Published online Nov 26, 2018. doi: 10.4330/wjc.v10.i11.242
Peer-review started: August 9, 2018
First decision: August 24, 2018
Revised: September 8, 2018
Accepted: October 12, 2018
Article in press: October 12, 2018
Published online: November 26, 2018
Processing time: 109 Days and 0.5 Hours
To evaluate the safety and efficacy of surgical left atrial appendage occlusion (s-LAAO) during concomitant cardiac surgery.
We performed a comprehensive literature search through May 31st 2018 for all eligible studies comparing s-LAAO vs no occlusion in patients undergoing cardiac surgery. Clinical outcomes during follow-up included: embolic events, stroke, all-cause mortality, atrial fibrillation (AF), reoperation for bleeding and postoperative complications. We further stratified the analysis based on propensity matched studies and AF predominance.
Twelve studies (n = 40107) met the inclusion criteria. s-LAAO was associated with lower risk of embolic events (OR: 0.63, 95%CI: 0.53-0.76; P < 0.001) and stroke (OR: 0.68, 95%CI: 0.57-0.82; P < 0.0001). Stratified analysis demonstrated this association was more prominent in the AF predominant strata. There was no significant difference in the incidence risk of all-cause mortality, AF, and reoperation for bleeding and postoperative complications.
Concomitant s-LAAO during cardiac surgery was associated with lower risk of follow-up thromboembolic events and stroke, especially in those with AF without significant increase in adverse events. Further randomized trials to evaluate long-term benefits of s-LAAO are warranted.
Core tip: Surgical left atrial appendage occlusion (s-LAAO) is performed during cardiac surgeries in patients with atrial fibrillation. However, evidence to perform routinely during cardiac surgeries is conflicting and contrasting. It is currently given a class IIb recommendation in the professional medical society guidelines. We sought to perform a meta-analysis of all the studies published to date to evaluate the safety and efficacy of s-LAAO.
- Citation: Atti V, Anantha-Narayanan M, Turagam MK, Koerber S, Rao S, Viles-Gonzalez JF, Suri RM, Velagapudi P, Lakkireddy D, Benditt DG. Surgical left atrial appendage occlusion during cardiac surgery: A systematic review and meta-analysis. World J Cardiol 2018; 10(11): 242-249
- URL: https://www.wjgnet.com/1949-8462/full/v10/i11/242.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i11.242
The left atrial appendage (LAA) is considered to be the dominant source of embolism (> 90%) in patients with non-valvular atrial fibrillation (AF)[1]. Occlusion or resection of the left atrial appendage occlusion (LAAO) remains an important intervention for prevention of recurrent emboli in patients who are at risk of stroke. LAAO provides an opportunity to avoid systemic anticoagulation, thereby minimizing the risk of bleeding.
Surgical LAAO (s-LAAO) usually involves LAA closure while performing other cardiac surgeries. With the increasing prevalence of AF[2], there is a growing interest in the surgical community for s-LAAO. Prior studies assessing the clinical impact of surgical occlusion of the LAA during cardiac surgery have shown contradictory results[3-14]. Furthermore, there are no large scale randomized controlled trials evaluating routine s-LAAO during cardiac surgery. Therefore s-LAAO remains a class IIb recommendation in professional medical society guidelines[15,16]. Despite this recommendation, s-LAAO is routinely performed in patients with AF undergoing cardiac surgery. Therefore, we sought to perform a meta-analysis of the available studies published to date to evaluate the safety and efficacy of concomitant s-LAAO vs no occlusion during cardiac surgery[3,4,6-14].
The systematic review and meta-analysis was done in compliance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines[17]. The PRISMA checklist is presented in Supplementary Table 1. The initial search strategy was developed by two authors (V.A and M.A.N). We performed a systematic search, without language restriction, using PubMed, EMBASE, SCOPUS, Google Scholar, and ClinicalTrials.gov from inception to May 31st, 2018 for studies comparing s-LAAO vs no occlusion- only in patients undergoing concomitant cardiac surgery. We used the following keywords and medical subject heading: “Cardiac surgeries” OR “Heart surgeries” OR “Cardiac surgical procedures” AND “Left atrial appendage” OR “occlusion” OR “ligation” OR “resection” OR “excision” OR “amputation”.
| Study, yr | Country | Study period | Study design | Sample size | Cardiac surgery type | Follow up period (mo) | |
| s-LAAO | No occlusion | ||||||
| García-Fernández et al, 2003[3] | Spain | 2003 | retrospective | 58 | 147 | MVS | 69.4 ± 67 |
| Healey et al, 2005[7] | Germany | 2001-2002 | RCT | 52 | 25 | CABG | 13 ± 7 |
| Nagpal et al, 2009[8] | Canada | 2007-2007 | RCT | 22 | 21 | MVS | <1 |
| Whitlock et al, 2013[9] | Canada | 2009-2010 | RCT | 26 | 25 | CABG and VS | 1 |
| Zapolanski et al, 2013[4] | United States | 2005-2012 | retrospective | 808 | 969 | CABG and VS | NR |
| Kim et al, 2013[6] | United States | 2001-2010 | retrospective | 631 | 631 | CABG and MVS | 1 |
| Lee et al, 20141[5] | Korea | 1999-2011 | retrospective | 119 | 119 | MVS with AF ablation | 63 ± 44 |
| Melduni et al, 20171[10] | United States | 2000-2005 | prospective | 461 | 461 | CABG and VS | 109.2 |
| Elbadawi et al, 20172[11] | United States | 1998-2013 | retrospective | 652 | 652 | VS | In-hospital |
| Elbadawi et al, 2017[12] | United States | 2004-2013 | retrospective | 2519 | 12595 | CABG | In-hospital |
| Friedman et al, 2018[14] | United States | 2011-2012 | retrospective | 3892 | 6632 | CABG, MVS, AVS | 31.2 |
| Yao et al, 20181[13] | United States | 2009-2017 | retrospective | 4295 | 4295 | CABG, VS | 25.2 ± 22.8 |
Only studies comparing s-LAAO vs no occlusion during any cardiac surgery were included in our analysis. The reference lists of original studies, conference abstracts and relevant review articles were further reviewed. Two investigators (V.A and M.A.N) independently performed the literature search, reviewed the originally identified titles and abstracts and selected studies for pooled analysis based on the inclusion criteria. Any divergence was resolved through discussion with a third independent reviewer (M.K.T). The quality of observational studies was assessed using the Newcastle Ottawa scale, Supplementary Table 2.
| Study | Age (mean ± SD) | Hypertension | AF (%) | Technique of s-LAAO | |||
| s-LAAO | No occlusion | s-LAAO | No occlusion | s-LAAO | No occlusion | ||
| García-Fernández et al, 2003[3] | 63 ± 12 | 62 ± 10 | NR | NR | NR | Double suturing | |
| Healey et al, 2005[7] | 72 ± 6 | 71 ± 5 | 75 | 92 | 17 | 8 | Suture or stapler |
| Nagpal et al, 2009[8] | 57.8 ± 13.3 | 59.2 ± 11.9 | NR | NR | 18 | 29 | Resection |
| Whitlock et al, 2013[9] | 77.4 ± 6.8 | 74.6 ± 7.6 | 92.3 | 92 | 100 | 100 | Amputation and closure or stapler |
| Zapolanski et al, 2013[4] | 70.52 ± 11.83 | 83.9 | 80.6 | 19.9 | 10.7 | Double ligation | |
| Kim et al, 2013[6] | 66.6 ± 11.4 | 65.8 ± 11.6 | 80.9 | 73.1 | NR | NR | Ligation and excision |
| Lee et al, 2014[5] | 55.9 ± 12.2 | 50.7 ± 12.4 | 19.8 | 14.5 | 100 | 100 | Amputation |
| Melduni et al, 2017[10] | 67.4 ± 12.7 | 67.6 ± 13.5 | 59 | 61 | 47 | 45 | Amputation, suturing or stapler |
| Elbadawi et al, 2017[11] | 70.8 ± 10.2 | 71.2 ± 11.1 | 70.6 | 52.8 | 100 | 100 | NR |
| Elbadawi et al, 2017[12] | 71.3 ± 9 | 70.6 ± 8.7 | 78.5 | 76.1 | 100 | 100 | NR |
| Friedman et al, 2018[14] | 75 ± 5.9 | 76.4 ± 6.4 | 14.5 | 12.7 | 50.5 | 43.4 | Any technique |
| Yao et al, 2018[13] | 68.2 ± 10.6 | 65.8 ± 11.3 | 88.6 | 90.4 | 75.4 | 31.4 | |
We evaluated the following clinical outcomes during follow-up in each report: (1) embolic events; (2) stroke; (3) all-cause mortality; (4) AF; (5) postoperative complications; and (6) reoperation for bleeding. We further performed stratified meta-analysis to evaluate the potential source of heterogeneity across the included studies. Stratification factors are inclusion of only propensity matched studies and studies with AF predominant cohort (> 50% of study population having AF). The ischemic events attributed to embolic causes in the included studies were included in the embolic events. Complications included in the analysis are appendage tears, myocardial infarction, major bleeding, septicemia, pacemaker implants, renal failure, pericardial effusion, cardiac tamponade, and stroke.
The meta-analysis was done using Review Manager (RevMan), Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014. Due to methodological and clinical heterogeneity between the included studies, a random-effects model estimating the odds ratio (OR) and the estimated 95% confidence interval (CI) of the above-mentioned outcomes were used. The OR estimate of each study was calculated by the random-effects model obtained by the DerSimonian-Lariad method[18].
Heterogeneity was assessed using Higgins’ and Thompson’s I2 statistic, with I2 values of > 50% was considered significant. Publication bias was visually estimated by funnel plots. A 2-tailed P < 0.05 was considered statistically significant for all analyses.
A total of 1328 reports were retrieved during the initial search (Supplementary figure 1). 1049 reports were selected after removing 279 duplicates. 387 reports were screened and 354 were excluded. 33 reports were assessed for eligibility. Finally, after excluding 21 reports (no comparison groups-14, others-7) 12 studies were included. Among these 12 studies, three were randomized controlled trials (RCTs) and nine were observational studies. Among these nine observational studies, four were propensity matching studies[5,6,10,13]. one was case matching study[12]. The inter-reviewer agreement on study eligibility was 100%.
The characteristics of the included studies are presented in Table 1 and Table 2. Out of 40107 patients included, 13535 patients received s-LAAO during cardiac surgery while the remaining 26572 patients did not receive s-LAAO. The mean (SD) age of the study population ranged from 50.7 (12.4) years to 77.4 (6.8) years. The primary cardiac operation varied widely. The surgical procedures were primarily valve surgery in the studies by Garcia-Fernandez, Nagpal, Lee and Elbadawi[3,5,8,12], while they were primarily coronary artery bypass grafting (CABG) in the studies by Healey, and Elbadawi[7,11]. Remaining studies included a combination of valve surgery and CABG. Lee et al[5] also performed ablation of AF together with mitral valve surgery. The prevalence of AF varied in the study cohorts. The s-LAAO techniques varied; the methods variously included double suturing, exclusion, amputation, resection and stapling (Table 2). The follow-up period ranged from in-hospital only to 109.2 mo.
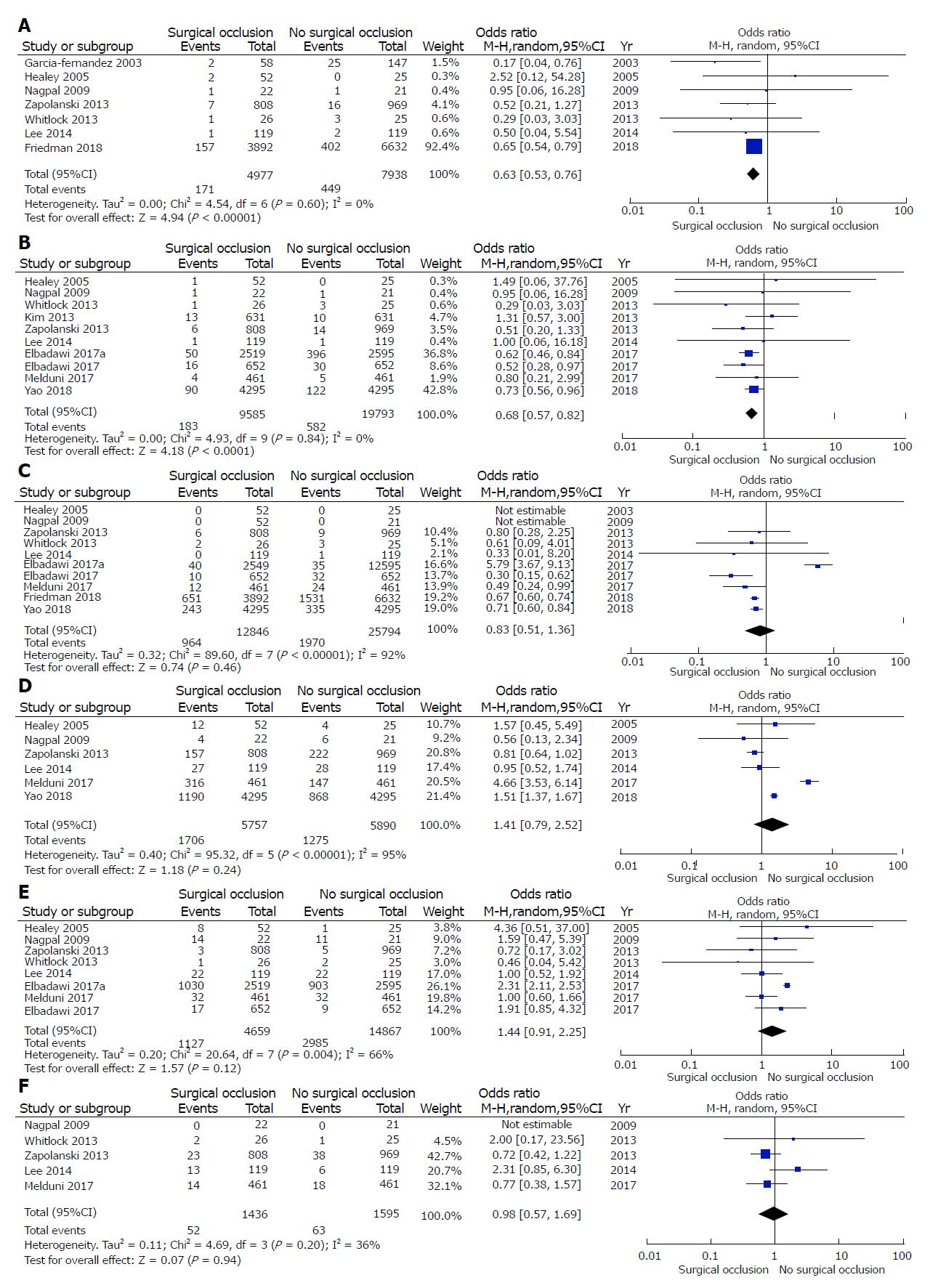
s-LAAO was associated with lower risk of embolic events (OR: 0.63, 95%CI: 0.53-0.76; P < 0.001) and a lower risk of stroke (OR: 0.68, 95%CI: 0.57-0.82; P < 0.0001) (Figure 1A and 1B). There was no significant difference in all-cause mortality between the two groups (OR: 0.83, 95%CI: 0.51-1.36; P = 0.46) (Figure 1C). There was no significant difference in the incidence of follow-up AF between the two groups (OR: 1.41, 95%CI: 0.79-2.52, P = 0.24) (Figure 1D).
With regard to postoperative complications, there was no significant difference between the groups (OR: 1.44, 95%CI: 0.91-2.25; P = 0.12) (Figure 1F). Similarly, there was no significant difference in the incidence of reoperation for bleeding between the two groups (OR: 0.98, 95%CI: 0.57-1.69; P = 0.94) (Figure 1G).
Test of heterogeneity was not significant for follow-up embolic events (P heterogeneity = 0.60, I2 = 0%) and stroke (P = 0.84, I2 = 0%), while it was significant for all-cause mortality (P < 0.001, I2 = 92%), AF (P < 0.001, I2 = 95%), postoperative complications (P = 0.004, I2 = 66%) and reoperation for bleeding (P = 0.20, I2 = 36%).
In subgroup analysis including only propensity matched studies, s-LAAO group had a trend towards lower risk of stroke (OR: 0.78, 95%CI: 0.60-1.00; P = 0.05), Supplementary Figure 2A. Test of heterogeneity was not significant (P = 0.63, I2 = 0%). There was no significant difference in the incidence of all-cause mortality (OR: 1.10, 95%CI: 0.34-3.60; P = 0.87), Supplementary Figure 2B. In subgroup analysis including only AF predominant studies (> 50%), s-LAAO was associated with lower risk of stroke (OR: 0.60, 95%CI: 0.46-0.78; P = 0.0002) (Supplementary Figure 3A). There was no significant difference in all-cause mortality (OR: 0.87, 95%CI: 0.11-7.12; P = 0.89) (Supplementary Figure 3B). Test of heterogeneity was not significant for stroke (P = 0.86, I2 = 0%) while it was significant for all-cause mortality (P < 0.001, I2 = 94%).
Funnel plot for visual inspection of publication bias is shown in Supplementary Figure 4.
The main findings of our meta-analysis of patients undergoing s-LAAO during concomitant cardiac surgery are the following: (1) s-LAAO was associated with lower rates of embolic events and stroke; and (2) there was no significant difference in the incidence of all-cause mortality, postoperative complications or reoperations for bleeding between the two groups. The reduced risk of embolic events and stroke with s-LAAO was retained in the subgroup analysis including only studies with AF predominant population (Table 3).
| Study | Total complications | Type of complications | |
| s-LAAO (%) vs No occlusion (%) | s-LAAO | No occlusion | |
| Healey et al, 2005[7] | 8 (52) vs 1 (4) | 8- intraoperative LAA tears | 1- LAA tear |
| Nagpal et al, 2009[8] | 14 (63.6) vs 11 (52.3) | 1- septicemia 1- myocardial infarction 2- RBC transfusion 8- temporary pacemaker 2- permanent pacemaker | 1- RBC transfusion 7- temporary pacemaker 3- permanent pacemaker |
| Whitlock et al, 2013[9] | 1 (3.8) vs 2 (25) | 1- major bleeding | 2- major bleeding |
| Zapolonski et al, 2013[4] | 3 (0.3) vs 5 (0.6) | 3- myocardial infarction | 5- myocardial infarction |
| Lee et al, 2014[5] | 22 (18.4) vs 22 (18.4) | 9- requirement of dialysis 4- permanent pacemaker insertion 1- wound revision 8- pericardial effusion | 1- low cardiac output syndrome 10- dialysis 2- permanent pacemaker insertion 1- mediastinitis 2- wound revision 6- pericardial effusion |
| Melduni et al, 2017[10] | 32 (6.9) vs 32 (6.9) | 14- pneumonia 18- acute renal failure | 14- pneumonia 18- acute renal failure |
| Elbadawi et al, 2017[11] | 17 (3.1) vs 9 (1.6) | 17- pericardial effusion | 7- pericardial effusion 2- hemorrhage |
| Elbadawi et al, 2017[12] | 1030 (40.8) vs 2903 (23) | 16- cardiac tamponade 68- pericardial effusion 917- hemorrhage 29- postoperative shock | 19- cardiac tamponade 151- pericardial effusion 2687- hemorrhage 46- postoperative shock |
The estimated global prevalence of AF is on the rise due to a demographic shift with more prevalent ageing population carrying a higher burden of comorbidities[19]. About 25% of the strokes in the United States are related to AF and about 90% of the strokes in non-valvular AF are caused by thrombi originating in LAA[20]. Anticoagulants, both warfarin and direct acting oral anticoagulants (DOACs) reduce the incidence of stroke by more than 60%[21,22] but they are associated with increasing risk of bleeding, and significant drug-drug interactions[16]. The benefits of anticoagulants are also limited by other issues including underutilization, poor compliance and cost[16].
The higher risk of stroke in the ageing population with AF has led to the increased adoption of LAA occlusion in clinical practice[23]. The two largest RCTs - PROTECT-AF (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) and PREVAIL (Watchman LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy) showed percutaneous LAAO being non-inferior to warfarin with respect to stroke rates and embolic events[24,25]. Following the success with percutaneous LAAO, there has been a resurgence of interest in s-LAAO within the surgical community, especially with increase in the aging population and rising prevalence of AF[6,10,14].
Our findings show that s-LAAO was associated with lower risk of follow-up embolic events and stroke. The association of lower risk of stroke was more prominent in subgroup with AF predominant population. S-LAAO theoretically prevents formation of thrombus in LAA. However, successful s-LAAO is largely influenced by LAA morphology, occlusion technique and also operator skill. A previous study showed that a complete LAA occlusion was achieved in only 40%-50% of the patient population[10,26]. The techniques of s-LAAO varied widely amongst the included studies as summarized in Table 2. The excision technique to exclude LAA has been shown to have a higher success rate than the other modalities of s-LAAO[24]. Currently, concomitant LAA closure is given a Class IIb (level of evidence B) by the European Society of Cardiology (ESC)/European Society for Cardio-Thoracic Surgery (EACTS) guidelines and a Class IIb (level of evidence C) by the 2017 Society of Thoracic Surgeons guidelines (STS)[16]. Therefore, there is a wide practice level variation in the utilization of s-LAAO during cardiac surgery. The number of studies with a particular technique is inadequate to perform individual technique based meta-analysis so we combined all different techniques of s-LAAO in our meta-analysis. It should be noted that none of the other studies except the study from Friedman et al[14] reported long-term benefits. However, Friedman et al[14] showed a remarkable reduction in postoperative embolism at follow up. Further studies with long-term follow up of embolic events are essential. Our results are similar to a previous meta-analysis comparing s-LAAO vs no occlusion[27,28]. However, we included additional studies by Friedman et al[14], Elbadawi et al[11] and Yao et al[13] yielding a larger sample size. In addition, we performed a subgroup analysis of the included studies to identify the patient population that is most likely to benefit from this procedure.
In the current study, we found no significant difference in the risk of postoperative complications and reoperation for bleeding. s-LAAO is associated with inherent risk of procedural complications including LAA tears as observed in the study by Healey et al[7] and so learning curve plays an essential role in success of the procedure. Hypothetically, avoidance of aggressive anticoagulation after s-LAAO might have contributed to some of the benefits observed with s-LAAO. However, only few studies reported the long-term details of anticoagulation. Lee et al[5] reported no difference in the utilization of anticoagulation between the two groups (62.2% vs 55.4%). In the study by Friedman et al[14], anticoagulation was prescribed to 68.9% of the patients in the s-LAAO group compared to only 60.3% in the group without s-LAAO. In contrast to percutaneous LAAO, evidence regarding the utilization of anticoagulation after s-LAAO is not clear. The 2016 ESC/EACTS guidelines still recommend therapeutic anti-coagulation in all patients despite s-LAAO (Class I, level of evidence B)[15]. With lack of long term data, there is need for prospective trials to address this issue. The ongoing LAAOS-III (left atrial appendage occlusion study III) and the ATLAS (AtriClip® Left Atrial Appendage Exclusion Concomitant to Structural Heart Procedures) trials should be able to provide further insights into the benefits of s-LAAO.
Our study should be viewed in the context of following limitations. First, due to the small number of studies with small sample sizes, except the study by Friedman et al[14], the results might be underpowered to detect the true clinical benefits of certain clinical outcomes. Second, there was a wide variation of surgical techniques of LAAO, so we were not able to address the effect of individual techniques. Third, only Friedman et al[14] reported long-term embolic events, whereas the other studies did not report long term outcomes. The study by Friedman et al[14] reported readmissions for embolic events, so some of the events which did not require hospitalization were not included. The effect of anticoagulation on postoperative outcomes remains unclear due to inadequate reporting in the included studies. Fourth, it is unclear if s-LAAO increases the duration of the surgical procedure as it was only reported in two studies. Fifth, the burden of AF varied among the included studies, thus carrying risk of a selection bias. Finally, publication bias is an inherent limitation of any meta-analysis.
In conclusion, our results support the safety of s-LAAO and favor its continued use in conjunction with concomitant cardiac surgery, especially in patients with AF. Randomized controlled trials are essential to evaluate the long-term benefits of s-LAAO.
The left atrial appendage (LAA) is a common site for intracardiac thrombus formation in patients with atrial fibrillation (AF). Surgical left atrial appendage occlusion (s-LAAO) during concomitant cardiac surgery has been evaluated as an effective treatment approach to reduce the risk of stroke and embolic events.
Percutaneous LAAO has been shown to be non-inferior compared with warfarin in reducing the risk of stroke and embolic events in two large randomized controlled trials, PROTECT-AF and PREVAIL. However, data regarding s-LAAO is conflicting and contrasting. So, we performed a systematic review and meta-analysis of all the studies published to date to evaluate if concomitant s-LAAO during cardiac surgery is safe and effective.
The purpose of this study is to evaluate the safety and efficacy of concomitant s-LAAO during cardiac surgery.
We searched five databases for studies comparing concomitant s-LAAO with no occlusion during cardiac surgery. We obtained a total of 12 studies for inclusion and performed a meta-analysis. The outcomes of interest were embolic events, stroke, all-cause mortality, AF, postoperative complications and reoperation for bleeding.
Concomitant s-LAAO during cardiac surgery was associated with lower risk of embolic events and stroke. This was evident in the AF predominant strata as well. There was no significant difference in the risk of all-cause mortality, AF, postoperative complications and reoperation for bleeding.
Our meta-analysis including all the studies published to date comparing concomitant s-LAAO against no occlusion during cardiac surgery supports the use of concomitant s-LAAO during cardiac surgeries. It was associated with lower risk of stroke and embolic events.
From this meta-analysis, it could be seen that concomitant s-LAAO during cardiac surgeries was associated with lower risk of stroke and embolic events compared with no occlusion. This association was prominent amongst the AF predominant strata as well. These beneficial effects could be seen due to the occlusion of LAA which is the source of 90% thrombi in non-valvular AF. Future randomized trials are needed to evaluate the long term benefits of s-LAAO.
Manuscript source: Invited Manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Vermeersch P, Said SAM S- Editor: Dou Y L- Editor: A E- Editor: Wu YXJ
| 1. | Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 460] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 2. | Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129:2371-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 348] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 3. | García-Fernández MA, Pérez-David E, Quiles J, Peralta J, García-Rojas I, Bermejo J, Moreno M, Silva J. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol. 2003;42:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 255] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 4. | Zapolanski A, Johnson CK, Dardashti O, O'Keefe RM, Rioux N, Ferrari G, Shaw RE, Brizzio ME, Grau JB. Epicardial surgical ligation of the left atrial appendage is safe, reproducible, and effective by transesophageal echocardiographic follow-up. Innovations (Phila). 2013;8:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Lee CH, Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Left atrial appendage resection versus preservation during the surgical ablation of atrial fibrillation. Ann Thorac Surg. 2014;97:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Kim R, Baumgartner N, Clements J. Routine left atrial appendage ligation during cardiac surgery may prevent postoperative atrial fibrillation-related cerebrovascular accident. J Thorac Cardiovasc Surg. 2013;145:582-9; discussion 589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Healey JS, Crystal E, Lamy A, Teoh K, Semelhago L, Hohnloser SH, Cybulsky I, Abouzahr L, Sawchuck C, Carroll S. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005;150:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 367] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 8. | Nagpal AD, Torracca L, Fumero A, Denti P, Cioni M, Alfieri O. Concurrent prophylactic left atrial appendage exclusion: results from a randomized controlled trial pilot study. Eur J Cardiothorac Surg. 2009;36:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Whitlock RP, Vincent J, Blackall MH, Hirsh J, Fremes S, Novick R, Devereaux PJ, Teoh K, Lamy A, Connolly SJ. Left Atrial Appendage Occlusion Study II (LAAOS II). Can J Cardiol. 2013;29:1443-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Melduni RM, Schaff HV, Lee HC, Gersh BJ, Noseworthy PA, Bailey KR, Ammash NM, Cha SS, Fatema K, Wysokinski WE. Impact of Left Atrial Appendage Closure During Cardiac Surgery on the Occurrence of Early Postoperative Atrial Fibrillation, Stroke, and Mortality: A Propensity Score-Matched Analysis of 10 633 Patients. Circulation. 2017;135:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Elbadawi A, Ogunbayo GO, Elgendy IY, Olorunfemi O, Saad M, Ha LD, Alotaki E, Baig B, Abuzaid AS, Shahin HI. Impact of Left Atrial Appendage Exclusion on Cardiovascular Outcomes in Patients With Atrial Fibrillation Undergoing Coronary Artery Bypass Grafting (From the National Inpatient Sample Database). Am J Cardiol. 2017;120:953-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Elbadawi A, Olorunfemi O, Ogunbayo GO, Saad M, Elgendy IY, Arif Z, Badran H, Saheed D, Ahmed HMA, Rao M. Cardiovascular Outcomes With Surgical Left Atrial Appendage Exclusion in Patients With Atrial Fibrillation Who Underwent Valvular Heart Surgery (from the National Inpatient Sample Database). Am J Cardiol. 2017;119:2056-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Yao X, Gersh BJ, Holmes DR Jr, Melduni RM, Johnsrud DO, Sangaralingham LR, Shah ND, Noseworthy PA. Association of Surgical Left Atrial Appendage Occlusion With Subsequent Stroke and Mortality Among Patients Undergoing Cardiac Surgery. JAMA. 2018;319:2116-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 14. | Friedman DJ, Piccini JP, Wang T, Zheng J, Malaisrie SC, Holmes DR, Suri RM, Mack MJ, Badhwar V, Jacobs JP. Association Between Left Atrial Appendage Occlusion and Readmission for Thromboembolism Among Patients With Atrial Fibrillation Undergoing Concomitant Cardiac Surgery. JAMA. 2018;319:365-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 15. | Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1278] [Cited by in RCA: 1351] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 16. | January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2373] [Cited by in RCA: 2925] [Article Influence: 265.9] [Reference Citation Analysis (0)] |
| 17. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. [PubMed] |
| 18. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30337] [Article Influence: 777.9] [Reference Citation Analysis (0)] |
| 19. | Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2658] [Cited by in RCA: 3329] [Article Influence: 277.4] [Reference Citation Analysis (0)] |
| 20. | Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67-e492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4163] [Cited by in RCA: 4800] [Article Influence: 685.7] [Reference Citation Analysis (1)] |
| 21. | Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3260] [Cited by in RCA: 3422] [Article Influence: 190.1] [Reference Citation Analysis (0)] |
| 22. | Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1201] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 23. | Passman R, Bernstein RA. New Appraisal of Atrial Fibrillation Burden and Stroke Prevention. Stroke. 2016;47:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1498] [Cited by in RCA: 1613] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 25. | Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1357] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 26. | Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol. 2008;52:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 411] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 27. | Ando M, Funamoto M, Cameron DE, Sundt TM 3rd. Concomitant surgical closure of left atrial appendage: A systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2018;156:1071-1080.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Tsai YC, Phan K, Munkholm-Larsen S, Tian DH, La Meir M, Yan TD. Surgical left atrial appendage occlusion during cardiac surgery for patients with atrial fibrillation: a meta-analysis. Eur J Cardiothorac Surg. 2015;47:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |