Copyright
©The Author(s) 2018.
World J Cardiol. Sep 26, 2018; 10(9): 74-86
Published online Sep 26, 2018. doi: 10.4330/wjc.v10.i9.74
Published online Sep 26, 2018. doi: 10.4330/wjc.v10.i9.74
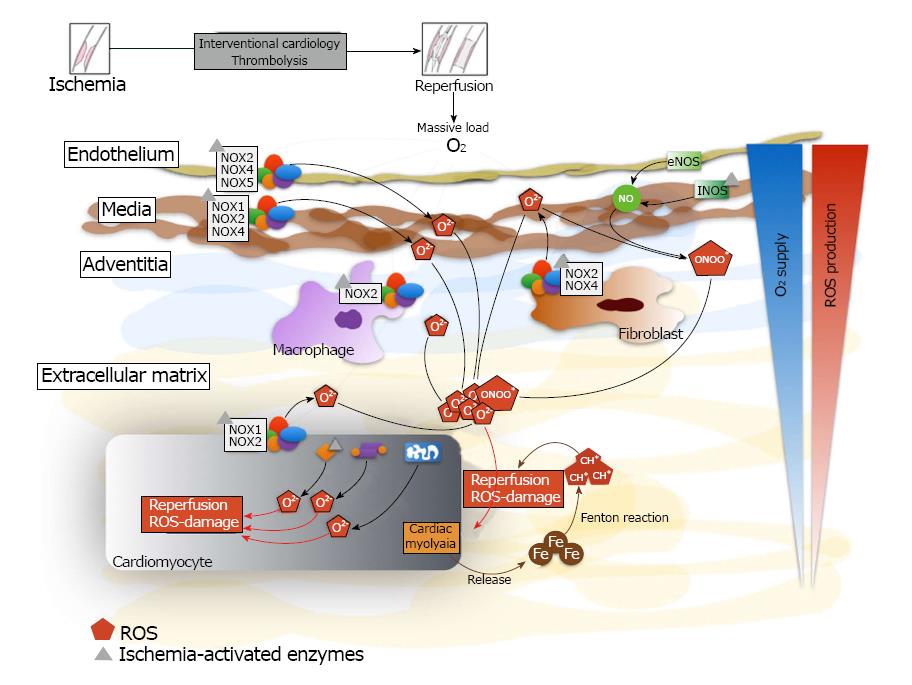
Figure 1 Generation of reactive oxygen species and mobilization of iron after myocardial reperfusion.
There is a massive production of reactive oxygen species and iron mobilization by the different cellular types of the myocardial tissue. The iron reacts with superoxide anion to produce hydroxyl radical by the Fenton reaction. Inside cardiomyocyte, there is intracellular production of reactive oxygen species through NADPH oxidase, eNOS uncoupled, xanthine oxidase and mitochondrion. NOX: NADPH oxidase; ROS: Reactive oxygen species; Fe: Iron; eNOS: Endothelial nieric oxide synthases.
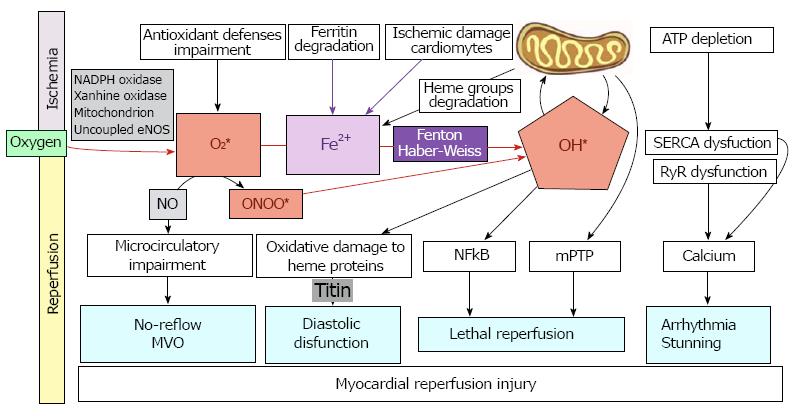
Figure 2 Role of reactive oxygen species and iron mobilization in myocardial reperfusion injury and its clinical implications.
MVO: Microvascular obstruction; ONOO*: Peroxynitrite; NO: Nitric oxide; OH *: Radical hydroxyl; Fe: Iron; RyR: Ryanodine receptor channel; SERCA: Sarco/endoplasmic reticulum Ca2+-ATPase.
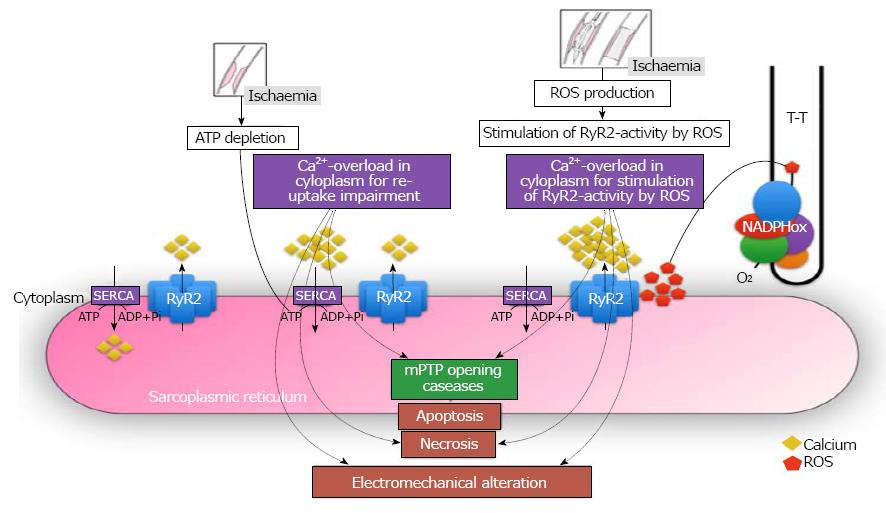
Figure 3 Central role of calcium in the electro-mechanical dissociation of cardiomyocyte after myocardial reperfusion.
RyR: Ryanodine receptor channel; SERCA: Sarco / endoplasmic reticulum Ca2+-ATPase; mPTP: Mitochondrial permeability transition pore; Ca: Calcium; ROS: Reactive oxygen species.
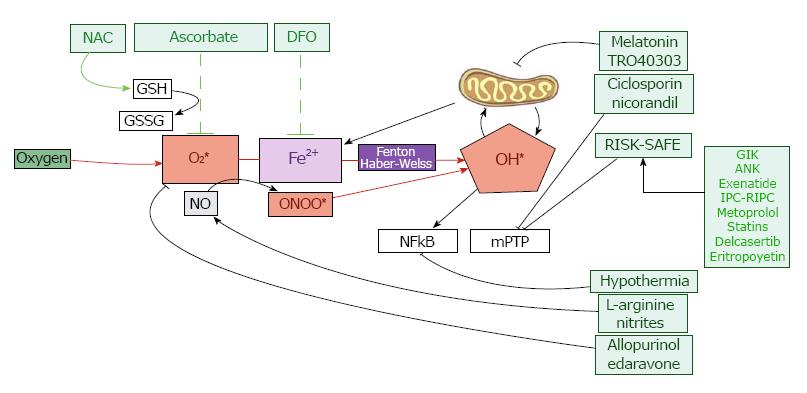
Figure 4 Experimental, pharmacological and clinical approaches to prevent myocardial reperfusion injury at cellular level.
RISK: Reperfusion injury salvage kinase pathway; SAFE: Survivor activating factor enhancement pathway; GSH: Reduced glutathione; GSSG: Oxidized glutathione; NAC: N-acetylcysteine; DFO: deferoxamine; ONOO*: Peroxynitrite; NO: Nitric oxide; OH *: Radical hydroxyl; mPTP: Mitochondrial permeability transition pore.
- Citation: González-Montero J, Brito R, Gajardo AI, Rodrigo R. Myocardial reperfusion injury and oxidative stress: Therapeutic opportunities. World J Cardiol 2018; 10(9): 74-86
- URL: https://www.wjgnet.com/1949-8462/full/v10/i9/74.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i9.74