprotein cholesterol (P = 0.043) and higher triglycerides (P = 0.011). This group also showed a higher percentage of patients with intermediate-to-high PTP for CAD and lower percentage of cardiovascular risk factor–free subjects (P < 0.001). Regarding basal echocardiography, there were no differences in basal ejection fraction, but CP subjects had more mitral regurgitation (P = 0.001). Heart rate (P < 0.001), exercise time (P = 0.011) and metabolic equivalents (METs) (P = 0.015) were lower in the CP group, whereas mean CIMT (P < 0.001) and CIMT > 0.9 mm (P < 0.001) were higher. Patients with CP were more frequently on angiotensin II receptor blockers (P = 0.001), calcium channel blockers (P = 0.011), statins (P = 0.043) and oral antidiabetic (P = 0.030) treatment. The baseline characteristics are summarized in Tables 1 and 2.
| n = 226 | No plaque, n = 150 | Plaque, n = 76 | P value | |
| Age | 63.2 (11.4) | 60.6 (12.1) | 68.2 (7.3) | < 0.001a |
| Male sex | 106 (46.9%) | 64 (42.7%) | 42 (55.3%) | 0.099 |
| Hypertension | 166 (73.5%) | 100 (66.7%) | 66 (86.8%) | 0.002a |
| Diabetes mellitus | 45 (19.9%) | 24 (16.0%) | 21 (27.6%) | 0.058 |
| Dyslipidaemia | 124 (54.9%) | 74 (49.3%) | 50 (65.8%) | 0.027a |
| Current smoker | 55 (24.3%) | 36 (24.0%) | 19 (25.0%) | 0.999 |
| Family history of premature CAD | 17 (7.5%) | 13 (8.7%) | 4 (5.3%) | 0.516 |
| BMI, kg/m2 | 33.3 (4.1) | 33.2 (4.5) | 33.4 (3.0) | 0.694 |
| Obesity | 0.033a | |||
| Grade 1 | 179 (79.2%) | 126 (84.0%) | 53 (69.7%) | |
| Grade 2 | 39 (17.3%) | 19 (12.7%) | 20 (26.3%) | |
| Grade 3 | 8 (3.5%) | 5 (3.3%) | 3 (3.9%) | |
| No cardiovascular risk factors | 29 (12.8%) | 28 (18.7%) | 1 (1.3%) | < 0.001a |
| SCORE | < 0.001a | |||
| Low-risk, < 1% | 29 (12.8%) | 28 (18.7%) | 1 (1.3%) | |
| Moderate-risk, 1%-5% | 113 (50.0%) | 78 (52.0%) | 35 (46.1%) | |
| High risk, 5%-10% | 33 (14.6%) | 20 (13.3%) | 13 (17.1%) | |
| Very high-risk, ≥ 10% | 49 (21.7%) | 24 (16.0%) | 25 (32.9%) | |
| Not classifiable | 2 (0.9%) | 0 | 2 (2.6%) | |
| CAD PTP | 0.017a | |||
| < 15% | 10 (4.4%) | 10 (6.7%) | 0 | |
| 15-65% | 180 (79.6%) | 121 (80.7%) | 59 (77.6%) | |
| 65-85% | 36 (15.9%) | 19 (12.7%) | 17 (22.4%) | |
| > 85% | 0 (0%) | 0 (0%) | 0 (0%) | |
| Fasting plasma glucose, mg/dL | 113.8 (32.2) | 112.5 (33.4) | 116.6 (29.5) | 0.369 |
| Glomerular filtration rate, mL/min/1.73 m2 | 87.6 (25.4) | 91.4 (27.1) | 79.7 (20.3) | < 0.001a |
| Total cholesterol, mg/dL | 199.6 (40.5) | 196.9 (38.5) | 205.0 (43.9) | 0.159 |
| HDL cholesterol, mg/dL | 48.6 (12.3) | 49.6 (13.4) | 46.5 (9.1) | 0.043a |
| Triglycerides, mg/dL | 143.6 (75.9) | 133.0 (60.0) | 164.8 (97.6) | 0.011a |
| LDL cholesterol, mg/dL | 122.1 (34.6) | 120.2 (34.1) | 125.9 (35.8) | 0.257 |
| Atrial fibrillation | 26 (11.5%) | 14 (9.3%) | 12 (15.8%) | 0.224 |
| Treatment after EE | ||||
| Angiotensin-converting enzyme inhibitor | 29 (12.8%) | 16 (10.7%) | 13 (17.1%) | 0.247 |
| Angiotensin II receptor blockers | 99 (43.8%) | 54 (36.0%) | 45 (59.2%) | 0.001a |
| Beta-blockers | 72 (31.9%) | 44 (29.3%) | 28 (36.8%) | 0.32 |
| Calcium channel blockers | 53 (23.5%) | 27 (18.0%) | 26 (34.2%) | 0.011a |
| Nitrates | 21 (9.3%) | 13 (8.7%) | 8 (10.5%) | 0.832 |
| Statins | 111 (49.1%) | 66 (44.0%) | 45 (59.2%) | 0.043a |
| Ezetimibe | 6 (2.7%) | 3 (2.0%) | 3 (3.9%) | 0.673 |
| Fibrates | 10 (4.4%) | 5 (3.3%) | 5 (6.6%) | 0.476 |
| Omega−3 fatty acids | 4 (1.8%) | 3 (2.0%) | 1 (1.3%) | 1 |
| Antiplatelet drugs | 109 (48.2%) | 67 (44.7%) | 42 (55.3%) | 0.172 |
| Anticoagulants drugs | 17 (7.5%) | 8 (5.3%) | 9 (11.8%) | 0.137 |
| Oral antidiabetic drugs | 28 (12.4%) | 13 (8.7%) | 15 (19.7%) | 0.030a |
| Insulin treatment | 5 (2.2%) | 2 (1.3%) | 3 (3.9%) | 0.338 |
| n = 226 | No plaque, n = 150 | Plaque, n = 76 | P value | |
| Baseline echocardiography | ||||
| Baseline ejection fraction, % | 64.6 (5.2) | 64.5 (5.2) | 64.7 (5.3) | 0.823 |
| Mitral regurgitation | 82 (36.3%) | 43 (28.7%) | 39 (51.3%) | 0.001a |
| Aortic regurgitation | 57 (25.2%) | 33 (22.0%) | 24 (31.6%) | 0.16 |
| Tricuspid regurgitation | 116 (51.3%) | 73 (48.7%) | 43 (56.6%) | 0.325 |
| Pulmonary regurgitation | 3 (1.3%) | 3 (2.0%) | 0 (0%) | 0.553 |
| Pulmonary artery systolic pressure, mmHg | 32.6 (7.3) | 30.5 (5.8) | 35.1 (6.2) | 0.013a |
| Treadmill exercise stress echocardiography | ||||
| Stress protocol | 0.778 | |||
| Naughton | 14 (6.2%) | 10 (6.7%) | 4 (5.3%) | |
| Bruce | 212 (93.8%) | 140 (93.3%) | 72 (94.7%) | |
| Systolic blood pressure | ||||
| Baseline | 141.8 (18.8) | 140.7 (18.7) | 143.9 (18.9) | 0.222 |
| Peak | 197.6 (23.8) | 196.6 (23.0) | 199.7 (25.4) | 0.361 |
| Heart rate | ||||
| Baseline | 73.6 (12.4) | 74.0 (11.9) | 72.8 (13.5) | 0.409 |
| Peak | 146.0 (13.1) | 148.2 (13.4) | 141.7 (11.5) | < 0.001a |
| Percentage | 93.1 (5.6) | 93.0 (5.5) | 93.3 (5.8) | 0.718 |
| Maximal stress test | 26 (11.5%) | 17 (11.3%) | 9 (11.8%) | 1 |
| Rate-pressure, × 103 mmHg beats/min | ||||
| Basal | 10.5 (2.4) | 10.4 (2.3) | 10.5 (2.6) | 0.831 |
| Peak | 28.9 (4.4) | 29.1 (4.4) | 28.3 (4.3) | 0.174 |
| Exercise time, min | 8.6 (2.9) | 8.1 (2.9) | 7.1 (2.4) | 0.011a |
| METs | 8.5 (2.9) | 8.8 (3.1) | 7.9 (2.3) | 0.015a |
| Carotid ultrasonography | ||||
| CIMT, mm | 0.80 (0.20) | 0.74 (0.18) | 0.91 (0.18) | < 0.001a |
| CIMT > 0.9 mm | 62 (27.4%) | 27 (18.0%) | 35 (46.1%) | < 0.001a |
During a mean follow-up of 8.0 (2.2) years, six (2.7%) non-ST elevation myocardial infarctions, two (0.9%) ST elevation myocardial infarctions, nine (4.0%) strokes and 15 (6.6%) deaths were recorded, for a total of 27 (11.9%) AE.
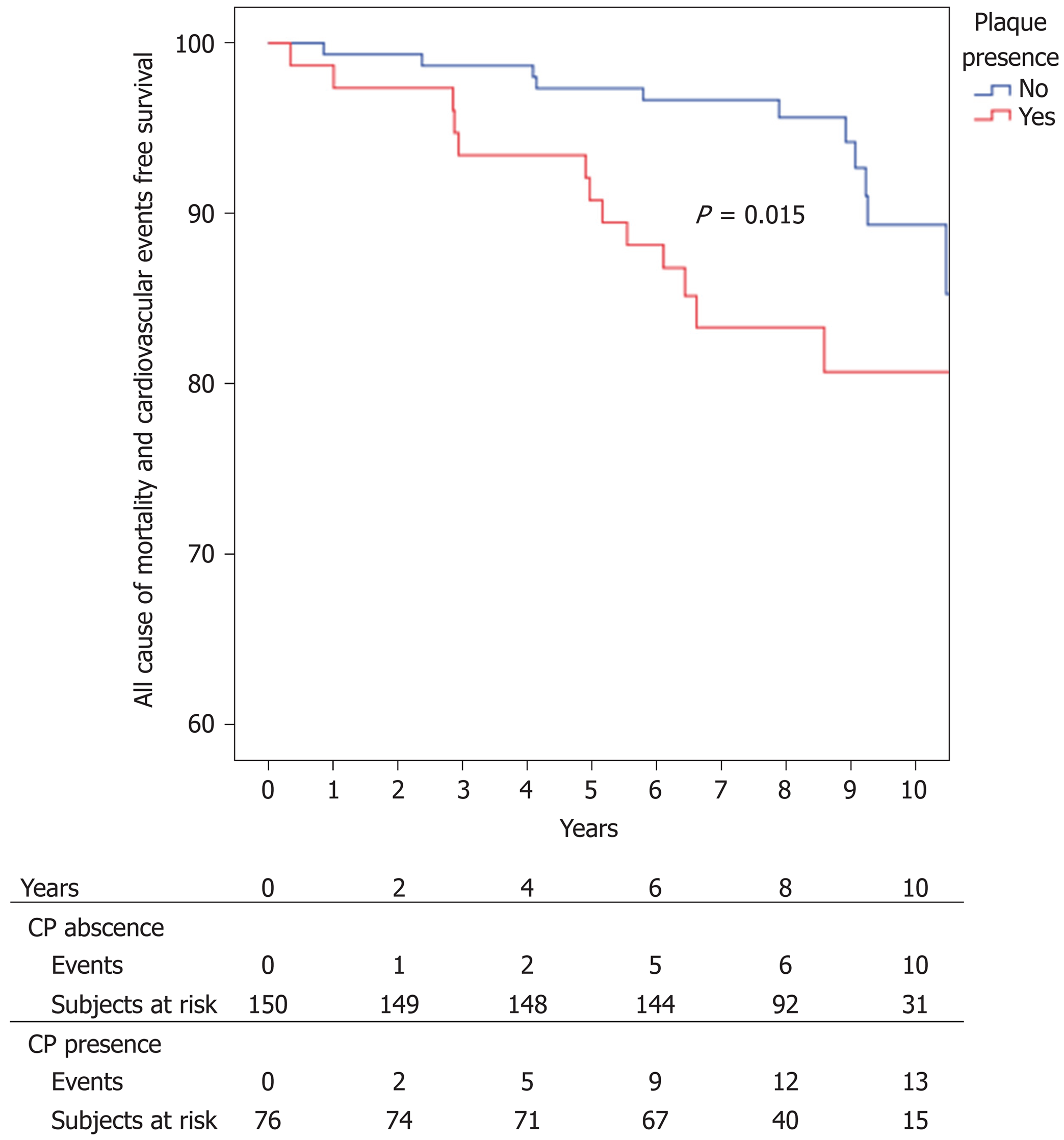
Kaplan–Meier adverse event-free survival at 1, 2, 3, 5 and 10 years was 99.1% (0.6), 98.7% (0.8), 96.9% (1.2), 95.1 (1.4%) and 86.5% (2.7%), respectively. Kaplan-Meier event-free survival was significantly higher in the non-CP group, with 99.3% (0.7) event-free survival at 1 and 2 years, 98.7% (0.9) at 2 and 3 years, 97.3% (1.3) at 5 years and 89.3% (3.5) at 10 years vs 98.7% (1.3) at 1 year, 97.4 (1.8%) at 2 and 3 years, 93.4 (2.8) at 5 years and 80.7% (5.0) at 10 years in the CP group (P = 0.015) ( Figure 2).
Age (P < 0.001), glomerular filtration rate (P = 0.002), moderate mitral regurgitation (P = 0.007), cardiorespiratory fitness expressed in METs (P = 0.001) and CP presence (P = 0.019) were associated in univariate analysis with AE.
Multivariable analysis showed that glomerular filtration rate (P = 0.023), moderate mitral regurgitation (P = 0.012), peak METs during the EE (P = 0.034) and CP (P = 0.041) were independent predictors of AE. Tables 3 and 4 show the univariate and multivariate analysis results.
| Hazard ratio | 95% Confidence interval | P value | |
| Age | 1.1 | 1.05-1.15 | < 0.001a |
| Male sex | 0.68 | 0.31-1.50 | 0.338 |
| Hypertension | 2.52 | 0.75-8.44 | 0.134 |
| Diabetes mellitus | 1.42 | 0.60-3.38 | 0.427 |
| Dyslipidaemia | 1.89 | 0.82-4.36 | 0.134 |
| Current smoker | 1.23 | 0.52-2.95 | 0.635 |
| Family history of premature CAD | 2.17 | 0.65-7.26 | 0.21 |
| No cardiovascular risk factors | 0.3 | 0.04-2.24 | 0.242 |
| High/very high SCORE | 2.15 | 0.98-4.71 | 0.055 |
| Atrial fibrillation | 2.45 | 0.98-6.10 | 0.055 |
| CAD PTP ≥ 65% | 1.44 | 0.57-3.60 | 0.441 |
| BMI, kg/m2 | 0.93 | 0.80-1.09 | 0.381 |
| Fasting plasma glucose | 1 | 0.99-1.01 | 0.863 |
| Glomerular filtration rate, mL/min/1.73 m2 | 0.98 | 0.96-0.99 | 0.002a |
| Total cholesterol, mg/dL | 1 | 0.99-1.00 | 0.333 |
| HDL cholesterol, mg/dL | 1 | 0.95-1.02 | 0.409 |
| Triglycerides, mg/dL | 1 | 0.99-1.01 | 0.189 |
| LDL cholesterol, mg/dL | 0.99 | 0.98-1.01 | 0.294 |
| Left ventricular ejection fraction, % | 0.98 | 0.91-1.06 | 0.563 |
| Moderate mitral regurgitation | 5.29 | 1.57-17.84 | 0.007a |
| Moderate aortic regurgitation | 4.24 | 0.57-31.55 | 0.158 |
| Moderate tricuspid regurgitation | 2.03 | 0.27-15.19 | 0.492 |
| METs | 0.77 | 0.66-0.90 | 0.001a |
| CIMT | 0.91 | 0.14-6.19 | 0.926 |
| CIMT > 0.9 mm | 0.79 | 0.33-1.91 | 0.603 |
| CP presence | 2.52 | 1.17-5.46 | 0.019a |
To the best of our knowledge, this is the first article that correlates subclinical atherosclerosis with AE in obese patients, and specifically in obese patients with suspicion of ischaemic heart disease and good prognoses from EE.
Recent data from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation show that mortality in stable CAD is not negligible, with nearly 25% of patients dying during a mean follow-up of 10.5 years[26]. Moreover, the composite outcome of death, nonfatal myocardial infarction and stroke at a median follow-up period of 4.6 years has been approximately 20%[27]. For that reason, it is important to find predictors of evolution beyond the classic clinical, echocardiography, non-invasive and invasive CAD risk factors[17].
Our study shows that CP increased the probability of an AE in obese patients with CAD suspicion and negative EE by 2.26. Similar findings were obtained in other studies performed in ischaemic patients[28-34]. In the Angina Prognosis Study in Stockholm[28], CIMT could not predict AE defined as cardiovascular death or cardiovascular events, while CP had a tendency (P = 0.056) to predict them in 809 patients younger than 70 years with clinical suspicion of CAD. Compared to our study, their patients were younger, more frequently male, with a lower percentage of traditional cardiovascular risk factors. More importantly, 14% of subjects had previous myocardial infarction, BMI was not reported, there was no prognosis assessment by non-invasive stress tests, and the CP definition was different from ours. Petersen et al[29] reported CP presence, especially heterogeneous plaques, as a predictor of all-cause deaths in 541 hospitalized cardiological patients, 25% of them with a BMI > 30 kg/m2, after a median follow-up of 34 mo. Recently, Sirimarco et al[30] detected CP presence as a predictor of a composite of first occurrence of cardiovascular death, myocardial infarction, or coronary hospitalization during a follow-up period of 4 years in 45,227 middle-aged patients (45 years or more). In addition, CP in this study also predicted three or more cardiovascular risk factors or established CAD, cerebrovascular disease or peripheral artery disease in these patients, 28.1% of whom had BMI ≥ 30 kg/m2. Both studies had heterogeneous populations, with 64% of patients diagnosed with ischaemic heart disease in the Petersen study and 55.6% with CAD (defined as stable angina, prior acute coronary event, history of percutaneous coronary intervention or coronary artery bypass grafting) in the Reduction of Atherothrombosis for Continued Health Registry. A non-invasive stress test was not performed in the CAD patients. Like ours, their patients with CP were older and had a higher prevalence of cardiovascular risk factors. Studies involving patients with CAD assessed by angiography have also been published. Komorovsky et al[31] identified echogenic or calcified CP as a predictor of cardiac death, non-fatal myocardial infarction, and rehospitalization for unstable angina in 337 consecutive patients with acute coronary syndrome submitted to coronary angiography. Along the same lines, Zielinski et al[32] found a significant association between CIMT and death from all causes, stroke, or myocardial infarction (P = 0.010) in hypertensive patients with CAD, defined as ≥ 50% stenosis by coronary angiography and a mean BMI of 28.6 (3.8) kg/m2. Park et al[33] found CP as a predictor of cardiac death and hard major AE (death, stroke or myocardial infarction) in a cohort of 1,390 consecutive patients with angiographically-proven CAD and a mean BMI of 24.7 (3.4) kg/m2 followed up during a mean of 54.2 mo. However, they did not find a significant relationship with CIMT. Although their inclusion criteria differed from ours, their findings were similar to other studies and ours in that the CP patients were older and had a greater prevalence of cardiovascular risk factors. One important issue is that 33.9% of patients had previous CAD, > 60% had left ventricular ejection fraction < 50%, 41.2% were treated with percutaneous coronary angioplasty or coronary artery bypass grafting, and they included stent restenosis and target vessel revascularization in the end point. Notably, these events were not only due to atherosclerosis progression. Finally, Steinvil et al[34] found significant associations between carotid stenosis and all-cause mortality, myocardial infarction, stroke, and any coronary revascularization procedure in 1,015 patients with significant CAD (defined as stenosis > 70% determined by angiography). However, they did not indicate which treatment was performed (medical, percutaneous intervention or surgical) or which medication was administered, and they did not specify other important prognostic factors, such as left ventricular ejection fraction.
Although CIMT was associated in classic[12-14] and contemporary studies[16] with overall mortality and cardiovascular events, we were not able to make this association in this research. CIMT as a surrogate marker of atherosclerosis and predictor of AE is penalized by the highly variability association in the different studies published so far[12-14,16,28,35]. Possible explanations for this discrepancy are differences in measurement methods, definitions of abnormal CIMT, atherosclerosis development between the vascular beds and in the adaptive response[16,35]. Recent studies have shown CP as a better predictor of cardiovascular events than CIMT[15]. It is possible that CP represents a more advanced atherogenesis stage than CIMT[15,36,37]. This issue explains why CP groups have a consistently higher prevalence of cardiovascular risk factors and are older[29,33], and why there was a lower percentage of patients with “healthy metabolic obesity” in our CP group. Our findings are in consonance with current European Guidelines on Cardiovascular Disease Prevention in Clinical Practice, where CIMT screening for cardiovascular risk assessment is not recommended (Class III level A indication), whereas CP assessment is a IIb B recommendation for the same purpose[1]. In this sense, patients with CP might benefit from aggressive preventive measures, and it is important to highlight that in our study not all patients with subclinical atherosclerosis were treated after EE, such as very high-risk patients, with only 59.2% of the CP group receiving statins.
In addition to carotid disease, functional capacity was associated with AE. As previously mentioned, functional capacity has been associated with mortality in obese patients. Barry et al[10] meta-analysis showed that overweight and obese fit people presented similar mortality risks to normal weight fit subjects (odds ratio 1.21; 95% confidence interval (CI) 0.95 to 1.52), whereas obese unfit patients had higher overall mortality compared to normal weight fit individuals (odds ratio 2.46; 95%CI 1.92 to 3.14). Focusing on obese patients with CAD, Goel et al[38] found a statistical association between low fitness and mortality in patients with central obesity and a tendency towards such an association in obese and overweight patients. This was assessed by measuring the BMI of 855 patients who were enrolled in the Mayo Clinic cardiac rehabilitation programme, ultimately revealing that the association of BMI with mortality is complex and altered by fitness level.
It was not surprising to find glomerular filtration rate and mitral valve regurgitation as AE predictors. Several articles have found a significant relationship between CP and/or CIMT and CAD presence and extension in dialysis or end-stage renal disease patients[39,40]. Moreover, renal disease has been associated with a worse prognosis after acute coronary syndrome[41]. Focusing in obese patients with angiographic CAD, chronic kidney disease, defined as glomerular filtration rate < 60 mL/min/1.73 m2, was a strong predictor of cardiac events [hazard ratio (HR) 1.63, 95%CI 1.05-2.53] and overall mortality (HR 2.17, 95%CI 1.54-3.07) in Asiatic subjects with BMI > 25 kg/m2[42]. On the other hand, mitral valve regurgitation has been identified as an important long-term predictor of adverse outcomes in patients with ischaemic heart disease in different clinical scenarios, such as after acute myocardial infarction[43], coronary artery bypass graft surgery[44], percutaneous coronary intervention[45] and even stable CAD[46]. Recently, it has also been associated with a worse prognosis in patients referred for non-invasive stress testing (dobutamine stress echocardiography)[47].
The main strength of our study is the restrictive inclusion criteria, including obese patients with a good prognosis from EE, and the exclusion of potential confounding factors such as decreased left ventricular ejection fraction, previous CAD (and a subsequent different treatment approach), valvular heart disease that can evolve and produce AE (like aortic stenosis) and hereditary cardiac disease. Moreover, and in contrast to the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation study where patients were included after coronary angiography[26,27], our study is in consonance with European guidelines where PTP is first determined and then non-invasive testing is performed to establish CAD diagnosis and prognosis[17]. The main limitation of our study is that it is a retrospective and single centre study. For that reason, circulating or urinary biomarkers that might be helpful for guiding therapy in certain situations (e.g., albuminuria in hypertension or DM) were not analysed. Nevertheless, this strategy is in consonance with 2016 European guidelines on CVD prevention in clinical practice, which advise against the routine assessment of circulating or urinary biomarkers as a method to reclassify cardiovascular risk[1]. Another limitation is the number of patients studied. However, even with a small sample size, this study was big enough to reveal significant differences in several issues traditionally related to AE in obese and non-obese patients, such as carotid disease, cardiorespiratory fitness, moderate mitral regurgitation and glomerular filtration rate. It is possible, however, that other clinical conditions like traditional cardiovascular risk factors may not be represented in the multivariate analysis due to insufficient statistical power. Finally, treatments were not included in the AE analysis. The main reason for this is because baseline medications are difficult to maintain throughout the study (mean follow-up time 8.2 ± 2.1 years) and can skew the results, since they can be easily added or removed by different professionals who are in charge of the patient throughout this extended period of time.
In conclusion, subclinical atherosclerosis defined by CP presence predicts AE in obese patients with negative EE. These patients could benefit from aggressive prevention measures.
Obesity is independently associated with mortality and cardiovascular disease. However, once cardiovascular disease is established, the studies published so far show contradictory results. On the other hand, several epidemiological studies have demonstrated an independent association of carotid disease, defined as carotid plaques or carotid intima media thickness, with overall mortality and cardiovascular events.
There are no studies addressing the value of subclinical atherosclerosis, defined as carotid disease, and adverse events in obese patients with or without cardiovascular disease.
This study aimed to determine if carotid disease is a predictor of adverse events in obese patients with coronary artery disease suspicion and negative treadmill stress echocardiography.
A retrospective cohort study of patients without significant heart or vascular disease, body mass index ≥ 30 kg/m2 and coronary artery disease suspicion with negative exercise echocardiography (EE) submitted to carotid ultrasonography. Between January 2006 and December 2010, 2000 patients were submitted for stress echocardiography and carotid ultrasonography in our centre. Of them, 226 (11.3%) were included. Adverse events were defined as all-cause mortality, myocardial infarction and cerebrovascular accident.
We found that 226 patients had body mass indexes ≥ 30 kg/m2, and 76 of them (33.6%) had carotid plaques. During a mean follow-up time of 8.2 (2.1) years, 27 adverse events were found (11.9%). Mean event-free survival at 1, 5 and 10 years was 99.1% (0.6), 95.1% (1.4) and 86.5% (2.7), respectively. In univariate analysis, carotid plaques predicted adverse events (hazard ratio (HR) 2.52, 95% confidence interval (CI) 1.17-5.46; P = 0.019). In multivariable analysis, the presence of carotid plaques remained a predictor of adverse events (HR 2.26, 95%CI 1.04-4.95, P = 0.041). Other predictors identified were glomerular filtration rate, metabolic equivalents and moderate mitral regurgitation.
This study demonstrates that subclinical atherosclerosis defined by carotid plaques predicts adverse events in obese patients with negative EE.
To the best of our knowledge, this is the first article that correlates subclinical atherosclerosis and adverse events in obese patients, and specifically in obese patients with suspicion of ischaemic heart disease and a good prognosis from EE. One lesson from this study is that these good prognosis patients could be further stratified with carotid imaging and, in the case of plaque presence, benefit from more aggressive prevention measures.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Avtanski D, Fabbian F, Karatza AA, Kharlamov AN S- Editor: Yan JP L- Editor: Filipodia E- Editor: Song H
| 1. | Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention and amp; Rehabilitation (EACPR). Eur Heart J. 2016;37:2315-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5080] [Cited by in RCA: 4710] [Article Influence: 523.3] [Reference Citation Analysis (1)] |
| 2. | McGee DL; Diverse Populations Collaboration. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 349] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | Emerging Risk Factors Collaboration; Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085-1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 945] [Cited by in RCA: 854] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 4. | Burke GL, Bertoni AG, Shea S, Tracy R, Watson KE, Blumenthal RS, Chung H, Carnethon MR. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: The Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:928-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Blaha MJ, Rivera JJ, Budoff MJ, Blankstein R, Agatston A, O’Leary DH, Cushman M, Lakoski S, Criqui MH, Szklo M, Blumenthal RS, Nasir K. Association between obesity, high-sensitivity C-reactive protein ≥2 mg/L, and subclinical atherosclerosis: implications of JUPITER from the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:1430-1438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Oreopoulos A, Padwal R, Norris CM, Mullen JC, Pretorius V, Kalantar-Zadeh K. Effect of obesity on short- and long-term mortality postcoronary revascularization: a meta-analysis. Obesity (Silver Spring). 2008;16:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: A systematic review of cohort studies. Lancet. 2006;368:666-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1088] [Cited by in RCA: 1163] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 8. | Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med. 2013;159:758-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 708] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 9. | van der A DL, Nooyens AC, van Duijnhoven FJ, Verschuren MM, Boer JM. All-cause mortality risk of metabolically healthy abdominal obese individuals: the EPIC-MORGEN study. Obesity (Silver Spring). 2014;22:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Barry VW, Baruth M, Beets MW, Durstine JL, Liu J, Blair SN. Fitness vs fatness on all-cause mortality: A meta-analysis. Prog Cardiovasc Dis. 2014;56:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 438] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 11. | Ekelund U, Ward HA, Norat T, Luan J, May AM, Weiderpass E, Sharp SJ, Overvad K, Østergaard JN, Tjønneland A, Johnsen NF, Mesrine S, Fournier A, Fagherazzi G, Trichopoulou A, Lagiou P, Trichopoulos D, Li K, Kaaks R, Ferrari P, Licaj I, Jenab M, Bergmann M, Boeing H, Palli D, Sieri S, Panico S, Tumino R, Vineis P, Peeters PH, Monnikhof E, Bueno-de-Mesquita HB, Quirós JR, Agudo A, Sánchez MJ, Huerta JM, Ardanaz E, Arriola L, Hedblad B, Wirfält E, Sund M, Johansson M, Key TJ, Travis RC, Khaw KT, Brage S, Wareham NJ, Riboli E. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: The European Prospective Investigation into Cancer and Nutrition Study (EPIC). Am J Clin Nutr. 2015;101:613-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 241] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 12. | Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997;146:483-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1410] [Cited by in RCA: 1441] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 13. | Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2110] [Cited by in RCA: 2215] [Article Influence: 123.1] [Reference Citation Analysis (0)] |
| 14. | Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke. 2006;37:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 15. | Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 539] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 16. | Lorenz MW, Gao L, Ziegelbauer K, Norata GD, Empana JP, Schmidtmann I, Lin HJ, McLachlan S, Bokemark L, Ronkainen K, Amato M, Schminke U, Srinivasan SR, Lind L, Okazaki S, Stehouwer CDA, Willeit P, Polak JF, Steinmetz H, Sander D, Poppert H, Desvarieux M, Ikram MA, Johnsen SH, Staub D, Sirtori CR, Iglseder B, Beloqui O, Engström G, Friera A, Rozza F, Xie W, Parraga G, Grigore L, Plichart M, Blankenberg S, Su TC, Schmidt C, Tuomainen TP, Veglia F, Völzke H, Nijpels G, Willeit J, Sacco RL, Franco OH, Uthoff H, Hedblad B, Suarez C, Izzo R, Zhao D, Wannarong T, Catapano A, Ducimetiere P, Espinola-Klein C, Chien KL, Price JF, Bergström G, Kauhanen J, Tremoli E, Dörr M, Berenson G, Kitagawa K, Dekker JM, Kiechl S, Sitzer M, Bickel H, Rundek T, Hofman A, Mathiesen EB, Castelnuovo S, Landecho MF, Rosvall M, Gabriel R, de Luca N, Liu J, Baldassarre D, Kavousi M, de Groot E, Bots ML, Yanez DN, Thompson SG; PROG-IMT study group. Predictive value for cardiovascular events of common carotid intima media thickness and its rate of change in individuals at high cardiovascular risk - Results from the PROG-IMT collaboration. PLoS One. 2018;13:e0191172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL; Task Force Members; ESC Committee for Practice Guidelines; Document Reviewers. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2772] [Cited by in RCA: 2994] [Article Influence: 249.5] [Reference Citation Analysis (0)] |
| 18. | Bouzas-Mosquera A, Peteiro J, Alvarez-García N, Broullón FJ, Mosquera VX, García-Bueno L, Ferro L, Castro-Beiras A. Prediction of mortality and major cardiac events by exercise echocardiography in patients with normal exercise electrocardiographic testing. J Am Coll Cardiol. 2009;53:1981-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Talavera-Garcia E, Delgado-Lista J, Garcia-Rios A, Delgado-Casado N, Gomez-Luna P, Gomez-Garduño A, Gomez-Delgado F, Alcala-Diaz JF, Yubero-Serrano E, Marin C, Perez-Caballero AI, Fuentes-Jimenez FJ, Camargo A, Rodriguez-Cantalejo F, Tinahones FJ, Ordovas JM, Perez-Jimenez F, Perez-Martinez P, Lopez-Miranda J. Influence of Obesity and Metabolic Disease on Carotid Atherosclerosis in Patients with Coronary Artery Disease (CordioPrev Study). PLoS One. 2016;11:e0153096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Laing ST, Smulevitz B, Vatcheva KP, Rahbar MH, Reininger B, McPherson DD, McCormick JB, Fisher-Hoch SP. Subclinical atherosclerosis and obesity phenotypes among Mexican Americans. J Am Heart Assoc. 2015;4:e001540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 529] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 22. | Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Fatar M, Hernandez Hernandez R, Jaff M, Kownator S, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS, Zannad F, Zureik M. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 914] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 23. | Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 1282] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 24. | Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Desvarieux M, Ebrahim S, Fatar M, Hernandez Hernandez R, Kownator S, Prati P, Rundek T, Taylor A, Bornstein N, Csiba L, Vicaut E, Woo KS, Zannad F; Advisory Board of the 3rd Watching the Risk Symposium 2004, 13th European Stroke Conference. Mannheim intima-media thickness consensus. Cerebrovasc Dis. 2004;18:346-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 453] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 25. | Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126:2020-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2200] [Cited by in RCA: 2477] [Article Influence: 190.5] [Reference Citation Analysis (0)] |
| 26. | Sedlis SP, Hartigan PM, Teo KK, Maron DJ, Spertus JA, Mancini GB, Kostuk W, Chaitman BR, Berman D, Lorin JD, Dada M, Weintraub WS, Boden WE; COURAGE Trial Investigators. Effect of PCI on Long-Term Survival in Patients with Stable Ischemic Heart Disease. N Engl J Med. 2015;373:1937-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 27. | Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3259] [Cited by in RCA: 3208] [Article Influence: 178.2] [Reference Citation Analysis (0)] |
| 28. | Held C, Hjemdahl P, Eriksson SV, Björkander I, Forslund L, Rehnqvist N. Prognostic implications of intima-media thickness and plaques in the carotid and femoral arteries in patients with stable angina pectoris. Eur Heart J. 2001;22:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Petersen C, Peçanha PB, Venneri L, Pasanisi E, Pratali L, Picano E. The impact of carotid plaque presence and morphology on mortality outcome in cardiological patients. Cardiovasc Ultrasound. 2006;4:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Sirimarco G, Amarenco P, Labreuche J, Touboul PJ, Alberts M, Goto S, Rother J, Mas JL, Bhatt DL, Steg PG; REACH Registry Investigators. Carotid atherosclerosis and risk of subsequent coronary event in outpatients with atherothrombosis. Stroke. 2013;44:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Komorovsky R, Desideri A, Coscarelli S, Cortigiani L, Tonello D, Visonà A, Celegon L. Predictive value of associations between carotid and coronary artery disease in patients with acute coronary syndromes. Am J Cardiol. 2005;95:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Zielinski T, Dzielinska Z, Januszewicz A, Rynkun D, Makowiecka Ciesla M, Tyczynski P, Prejbisz A, Demkow M, Kadziela J, Naruszewicz M, Januszewicz M, Juraszynski Z, Korewicki J, Ruzyllo W. Carotid intima-media thickness as a marker of cardiovascular risk in hypertensive patients with coronary artery disease. Am J Hypertens. 2007;20:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Park HW, Kim WH, Kim KH, Yang DJ, Kim JH, Song IG, Kwon TG, Bae JH. Carotid plaque is associated with increased cardiac mortality in patients with coronary artery disease. Int J Cardiol. 2013;166:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Steinvil A, Sadeh B, Bornstein NM, Havakuk O, Greenberg S, Arbel Y, Konigstein M, Finkelstein A, Banai S, Halkin A. Impact of carotid atherosclerosis on the risk of adverse cardiac events in patients with and without coronary disease. Stroke. 2014;45:2311-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7:1025-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 455] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 36. | Spence JD. Technology Insight: ultrasound measurement of carotid plaque--patient management, genetic research, and therapy evaluation. Nat Clin Pract Neurol. 2006;2:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Spence JD, Hegele RA. Noninvasive phenotypes of atherosclerosis: similar windows but different views. Stroke. 2004;35:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Goel K, Thomas RJ, Squires RW, Coutinho T, Trejo-Gutierrez JF, Somers VK, Miles JM, Lopez-Jimenez F. Combined effect of cardiorespiratory fitness and adiposity on mortality in patients with coronary artery disease. Am Heart J. 2011;161:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Fabbian F, Cacici G, Franceschini L, Russo G, Vassanelli C, Catizone L, Lupo A. The relationship between carotid and coronary atherosclerotic damage in dialysis patients. Int J Artif Organs. 2007;30:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 40. | Modi N, Kapoor A, Kumar S, Tewari S, Garg N, Sinha N. Utility of carotid intimal medial thickness as a screening tool for evaluation of coronary artery disease in pre-transplant end stage renal disease. J Postgrad Med. 2006;52:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Keough-Ryan TM, Kiberd BA, Dipchand CS, Cox JL, Rose CL, Thompson KJ, Clase CM. Outcomes of acute coronary syndrome in a large Canadian cohort: impact of chronic renal insufficiency, cardiac interventions, and anemia. Am J Kidney Dis. 2005;46:845-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Li YH, Lin GM, Lin CL, Wang JH, Han CL. Relation of estimated glomerular filtration rate and body mass index to mortality in non-dialysis patients with coronary artery disease: a report from the ET-CHD registry, 1997-2003. J Cardiol. 2013;62:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Barzilai B, Gessler C, Pérez JE, Schaab C, Jaffe AS. Significance of Doppler-detected mitral regurgitation in acute myocardial infarction. Am J Cardiol. 1988;61:220-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Fattouch K, Sampognaro R, Speziale G, Salardino M, Novo G, Caruso M, Novo S, Ruvolo G. Impact of moderate ischemic mitral regurgitation after isolated coronary artery bypass grafting. Ann Thorac Surg. 2010;90:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Ellis SG, Whitlow PL, Raymond RE, Schneider JP. Impact of mitral regurgitation on long-term survival after percutaneous coronary intervention. Am J Cardiol. 2002;89:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Gahl K, Sutton R, Pearson M, Caspari P, Lairet A, McDonald L. Mitral regurgitation in coronary heart disease. Br Heart J. 1977;39:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | O’Driscoll JM, Gargallo-Fernandez P, Araco M, Perez-Lopez M, Sharma R. Baseline mitral regurgitation predicts outcome in patients referred for dobutamine stress echocardiography. Int J Cardiovasc Imaging. 2017;33:1711-1721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |