Published online Aug 26, 2014. doi: 10.4331/wjbc.v5.i3.269
Revised: May 6, 2014
Accepted: June 10, 2014
Published online: August 26, 2014
Processing time: 203 Days and 11.4 Hours
Doxorubicin (Dox) is one of the most effective chemotherapeutic agents used in the treatment of several types of cancer. However the use is limited by cardiotoxicity. Despite extensive investigation into the mechanisms of toxicity and preventative strategies, Dox-induced cardiotoxicity still remains a major cause of morbidity and mortality in cancer survivors. Thus, continued research into preventative strategies is vital. Short-term fasting has proven to be cardioprotective against a variety of insults. Despite the potential, only a few studies have been conducted investigating its ability to prevent Dox-induced cardiotoxicity. However, all show proof-of-principle that short-term fasting is cardioprotective against Dox. Fasting affects a plethora of cellular processes making it difficult to discern the mechanism(s) translating fasting to cardioprotection, but may involve suppression of insulin and insulin-like growth factor-1 signaling with stimulated autophagy. It is likely that additional mechanisms also contribute. Importantly, the literature suggests that fasting may enhance the antitumor activity of Dox. Thus, fasting is a regimen that warrants further investigation as a potential strategy to prevent Dox-induced cardiotoxicity. Future research should aim to determine the optimal regimen of fasting, confirmation that this regimen does not interfere with the antitumor properties of Dox, as well as the underlying mechanisms exerting the cardioprotective effects.
Core tip: Doxorubicin (Dox)-induced cardiotoxicity remains a significant cause of morbidity and mortality in cancer survivors, despite the intensive investigation of potential protective strategies. Studies have shown that short-term fasting induces cardioprotective effects against Dox-induced injury. Importantly, evidence suggests that fasting may enhance the antitumor effects of Dox. Thus, short-term fasting may be a feasible practice that can easily be incorporated into the treatment plans of cancer patients.
- Citation: Dirks-Naylor AJ, Kouzi SA, Yang S, Tran NT, Bero JD, Mabolo R, Phan DT, Whitt SD, Taylor HN. Can short-term fasting protect against doxorubicin-induced cardiotoxicity? World J Biol Chem 2014; 5(3): 269-274
- URL: https://www.wjgnet.com/1949-8454/full/v5/i3/269.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i3.269
Doxorubicin (Dox) is one of the most effective chemotherapeutic agents currently used in the treatment of haematological malignancies and solid tumors such as breast cancer. It is a quinone-containing anthracycline antibiotic. Its mechanism of antitumor activity has been shown to involve binding to topoisomerase-II α, thereby resulting in DNA strand breaks and apoptosis[1]. Despite its effectiveness, its use is limited due to cardiotoxicity. In animal models, Dox has been shown to be hepatotoxic as well, but in humans it is the cardiotoxicity which primarily limits its use[2,3]. High cumulative doses of Dox are the most powerful predictor of Dox-induced congestive heart failure[4]. One early study reported that 4% of patients receiving a dose of 500-550 mg/m2 and 18% and 36% of patients receiving 551 mg/m2 or higher, respectively, experienced heart failure, which is often refractory to conventional therapy[4,5]. The clinical outcome for these patients is poor[4]. A variety of approaches to prevent cardiotoxicity have been tested, however, their efficacy has been limited[4]. Thus, continued investigation of viable strategies to protect the heart from Dox-induced toxicity is of vital importance.
Mechanisms of Dox-induced cardiotoxicity have not been clearly elucidated, but have been shown to involve oxidative stress, mitochondrial dysfunction, and apoptosis. For example, Dox treatment has been shown to increase mitochondrial depolarization, fission, and ROS production while decreasing the rate of ATP synthesis and content[6-10]. Lipid peroxidation, reduced aconitase activity (a marker of oxidative stress), and alterations in the expression and activity of antioxidant enzymes, such as superoxide dismutase (SOD), are also evident[11,12]. Oxidative stress and mitochondrial dysfunction can induce apoptosis which leads to loss of post-mitotic myocytes and altered cardiac function[8,13,14]. It has long been known that Dox can induce oxidative stress via semiquinone redox cycling, however it is unclear if this is the specific mechanism of cardiotoxicity since ROS scavengers failed to prevent cardiac toxicity in several studies[15,16]. Recently, topoisomerase-II β has been shown to be a molecular target of Dox in cardiomyocytes[17]. Cardiac myocytes do not express topoisomerase-II α, the molecular target in tumor cells[18]. Zhang et al[17] demonstrated that cardiomyocyte-specific deletion of topoisomerase-II β prevented Dox-induced cardiotoxicity. Furthermore, the deletion prevented Dox-induced DNA damage and transcriptional changes that are responsible for impaired mitochondrial biogenesis, ROS formation, and apoptosis. Thus, the mechanism of Dox-induced cardiotoxicity may involve molecular targeting of topoisomerase-II β as well as the potential contribution of semiquinone redox cycling.
Currently, available therapies to effectively prevent cardiotoxicity in patients treated with Dox are limited. Thus, the first line of defense is to limit the cumulative dose of Dox. However, lowering cumulative dose may translate to reduced treatment efficacy[19]. Another strategy to protect against cardiotoxicity has been to alter the mode of delivery of Dox, such as encapsulation in liposomes, which aims to target the delivery to the tumor, thereby, reducing plasma concentrations of Dox. The United States Food and Drug Administration has approved one liposomal doxorubicin, Doxil[19]. Shorter-term clinical trials have shown that liposomal doxorubicin can reduce early cardiotoxicity while having the same antineoplastic efficacy as conventional doxorubicin[19]. Although, liposomal doxorubicin has shown promise in reducing cardiotoxicity, currently, it is still mainstream to use conventional Dox. Utilizing antioxidants or iron chelators to reduce Dox-induced oxidative stress has been another tested strategy, but with limited success[19]. Dexrazoxane, an iron chelating agent, has shown the most promise in reducing oxidative stress and cardiotoxicity, however, with some limitations. Most studies have shown that Dexrazoxane is safe, however, some have shown that dexrazoxane may cause myelosuppression and also increase the risk of second malignancies[20,21]. Furthermore, it has been shown that the efficacy of dexrazoxane may vary between sexes, with less benefit in males[19,22]. Despite extensive investigation and numerous tested strategies to prevent cardiotoxicity, success has been limited. Dox-induced cardiotoxicity still remains a major cause of morbidity and mortality in cancer survivors[19]. Thus, exploration of additional strategies to prevent Dox-induced cardiotoxicity is paramount.
A cardioprotective strategy that warrants further exploration is fasting. Fasting and/or caloric restriction (CR) has been shown to protect the heart from a variety of conditions and insults. For example, intermittent fasting protects the heart from ischemic damage and attenuates post-MI cardiac remodeling[23]. Furthermore, calorie restriction has proven protective against coronary artery disease, the process of aging on the cardiovascular system, as well as drug toxicities, including doxorubicin-induced cardiotoxicity[24-27]. Mitra et al[26] demonstrated that 40+ days of a 35% calorie restricted diet lead to 100% protection against Dox-induced cardiotoxicity and death while all of the rodents administered with Dox in the ad libitum fed group died. However, long term CR regimens, such as this, are not feasible in cancer patients since they typically suffer from malnutrition and other complications. Therefore, short-term fasting may be an alternative approach. Indeed, Raffaghello et al[28] reported that 48-60 h of complete fasting prevented organ toxicity induced by chemotherapy in various species of female mice, however, etoposide rather than Dox was used in the study. Kawaguchi et al[29] demonstrated that 48 h of complete fasting prior to Dox administration mitigated the Dox-induced impairment in cardiac function in adult GFP-LC-3 transgenic mice, as determined by left ventricular ejection fraction (LVEF), systolic pressure (LVSP), end diastolic pressure (LVEDP), and +dP/dt. Microscopy revealed attenuation of LV dilatation, myocardial atrophy, and fibrosis[29]. In vitro, a caloric restriction mimetic, 2-deoxyglucose (2-DG), was shown to exhibit cardioprotective properties against Dox using neonatal rat cardiomyocytes isolated from 0-2 d old Harlan Sprague-Dawley rat neonates[30]. Thus, the literature supports that fasting may be an effective regimen to protect against Dox-induced cardiotoxicity.
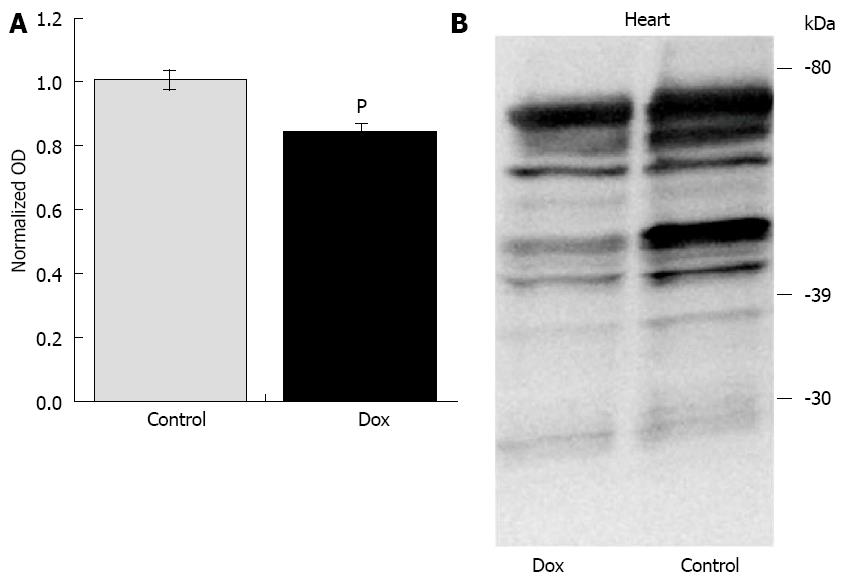
Unpublished data from our laboratory (Table 1) may also suggest that short-term fasting may provide cardioprotection against Dox. Six-week old male F344 rats were treated with a single injection of Dox (20 mg/kg) or saline. Tissues were harvested for analysis 24-h post injection with the aim of determining the effects of Dox on the mitochondrial dynamics and mitophagy machinery. In order to remove the external variable of Dox-induced anorexia, we fasted both groups of animals upon treatment. Studies have shown that animals treated with Dox reduce their food and water intake by up to 70% for several days[31]. Using this experimental design, the results were unexpected. Dox did not affect any markers of oxidative stress or apoptosis that were assessed in the heart. Dox did not affect aconitase activity, superoxide dismutase (SOD) activity, nor the protein content of cytosolic SOD1 and mitochondrial SOD2. Expression and activation of caspase-12, caspase-9, and caspase-8 were assessed via Western analysis, as well as caspase-3 and -9 enzyme activities, and were not affected by Dox. As previously mentioned, the original aim of the study was to investigate the effects of Dox on the mitochondrial dynamics and mitophagy machinery with the hypothesis that Dox treatment would increase the protein content of FIS1 and DRP1 (fission regulators) and decrease the content of MFN1, MFN2 and OPA1 (fusion regulators) thus favoring mitochondrial fission, which is most often associated with oxidative stress, mitochondrial dysfunction and apoptosis[32-34]. Under the current fasting conditions, Dox did not affect the content of any of these primary regulators. Regulators of mitophagy were also assessed. Dox did not affect the content of PINK1, Parkin, or p62 (a marker of mitophagy) under these fasting conditions. We do know that Dox exerted a biological effect in these animals since many of these variables were altered in the liver. Furthermore, the treatment significantly affected the proteome lysine acetylation status in the heart, inducing deacetylation (Figure 1), although the significance of this observation is currently unknown. Because previously published studies have reported that acute Dox treatment does affect many of these variables and processes[8-11,35-37], we believe that complete fasting of the animals in our study may have exerted an unintended cardioprotective effect against the Dox-induced insult. However, further investigation is required to confirm our interpretation of the data. Although this work was done using an acute model of Dox cardiotoxicity, since short-term fasting may be able to protect against the high dose used in the acute model, it is likely that it may also be protective against lower doses used in chronic models of Dox cardiotoxicity which mimics more closely the clinical use of Dox in patients. In summary, short-term fasting may extend similar benefits as longer term CR in regards to cardioprotection against Dox-induced injury.
| Dependent variable | Control (mean ± SEM) | Dox (mean ± SEM) | P value |
| Aconitase activity (nmol/min per milligram protein) | 14.46 ± 3.68 | 23.74 ± 3.25 | 0.08 |
| SOD activity (units/mg protein) | 0.026 ± 0.003 | 0.026 ± 0.002 | 0.904 |
| SOD1 content | 1025 ± 110.2 | 949 ± 91.6 | 0.603 |
| SOD2 content | 275.6 ± 23.25 | 288.1 ± 23.71 | 0.715 |
| Procaspase-12 content | 36.90 ± 6.14 | 24.28 ± 4.19 | 0.1 |
| Procaspase-9 content | 28.59 ± 1.57 | 25.33 ± 3.61 | 0.5 |
| Procaspase-8 content | 68.10 ± 11.90 | 82.90 ± 0.93 | 0.34 |
| Caspase-3 activity (arbitrary OD/mg protein) | 0.951 ± 0.676 | 0.490 ± 0.295 | 0.524 |
| Caspase-9 activity (arbitrary OD/mg protein) | 1.084 ± 0.809 | 0.462 ± 0.255 | 0.451 |
| FIS1 content | 563.6 ± 76.6 | 474.3 ± 68.8 | 0.4 |
| DRP1 content | 1294.9 ± 109.8 | 1187.5 ± 73.5 | 0.421 |
| MFN1 content | 5443.5 ± 786.8 | 4607.8 ± 627.0 | 0.417 |
| MFN2 content | 2001.5 ± 456.8 | 2053.6 ± 330.2 | 0.926 |
| OPA1 content | 6019.5 ± 739.3 | 6143.6 ± 601.0 | 0.897 |
| PINK1 content | 3343.0 ± 206.9 | 3422.0 ± 263.4 | 0.824 |
| Parkin content | 4192.0 ± 1009.0 | 4157.0 ± 1629.0 | 0.986 |
| p62 content | 1895.7 ± 272.7 | 1896.7 ± 252.2 | 0.998 |
Fasting and caloric deprivation affect a plethora of cellular processes such as mitochondrial dynamics and biogenesis, energy metabolism, oxidative stress, autophagy, and survival signaling pathways, thus making it difficult to discern the mechanism(s) responsible for the cardioprotection[38-42]. Kawaguchi et al[29] concluded that the protection against Dox-induced injury extended by 48-h of fasting prior to treatment was due to restoration of autophagy. Autophagy is a conserved process among eukaryotic cells that sequesters cellular material via formation of a multimembrane-bound vacuole, an autophagosome, followed by degradation of the material via fusion of the autophagosome with a lysosome[43]. Autophagy can enhance cellular function and survival by degrading damaged or unwanted proteins and organelles such as mitochondria, as well as by modulating apoptosis[44]. Indeed, stimulation of autophagy has been shown to be cardioprotective from a variety of damaging stimuli[44]. Kawaguchi et al[29] reported that the inhibition of autophagy by Dox was due to inhibition of AMP-activated protein kinase (AMPK). Prior fasting prevented the Dox-induced inhibition of AMPK. Although fasting was able to reverse the effects of Dox on autophagy, no experimental methods were employed to identify restoration of autophagy as the underlying factor for cardioprotection. Furthermore, no other processes known to be affected by fasting were assessed in the study. Moreover, several studies have shown that stimulation of autophagy contributes to Dox-induced cardiotoxicity and protection is provided via inhibition of autophagy[43]. Thus the role of autophagy in Dox-induced cardiotoxicity, whether protective or pathological, is still under question. Therefore, the underlying mechanism(s) of fasting-induced cardioprotection against Dox remains to be determined and is likely due to a combination of mechanisms[30].
It is critical that a potential cardioprotective agent or regimen does not interfere with the goal of cancer treatment. CR has long been shown to have antineoplastic effects. CR can slow the intrinsic rate of aging and prevent the onset of age-related pathologies, including cancer[45,46]. Furthermore, CR mimetics, such as 2-DG, have been shown to inhibit tumor growth[47]. Moreover, 2-DG has been shown to enhance the antitumor efficacy of Dox both in vitro and in vivo[48,49]. Short-term (48-60 h) fasting was shown to enhance death of cancer cells, prevent organ toxicity, and increase survival in chemotherapy treated mice, however the chemotherapy tested was etoposide, not Dox[28]. Interestingly, Raffaghello et al[28] noted that fasting longer than 60 h worsened outcomes. Thus, there may be a window of optimal duration of fasting to maximize beneficial effects. Many of the benefits of fasting and caloric restriction have been shown to be, at least in part, due to decreased circulating levels of insulin and reduced insulin-like growth factor-1 receptor (IGF-1R) signaling[50,51]. Seventy-two hours of fasting reduced circulating IGF-1 by 70% and increased the level of the IFG-1 binding protein (IGFBP) by 11x[52]. Survival time, after Dox treatment, was extended by delaying metastasis of highly aggressive melanoma and prevented Dox-induced toxicity in liver-specific IGF-1-deficient (LID) mice compared to non-LID mice[52]. Ninety days after inoculation with the melanoma cancer cells, all non-LID mice that were treated with Dox had died from either cancer metastases or Dox toxicity. 60% of LID mice treated with Dox were cancer-free with no signs of toxicity[52]. Thus, the evidence supports that fasting may be a safe regimen to use in conjunction with Dox in order to prevent cardiotoxicity.
In conclusion, Dox-induced cardiotoxicity remains a significant cause of morbidity and mortality in cancer survivors despite the intensive investigation of potential protective strategies. Studies have shown that short-term fasting induces cardioprotective effects against Dox-induced injury. Importantly, evidence suggests that fasting may enhance the antitumor effects of Dox. It seems that short-term fasting would be a feasible practice that can easily be incorporated into the treatment plans of cancer patients. Thus, short-term fasting is a strategy warranting further exploration. Further studies, both preclinical and clinical, should reveal the optimal regimen of fasting, confirmation that this regimen does not interfere with the antitumor properties of Dox, as well as the underlying mechanisms exerting the cardioprotective effects.
P- Reviewer: Shi NQ, Simkhovich BZ S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984;226:466-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1056] [Cited by in RCA: 1098] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 2. | Kalender Y, Yel M, Kalender S. Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats. The effects of vitamin E and catechin. Toxicology. 2005;209:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Patel N, Joseph C, Corcoran GB, Ray SD. Silymarin modulates doxorubicin-induced oxidative stress, Bcl-xL and p53 expression while preventing apoptotic and necrotic cell death in the liver. Toxicol Appl Pharmacol. 2010;245:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 652] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 5. | Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302-314. [PubMed] |
| 6. | Bugger H, Guzman C, Zechner C, Palmeri M, Russell KS, Russell RR. Uncoupling protein downregulation in doxorubicin-induced heart failure improves mitochondrial coupling but increases reactive oxygen species generation. Cancer Chemother Pharmacol. 2011;67:1381-1388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Danz ED, Skramsted J, Henry N, Bennett JA, Keller RS. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic Biol Med. 2009;46:1589-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Pointon AV, Walker TM, Phillips KM, Luo J, Riley J, Zhang SD, Parry JD, Lyon JJ, Marczylo EL, Gant TW. Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase 3 activation. PLoS One. 2010;5:e12733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Parra V, Eisner V, Chiong M, Criollo A, Moraga F, Garcia A, Härtel S, Jaimovich E, Zorzano A, Hidalgo C. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res. 2008;77:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Marechal X, Montaigne D, Marciniak C, Marchetti P, Hassoun SM, Beauvillain JC, Lancel S, Neviere R. Doxorubicin-induced cardiac dysfunction is attenuated by ciclosporin treatment in mice through improvements in mitochondrial bioenergetics. Clin Sci (Lond). 2011;121:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Ascensão A, Magalhães J, Soares JM, Ferreira R, Neuparth MJ, Marques F, Oliveira PJ, Duarte JA. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am J Physiol Heart Circ Physiol. 2005;289:H722-H731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Minotti G, Ronchi R, Salvatorelli E, Menna P, Cairo G. Doxorubicin irreversibly inactivates iron regulatory proteins 1 and 2 in cardiomyocytes: evidence for distinct metabolic pathways and implications for iron-mediated cardiotoxicity of antitumor therapy. Cancer Res. 2001;61:8422-8428. [PubMed] |
| 13. | Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2: Bax ratio. Cancer Res. 2002;62:4592-4598. [PubMed] |
| 14. | Ueno M, Kakinuma Y, Yuhki K, Murakoshi N, Iemitsu M, Miyauchi T, Yamaguchi I. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J Pharmacol Sci. 2006;101:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Myers C, Bonow R, Palmeri S, Jenkins J, Corden B, Locker G, Doroshow J, Epstein S. A randomized controlled trial assessing the prevention of doxorubicin cardiomyopathy by N-acetylcysteine. Semin Oncol. 1983;10:53-55. [PubMed] |
| 16. | Martin E, Thougaard AV, Grauslund M, Jensen PB, Bjorkling F, Hasinoff BB, Tjørnelund J, Sehested M, Jensen LH. Evaluation of the topoisomerase II-inactive bisdioxopiperazine ICRF-161 as a protectant against doxorubicin-induced cardiomyopathy. Toxicology. 2009;255:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1407] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 18. | Capranico G, Tinelli S, Austin CA, Fisher ML, Zunino F. Different patterns of gene expression of topoisomerase II isoforms in differentiated tissues during murine development. Biochim Biophys Acta. 1992;1132:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 203] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, Hudson MM, Kremer LC, Landy DC, Miller TL. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128:1927-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 406] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 20. | Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, Mendenhall NP, Sposto R, Chauvenet A, Schwartz CL. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol. 2007;25:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 373] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 21. | Curran CF, Narang PK, Reynolds RD. Toxicity profile of dexrazoxane (Zinecard, ICRF-187, ADR-529, NSC-169780), a modulator of doxorubicin cardiotoxicity. Cancer Treat Rev. 1991;18:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, Barry EV, Asselin BL, Athale U, Clavell LA. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11:950-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 331] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 23. | Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115-3121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 169] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 25. | Ahmet I, Tae HJ, de Cabo R, Lakatta EG, Talan MI. Effects of calorie restriction on cardioprotection and cardiovascular health. J Mol Cell Cardiol. 2011;51:263-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Mitra MS, Donthamsetty S, White B, Latendresse JR, Mehendale HM. Mechanism of protection of moderately diet restricted rats against doxorubicin-induced acute cardiotoxicity. Toxicol Appl Pharmacol. 2007;225:90-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Vucevic D, Mladenovic D, Ninkovic M, Aleksic V, Stankovic MN, Stankovic M, Jorgacevic B, Vukicevic RJ, Radosavljevic T. The effects of caloric restriction against ethanol-induced oxidative and nitrosative cardiotoxicity and plasma lipids in rats. Exp Biol Med (Maywood). 2013;238:1396-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci USA. 2008;105:8215-8220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 410] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 29. | Kawaguchi T, Takemura G, Kanamori H, Takeyama T, Watanabe T, Morishita K, Ogino A, Tsujimoto A, Goto K, Maruyama R. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc Res. 2012;96:456-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Chen K, Xu X, Kobayashi S, Timm D, Jepperson T, Liang Q. Caloric restriction mimetic 2-deoxyglucose antagonizes doxorubicin-induced cardiomyocyte death by multiple mechanisms. J Biol Chem. 2011;286:21993-22006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Gilliam LA, Ferreira LF, Bruton JD, Moylan JS, Westerblad H, St Clair DK, Reid MB. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol (1985). 2009;107:1935-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Palmer CS, Osellame LD, Stojanovski D, Ryan MT. The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal. 2011;23:1534-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 224] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 33. | Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001-5011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 850] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 34. | Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185-26192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 1049] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 35. | Kavazis AN, Smuder AJ, Min K, Tümer N, Powers SK. Short-term exercise training protects against doxorubicin-induced cardiac mitochondrial damage independent of HSP72. Am J Physiol Heart Circ Physiol. 2010;299:H1515-H1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Smuder AJ, Kavazis AN, Min K, Powers SK. Doxorubicin-induced markers of myocardial autophagic signaling in sedentary and exercise trained animals. J Appl Physiol (1985). 2013;115:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Abd El-Aziz MA, Othman AI, Amer M, El-Missiry MA. Potential protective role of angiotensin-converting enzyme inhibitors captopril and enalapril against adriamycin-induced acute cardiac and hepatic toxicity in rats. J Appl Toxicol. 2001;21:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Sorensen M, Sanz A, Gómez J, Pamplona R, Portero-Otín M, Gredilla R, Barja G. Effects of fasting on oxidative stress in rat liver mitochondria. Free Radic Res. 2006;40:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA. 2011;108:10190-10195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 909] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 40. | Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1178] [Cited by in RCA: 1512] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 41. | Di Simplicio P, Rossi R, Falcinelli S, Ceserani R, Formento ML. Antioxidant status in various tissues of the mouse after fasting and swimming stress. Eur J Appl Physiol Occup Physiol. 1997;76:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Hayashida S, Arimoto A, Kuramoto Y, Kozako T, Honda S, Shimeno H, Soeda S. Fasting promotes the expression of SIRT1, an NAD+ -dependent protein deacetylase, via activation of PPARalpha in mice. Mol Cell Biochem. 2010;339:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | Dirks-Naylor AJ. The role of autophagy in doxorubicin-induced cardiotoxicity. Life Sci. 2013;93:913-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 44. | Gottlieb RA, Finley KD, Mentzer RM. Cardioprotection requires taking out the trash. Basic Res Cardiol. 2009;104:169-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Dirks AJ, Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech Ageing Dev. 2006;127:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 46. | Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 47. | Zhang XD, Deslandes E, Villedieu M, Poulain L, Duval M, Gauduchon P, Schwartz L, Icard P. Effect of 2-deoxy-D-glucose on various malignant cell lines in vitro. Anticancer Res. 2006;26:3561-3566. [PubMed] |
| 48. | Zhang F, Aft RL. Chemosensitizing and cytotoxic effects of 2-deoxy-D-glucose on breast cancer cells. J Cancer Res Ther. 2009;5 Suppl 1:S41-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF, De Young LR, Lampidis TJ. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 358] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 50. | Katic M, Kahn CR. The role of insulin and IGF-1 signaling in longevity. Cell Mol Life Sci. 2005;62:320-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Sell Ch. Caloric restriction and insulin-like growth factors in aging and cancer. Horm Metab Res. 2003;35:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564-1572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |