INTRODUCTION
Many tumors contain phenotypically and functionally heterogeneous cancer cells. Among them, a minority of cell subpopulation, termed cancer stem cell (CSC), which possesses the self-renewal capacity and is able to generate the heterogeneous lineage of cancer cells, has been believed to be responsible for the initiation and maintenance of the tumors. Besides their self-renewal and differentiation properties shared with stem cells, CSCs are characterized with high tumorigenic potential and are also referred to as tumor-initiating cells[1]. Recent data suggest that CSCs are more resistant to chemotherapy and radiotherapy than non-stem cells[2]. Relatively successful cancer treatments shrink the bulk of tumor but often fail to eliminate the CSCs, resulting in the recurrence of tumors. Combined with their high tumorigenic potential, CSCs are believed to be responsible for tumor therapy resistance, tumor relapse, and metastasis.
Many chemotherapeutic drugs, such as platinum-based agents as well as radiotherapy, kill cancer cells by inducing DNA damage. Therefore, cells that efficiently repair DNA damage can potentially survive therapeutic agents. Indeed, prompt activation of DNA damage responses (DDR) and enhanced DNA repair capacity have been implicated as contributors to increased resistance to therapy in the CSC population in gliomas and breast cancer[3,4].
DDR
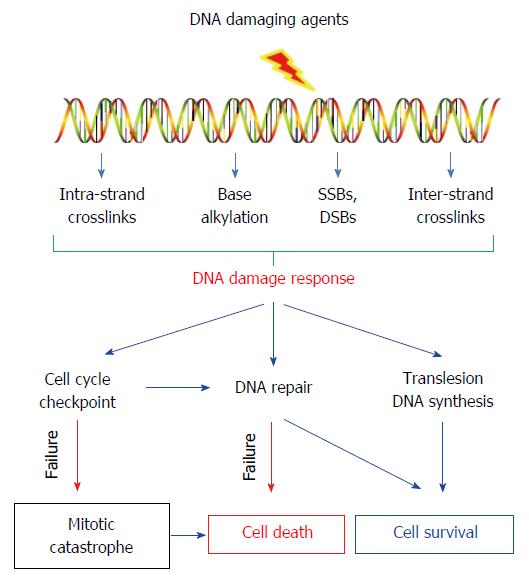
Cells incur a large number of DNA lesions from endogenous processes. Many lesions can block transcription, DNA replication, and chromosome segregation, resulting in cell death or gene mutations. Fortunately, cells have evolved an intricate machinery to deal with these lesions. This machinery, known as the DDR, can recognize these DNA lesions and repair them. Meanwhile, cells with genome injury can be arrested at specific checkpoints to allow repair of lesions before they are converted into permanent mutations. When damage is too significant, a cell may undergo apoptosis to avoid the passing of damage to their progeny. Although DDR is critical to the maintenance of genome stability, highly efficient DNA repair machinery can reduce the efficacy of DNA damaging agents in treating cancers. DNA damaging agents used for cancer therapy can induce various DNA lesions, such as covalent crosslinks between DNA bases (cisplatin, carboplatin, oxaliplatin and mitomycin C), base alkylation (methyl methanesulphonate and temozolomide), DNA single-strand breaks (SSB) and double-strand breaks (DSB) (camptothecin and etoposide). The DDR pathways in cancer cells can greatly affect the consequences of cancer treatment with DNA-damaging agents (Figure 1).
Figure 1 DNA damage responses and consequences in cancer treatment with DNA-damaging agents.
DNA-damaging agent-induced DNA lesions can (1) activate cell cycle checkpoint to arrest the cell cycle progression to allow time for repair before the damage is passed on to daughter cells; (2) activate specific DNA repair pathways to remove the lesions; (3) or enables continuous functioning of replication by bypassing the DNA lesions through translesion DNA synthesis. Thus, highly efficient DDR can promote the survival of cancer cells upon DNA-damaging agent treatment.
Repair of intra-strand crosslinks
Cisplatin induces primarily 1, 2-intra-strand crosslinks between adjoining purines in DNA, e.g., cis-Pt(NH3)2d(GpG) (Pt-GG), with Pt bound to two adjacent guanines, and cis-Pt(NH3)2d(ApG) (Pt-AG), with Pt bound to an adenine and an adjacent guanine. These damages contribute 90% of the total damage introduced by cisplatin[5-8]. The intra-strand crosslinks induced by platinum-based therapeutic agents are mainly processed by nucleotide excision repair (NER), which is carried out by a minimal set of proteins including XPA, XPC-RAD23B, XPG, RPA, ERCC1-XPF, TFIIH, PCNA, DNA polymerase δ or ε, and DNA ligase I, in human cells[9,10]. These proteins are sequentially assembled at the site of DNA lesions in chromatin[11-13], where they carry out the dual incision of the damage-containing DNA. Approximately 24-32 nucleotides are then removed, and a new fragment is synthesized to restore the DNA structure[14-16].
Repair of base alkylation and SSB
Many alkylating agents such as cyclophosphamide and temozolomide induce DNA damage by attaching an alkyl group to adenine or guanine, leading to replication fork stalling and subsequent apoptosis[17]. These DNA lesions are mainly repaired by base excision repair (BER). In BER, the alkylated bases are first excised from the DNA strand by the lesion specific DNA repair enzymes known as glycosylases. The resulting abasic sites or apyrimidinic/apurinic (AP) sites are further recognized and excised by an AP endonuclease, APE1. The resulted SSBs can be bound by poly(ADP-ribose) polymerase 1 (PARP1), which synthesizes PAR polymers attached to itself and other repair factors[18,19]. Afterwards, BER proteins such as DNA polymerase β and XRCC1-DNA ligase α are recruited to sites of DNA damage for DNA synthesis and gap filling. If the AP site is not repaired, the SSB will persist at the site of damage, cause replication fork collapse during the S-phase, and subsequently is converted to DSB.
Repair of DSBs
DSBs arise from ionizing radiation, free radicals, topoisomerase inhibitors, and replication of a SSB. Homologous recombination (HR) and nonhomologous end joining (NHEJ) are the two major DSB repair pathways that have been studied extensively. In NHEJ, the DSB ends are simply reconnected without the use of a repair template. This process requires Ku70, Ku80, DNA-dependent protein kinases (DNA-PKs), XRCC4, and DNA ligase IV[20]. The Ku70/Ku80 heterodimer first binds to DNA ends and recruits DNA-PKs, which function to keep the broken DNA ends in close proximity and to recruit end-processing factors, such as Artemis, polynucleotide kinase/phosphatase (PNKP), APE1, and tyrosyl-DNA phosphodiesterase 1 (TDP1), to prepare the DNA ends for ligation by XRCC4-DNA ligase IV[21].
NHEJ is active throughout the cell cycle. In contrast, HR-directed DSB repair is confined to the late S and G2 phases of proliferating cells when sister chromatids are available as repair templates. HR repair is initiated by the generation of 3’ single-stranded DNA (ssDNA) by resection of the 5’ end, and the binding of replication protein A (RPA). This initial processing of the DNA ends involves the activity of several nucleases, e.g., the MRN (MRE11-RAD50-NBS1) complex, Bloom’s syndrome helicase (BLM), CtBP-interacting protein (CtIP), exonuclease 1 (EXO1), and DNA replication ATP-dependent helicase (DNA2)[22]. A recombinase, e.g., RAD51 in eukaryotes, is loaded onto these specifically processed DNA ends to displace RPA to form a RAD51-ssDNA nucleofilament with the assist of breast cancer 1 (BRCA1), BRCA2, and several RAD51 paralogues[20]. The RAD51-ssDNA nucleofilament can invade homologous duplex DNA to search for and couple with a homologous sequence elsewhere in the genome to form a displacement D-loop in which DNA synthesis is initiated to replace the DNA surrounding the former break site.
Repair of inter-strand crosslinks
Inter-strand crosslinks (ICLs) are able to inhibit transcription and replication by blocking DNA strand separation. Among the chemotherapeutic agents, cisplatin, cyclophosphamide, melphalan, and mitomycin C (MMC) are considered inducers of ICLs[23]. The repair of ICLs is complex and involves a coordination of several DNA repair pathways such as NER, HR, Translesion synthesis (TLS), and Fanconi anemia (FA) pathway[23]. ICLs can be recognized by NER machinery and incised by ERCC1-XPF endonucleases, resulting in unhooking of the lesion, which is bypassed by TLS. When ICLs encounter a replication fork during S phase, the collapse of the replication fork can lead to formation of DSB, which can be further processed by HR[24]. However, the collapse of the replication fork can be avoided by FA complementation group M (FANCM)-mediated fork regression or stabilization.
Translesion DNA synthesis
TLS is a general DNA damage tolerance mechanism, which allows the cell to replicate over DNA lesions. DNA lesions that block replication can be bypassed by specialized low-fidelity DNA polymerases, which are capable of replicating across DNA damage. The most abundant class of these DNA polymerases belongs to the Y-family, including Pol η, Pol κ, Pol ι, and REV1[25]. TLS is facilitated by PCNA mono-ubiquitylation, which is regulated by Rad6-Rad18 E2-E3 ubiquitylating enzymes and USP1 deubiquitylating enzyme at sites of DNA damage[26-28]. During TLS, the first polymerase inserts a nucleotide opposite the lesion and the second polymerase, usually DNA polymerase ζ, extends beyond the lesion. TLS plays an important role in the development of cisplatin resistance and is critical in the repair of ICLs[29].
Activation of cell cycle checkpoint machinery
Cell cycle checkpoints can be activated to arrest cell cycle progression when DNA damage or replication stalls are sensed, so that cells will have sufficient time to repair the damage before it is passed on to daughter cells. The checkpoint pathways in the mammalian cells can be regulated by the ATM (Ataxia Telangiectasia mutated) and ATR (ATM and Rad3-related) protein kinases. ATM and ATR phosphorylate a number of substrates such as Chk1 and Chk2. The activated Chk1 and Chk2 further phosphorylate p53 to stabilize the protein, enhancing the effects of p53 in response to DNA damage. Cdc25A is also phosphorylated by Chk1 and Chk2, leading to a rapid degradation of G1/S transition activators, and enforcing the G1-arrested state[30]. Cdc25C phosphorylation by Chk1 and Chk2 triggers G2 checkpoints to make sure DNA is fully replicated and undamaged before cells undergo chromosome condensation and nuclear division. Targeting cell cycle checkpoints has been proposed as a novel anticancer strategy. ATM inhibitors (such as KU55933) have been shown to effectively increase the sensitivity of cancer cells to DNA damaging agents[31]. The inhibitors of Chk1, Chk2, and Cdc25 have also been suggested to enhance the cytotoxicity of various antitumor agents[32].
DDR IN CSCS
Stem cells persist and function throughout the entire life of an organism to regulate tissue homeostasis and regeneration. The functional importunity and the long life of stem cells suggest that they must be armed to maintain genomic integrity in unique ways different from those of somatic cells[2]. Indeed, human embryonic stem (ES) cells have been shown to display high levels of DNA repair capacity[33]. While reduced DNA repair capacity and loss of genomic integrity have been linked to differentiation or impaired function of stem cells[34]. However, contradictory results also showed that human ES cells are deficient in DNA repair capacity and cell cycle arrest but use high propensity to apoptosis as a strategy to ensure genomic integrity[35].
Given that CSCs share common properties with stem cells and are resistant to the treatment with various DNA damaging agents, CSCs were also proposed to possess the elevated DDR to process DNA damage more efficiently than bulk cancer cells. It has been reported that CD133+ glioma stem cells (GSCs) contribute to glioma radio-resistance and tumor regeneration through enhanced cell cycle checkpoint response and DNA repair. This is further supported by the finding that the radio-resistance of CD133+ GSCs can be overcome with a specific inhibitor of the Chk1 and Chk2 checkpoint kinases[3]. Further investigation demonstrated that L1CAM (CD171), which is preferentially expressed and is induced by DNA damage in GSCs, is responsible for the enhanced activation of cell cycle checkpoint and radio-resistance of GSCs by upregulating NBS1 expression[36]. Glioblastoma-initiating cells (GICs) also exhibit enhanced basal activation of SSB repair due to enhanced activation of the key SSB repair player PARP1. The growth, self-renewal, and DNA damage repair capacity of GICs can be inhibited by PARP inhibition, leading to an enhanced sensitization of GICs to radiation[37]. In addition, GICs exhibit hypermethylation in the promoter of O6-methylguanine DNA methyltransferase (MGMT) gene. The epigenetically silenced MGMT might be responsible for the resistance of GICs to temozolmide[38] due to MGMT’s ability to reverse alkylation at the O6 positon of guanine and neutralize the cytotoxicity of alkylating agents such as temozolmide[39].
In addition to glioma stem cells, enhanced DNA repair capacity was also found in other CSCs. For example, enhanced repair of DNA DSBs and upregulated expression of DSB repair genes were found in CD133+ CSCs isolated from the A549 human lung carcinoma cell line[40]. Similarly, DDR and the expression of various repair proteins are also found to be highly up-regulated in Lin-CD29HCD24H tumor-initiating cells isolated from mouse mammary gland tumors, indicating an elevated DDR in these CSCs[41]. Significant increase in the expression of DNA repair-related genes, such as BRCA1 and RAD51, have also been observed in pancreatic putative CSCs compared with bulk cells. These spheroid cells also repair DNA breaks more efficiently than bulk cells after treatment with gemcitabine[42]. Furthermore, a strong activation of Chk1 and cell cycle arrest were observed in patient-derived non-small-cell lung cancer (NSCLC) stem cells compared to their corresponding differentiated cells after exposure to DNA damaging agents. Treatment with Chk1 inhibitors in combination with chemotherapy dramatically reduced the NSCLC stem cell population in vitro by promoting premature cell cycle progression and inducing mitotic catastrophe[43].
Although considerable reports suggest that competent DNA repair machinery is utilized by CSCs to survive DNA damaging agent treatment, some controversial observations also exist. The DSB repair capacity was found to be significantly reduced in CD133+ glioma stem cells compared with the glioma cell lines. In addition, the intra-S-phase checkpoint was absent in these CD133+ cells, albeit G2 checkpoint activation was intact. Consistent with reduced DNA repair capacity, CD133+ glioma stem cells are more sensitive to radiation than the established glioma cell lines[44]. In another report, although CD133+ glioma stem cells were shown to display elongated cell cycle and enhanced basal activation of Chk1 and Chk2, neither BER nor SSB repair was found to be increased in CD133+ compared to CD133- glioma cells[45]. Interestingly, Lim et al[46] reported a more efficient HR repair in GICs, but neither a extended cell cycle nor augmented activation of checkpoint proteins was observed in these cells. The reduced DDR was also reported in other CSCs. For example, esophageal CSCs exhibited compromised induction of p53 and inhibited G1 checkpoint arrest, as well as attenuated DNA repair capacity upon exposure to DNA damaging agents, as compared to non-CSCs[47]. NSCLC TICs showed an impaired DDR due to deficient activation of various checkpoint factors, e.g., ATM, DNA-PKcs, Krűppel-associated protein 1 (KAP1), and FA complementation group D2 (FANCD2), leading to compromised cell cycle checkpoints[48]. We have recently determined the NER efficiency of ovarian CSCs, defined by either CD44+CD117+ or spheroid growing in serum-free culture, after treatment with cisplatin. Surprisingly, although ovarian CSCs exhibited resistance to cisplatin treatment compared to their corresponding bulk cancer cells, no increased capability to remove cisplatin-induced DNA lesions was found in these CSCs[49]. In contrast, we revealed an elevated expression of DNA polymerase η and constitutively high levels of mono-ubiquitylated PCNA in various ovarian CSCs, indicating that enhanced TLS could be responsible for cisplatin resistance in these cells[49].
The controversial observations of the DDR in CSCs indicate that enhanced DDR can be a contributor in the resistance of CSCs to DNA damaging agents, but this may not be applied to all CSCs. Due to the genetic heterogeneity in cancer, CSCs isolated from different cell lines and patients may have different responses to DNA damage. Furthermore, the heterogeneity in CSCs and uncertainty in CSC markers may also result in different subpopulations of CSCs from the same cell line or patient[50], which may also exhibit different DDR. Another possible explanation for the conflicting results is that the different culture conditions of CSCs may affect sensitivity and responses of CSCs to DNA damaging agents as well.
CHROMATIN REMODELING IN STEM CELLS
Chromatin creates a natural barrier against access to DNA in many nuclear functions. It is now clear that chromatin modifications have an integral role in DNA damage repair. Following DNA damage, the chromatin needs to be in an “open” state in order to allow the repair factors to access the DNA molecule. Increasing evidence indicates that DDR can be extensively impacted by the modulations to chromatin structure. Chromatin structure in stem cells is different from differentiated cells. Many stem and progenitor cells have been classically described as having a typical open chromatin conformation that is mostly devoid of heterochromatin[51]. Electron microscopy examination indicated that heterochromatin is predominant in differentiated cells but less common in ES cells[52]. Analysis of global chromatin compaction using nuclease also indicated that chromatin is more accessible, and thus more sensitive to nuclease digestion in ES cells than differentiated cells.
In addition to the histological evidence, the idea of “open chromatin” is also supported by other analyses. For example, immunoblotting and immunofluorescence analyses have demonstrated a reduced amount of histone H3 tri-methylation on Lys9 (H3K9me3), a marker of heterochromatin, suggesting that ES cells possess considerably less heterochromatin than differentiated cells. ChIP-seq analyses further showed that heterochromatic marks H3K9me3 and H3K27me3 cover 16% and 12% of the genome of differentiated IMR90 cells, respectively, but both of them cover only 4% in human ES cells[53]. On the other hand, it appears that ES cells have higher global level of histone acetylation, a general mark of open chromatin, as compared to differentiated cells. In addition, some indirect evidence also support “open chromatin” in ES cells. A fraction of loosely bound architectural chromatin proteins was found in ES cells by using Fluorescence recovery after photobleaching (FRAP) assay, while this fraction is absent in differentiated cells. Collectively, these findings indicate that chromatin in ES cells is globally decondensed and hyperdynamic compared with differentiated cells.
Given that stem cells use distinct characteristic of chromatin structure for regulation of self-renewal and differentiation, it is likely that histone modifications and chromatin remodeling upon DNA damage may be different between stem cells and differentiated cells. Among the limited data regarding DNA damage-induced chromatin modifications in stem cells, γH2AX was mostly investigated. It has been reported that γH2AX foci can be observed under microscope in an open chromatin structure even in the absence of exogenous DNA damage in pluripotent mouse ES (mES)[54]. Pluripotent mES cells can contain more than 100 large γH2AX foci, which is equivalent to the number of foci that are produced in differentiated cells in response to about 4 Gy X-rays. In comparison, intact differentiated mammalian cells normally exhibit fewer than 5 large γH2AX foci per cell. It was proposed that the decondensed chromatin structure of pluripotent mES cells, mainly maintained by enhanced global histone acetylation and reduced histone methylation, as well as abundant chromatin remodeling complexes, contributes to the development of larger γH2AX foci[54]. Further relaxation of ES cell chromatin by lessening the level of the linker histone H1 causes ES cells hyper-resistant to DNA damage, as the DDR is enhanced in the context of open chromatin[55]. Although DNA damage induces γH2AX foci in both differentiated and stem cells, the distribution of γH2AX in the nucleus of stem cells is distinct. Momcilovic et al[56] used a 405 nm laser to induce DNA damage in a defined subnuclear area of several ES cells and found that unlike in mouse embryonic fibroblasts (MEF), γH2AX staining was not confined to the affected nuclear region in ES cells. Instead, γH2AX specific signal was uniformly distributed through the entire nuclear area, perhaps due to the high dynamics of chromatin in pluripotent stem cells[56].
It is noteworthy that not all stem cells have unique pattern of γH2AX in response to DNA damage. Like differentiated cells, unirradiated hematopoietic stem cells displayed extremely low levels of γH2AX foci, while these cells showed an immediate induction of γH2AX foci after exposure to 2 Gy IR[57]. Similarly, the percentage of cells with nuclear foci of γH2AX after IR was not significantly different in stem glioma (CD133+) and non-stem glioma (CD133-) cells at both 1 and 24 h after treatment[45]. Thus, the distinct performance of histone H2AX phosphorylation upon DNA damage in various kinds of stem cells reflects various DNA damage responses in different stem cells.
TARGETING THE DDR TO FACILITATE ERADICATION OF CSCS
Genotoxic agents including ionizing radiation (IR) and many chemotherapeutic drugs that cause DNA damage have been used for cancer therapy for many years. As discussed above, the DDR is a determinant factor in cell sensitivity to DNA damaging agents. Thus, the protein kinases and enzymes that are involved in the DDR, such as ATM, ATR, Chk1, Chk2, and PARP1, have been identified as promising targets for cancer therapeutics[58]. Due to the enhanced DDR in most types of CSCs, modulation of the DDR in CSCs became a new therapy strategy in the treatment with DNA damaging agents. For example, a specific inhibitor of the checkpoint kinases debromohymenialdisine (DBH) has been demonstrated to reverse the radio-resistance of GSCs defined as CD133+[3]. PARP inhibitor (olaparib) was also able to sensitize GICs to radiation[37]. In addition, NSCLC-stem cell survival can be dramatically reduced by Chk1 inhibitors (SB218078 and AZD7762) in combination with chemotherapy[43]. Recently, the work in our lab revealed depleted miR-93 expression in ovarian CSCs, which is, at least partially, responsible for the upregulated DNA polymerase η expression. Transfection of miR-93 into ovarian CSCs increased their sensitivity to cisplatin treatment, probably through downregulation of DNA polymerase η and thus inhibition of TLS of CSCs[49]. These findings provide a new avenue to facilitate the eradication of CSCs by platinum-based chemotherapeutics. However, given that adult stem cells also possess enhanced DDR, the DDR inhibitors aimed at eliminating CSCs could also sensitize the adult stem cells to DNA damaging agent, resulting in severe side effects. Therefore, identifying the differences in the DDR between adult stem cells and CSCs, and finding the Achilles’ heel in the mechanisms protecting CSCs is critical to the development of potential drugs for facilitating eradication of CSCs by DNA damaging agents.
CONCLUSION
Dysregulated DDR is observed in many cancers and is responsible for the genomic instability that related to tumorigenesis and tumor progression[59]. The DDR defects provide an Achilles’s heel that can be exploited for cancer therapy[60-63]. The enhanced DDR found in CSCs is considered a protector from the insult of DNA damaging agents. Thus, reducing DDR in CSCs would be a promising therapy strategy for achieving elimination of CSCs with the conventional radio-/chemo-therapy.
Currently, most studies are focusing on the protein kinases and enzymes that are involved in the DDR. As discussed above, the DDR in CSCs can be affected by many factors, among which chromatin remodeling plays an important role in facilitating the DDR in cells following DNA damage. Although stem cells display an open chromatin conformation, there is lack of information on the chromatin structure in CSCs. Understanding the chromatin structure and chromatin remodeling in CSCs after the treatment with DNA damaging agents would provide a novel avenue to interfere with the DDR in this cells. Another question we need to address is how CSCs respond to DNA damaging agents in vivo. Most of the current studies were conducted using in vitro cultured cells. However, CSCs dwell in a specialized microenvironment denoted as “stem cell niche”, which contains diverse stromal cells, vascular networks, soluble factors, and extracellular matrix component. These components may affect the DDR in CSCs. In addition, the CSC niche can protect CSCs by sheltering them from diverse genotoxic insults, leading to an enhanced therapeutic resistance[64]. Thus, the future design of therapy to eliminate CSCs needs consideration of the tumor microenvironment. Finally, the phenotypic heterogeneity of CSCs in different tumors, and even in the same tumor, should be considered[65]. Identifying the dysregulated DDR components in different CSC subpopulations is critical to selecting targets that can be used to efficiently shut down their specific DDR.
ACKNOWLEDGMENTS
I thank Dr. Altaf Wani and Arman Rathod for critical reading of the manuscript. I also apologize to colleagues whose work was not cited because of space limitations.
P- Reviewer: Cui YP, Kiselev SL, Lee Y, Zaravinos A S- Editor: Ji FF L- Editor: A E- Editor: Wang CH