INTRODUCTION
Discovered more than two decades ago, cyclic adenosine diphosphate ribose (cADPR) has been established as a second messenger, according to criteria first proposed by Sutherland and co-workers[1]. Together with inositol 1,4,5-trisphosphate (IP3) and nicotinic acid adenine dinucleotide phosphate (NAADP), cADPR has been recognized as a principal second messenger involved in cellular Ca2+ mobilization. Extracellular stimuli can induce cADPR production, which leads to Ca2+ mobilization from intracellular stores as well as Ca2+ entry from the extracellular compartment to initiate diverse cellular responses. cADPR is synthesized by ADP-ribosyl cyclases and the major ADP-ribosyl cyclase in mammals is CD38 (Figure 1). In this review, we will first introduce the structure and function of cADPR. Next, the structure and topology of CD38 will be reviewed. Finally, the physiological functions of CD38/cADPR/Ca2+ signaling and their involvement in pathological processes will be summarized.
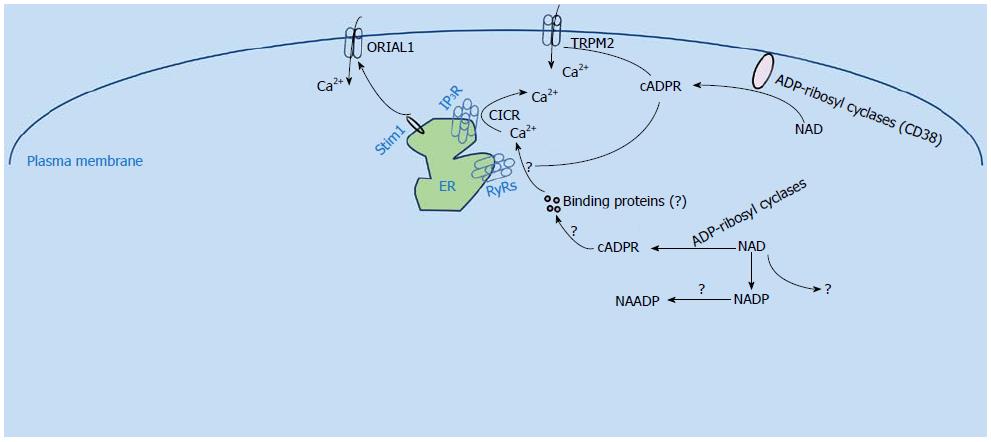
Figure 1 Cyclic adenosine diphosphate ribose mediated Ca2+ signaling.
TRPM2: Transient receptor potential cation channel M2; cADPR: Cyclic adenosine diphosphate ribose; NAADP: Nicotinic acid adenine dinucleotide phosphate; NAD: Nicotinamide adenine dinucleotide; ER: Endoplasmic reticulum.
THE STRUCTURE AND FUNCTION OF CADPR
A suitable model system is the foundation of any novel finding and this concept is also true for the discovery of cADPR. Sea urchin eggs are large and amenable for microinjection studies so that Ca2+ mobilizing activities during fertilization can be readily observed, and it is easy to isolate endoplasmic reticulum (ER) from sea urchin eggs, making them the perfect system to investigate mechanisms of intracellular Ca2+ mobilization[2]. Taking advantage of the sea urchin homogenate preparation and use of the fluorescent Ca2+ indicator Fura 2, Lee et al[3] and Clapper et al[4] found that the pyridine nucleotide nicotinamide adenine dinucleotide (NAD) can invoke a delayed Ca2+ release from ER independent of IP3. They then determined that this delay was due to enzymatic conversion of NAD to cADPR by the homogenate. Later, Lee et al[5] solved the structure of cADPR by x-ray crystallography and showed that it is a novel cyclic nucleotide formed by the covalent linkage of the N1 nitrogen of the adenine ring to the anomeric carbon of the terminal ribose to become a closed cyclic structure (Figure 2). Benefiting from the identified structure, multiple cADPR analogs have been synthesized, which greatly promoted research on the role and mechanism of cADPR-mediated Ca2+ signaling[6-9].
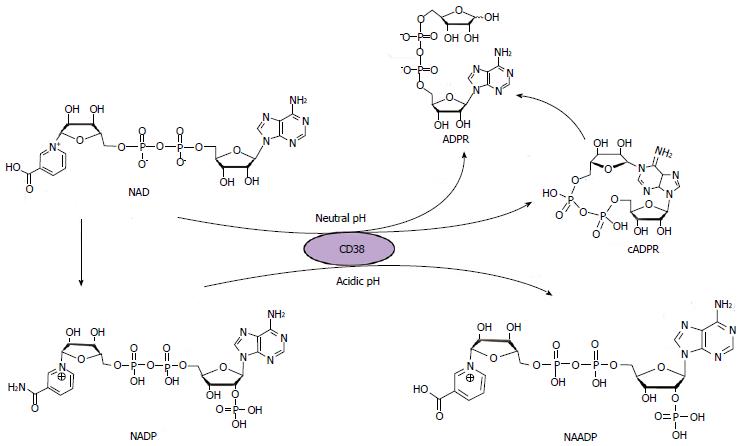
Figure 2 Schematic of the structure and synthesis of cyclic adenosine diphosphate ribose.
cADPR: Cyclic adenosine diphosphate ribose; NAADP: Nicotinic acid adenine dinucleotide phosphate; NAD: Nicotinamide adenine dinucleotide.
From the very beginning of research on cADPR, several pharmacological studies have clearly shown that the mechanism of cADPR-induced Ca2+ release is different from that of IP3. For example, desensitization experiments demonstrated that the sea urchin homogenates which were desensitized to IP3 would still respond to cADPR[4], and the IP3 inhibitor heparin had no effect on the cADPR-induced Ca2+ release[10]. Using the sea urchin homogenate as the model, Galione et al[11] proposed that calcium-induced calcium release (CICR) may be modulated by cADPR, since concentrations of cADPR in the nanomolar range could greatly increase the sensitivity to Ca2+ during the CICR process. Thus, ryanodine receptors (RyRs) were proposed to be the cADPR receptors through which the CICR functions, and this idea was supported by several subsequent studies. For example, cADPR was shown to directly activate RyR2 that was incorporated into lipid bilayers[12]. In HEK293 cells transfected with an islet type RyR, which is a splice variant of the RyR2 gene by alternative splicing of exons 4 and 75, Ca2+ release was enhanced in the presence of 100 μmol/L cADPR, and the effect could be reversed by preincubating with a cADPR antagonist, 8-bromo-cADPR (8-Br-cADPR)[13]. Similarly, cADPR triggered a marked Ca2+ transient in HEK293 cells that stably expressed RyR1 and RyR3, and this Ca2+ transient was abolished by dantrolene, an RyR antagonist[14]. In summary, all these results suggested that RyRs might serve as cADPR receptors (Figure 1).
However, further experiments argued that the action of cADPR on ryanodine receptors might require the assistance of additional protein factors (Figure 1). For example, both calmodulin and FK506 binding protein (FKBP) have been shown to be required for cADPR action[15-20]. These data suggested that cADPR does not directly bind to the ryanodine receptors, but acts through some intermediate proteins, whose definitive identities remain to be established. Zheng et al[21] demonstrated in mouse bladder smooth muscle that Ca2+ release induced by cADPR is actually mediated by FKBP12.6 proteins. Nevertheless, additional research such as genome-wide RNAi screening is needed to elucidate the direct receptor of cADPR.
In addition, growing evidence has shown that cADPR also evokes Ca2+ influx (Figure 1)[22]. It has been shown that cADPR can significantly potentiate the transient receptor potential cation channel M2 (TRPM2) channel activity in a temperature dependent manner[23]. Similarly, we recently synthesized a novel fluorescent caged cADPR analogue, coumarin caged isopropylidene-protected cIDPRE (Co-i-cIDPRE), and found that it is a potent and controllable cell permeant cADPR agonist. Moreover, we demonstrated that uncaging of Co-i-cIDPRE activates RyRs for Ca2+ mobilization and triggers Ca2+ influx via TRPM2[24]. Yet, another experiment showed that TRPM2 is not involved in the effect of another membrane-permeant cADPR agonist, 8-bromo-cyclic IDP-ribose (8-Br-N1-cIDPR), which induced Ca2+ entry in T cells[25]. Thus, the channel that mediates the cADPR induced Ca2+ influx still needs to be elucidated.
ENZYMATIC PATHWAY OF CADPR SYNTHESIS AND DEGRADATION
As mentioned above, the effect of NAD to induce Ca2+ release in sea urchin eggs was shown to result from its enzymatic conversion to cADPR. Subsequently, a similar enzymatic activity was shown to exist in a wide variety of mammalian tissues[26]. The first purified enzyme shown to produce cADPR from NAD was identified in Aplysia and was later named ADP-ribosyl cyclase[27]. Surprisingly, the amino acid sequence of Aplysia ADP-ribosyl cyclase, a soluble 30 kDa protein, showed overall about 68% homology with human CD38, a lymphocyte antigen[28,29]. CD38 was indeed able to catalyze the cyclization of NAD to cADPR in pancreatic beta-cells[30]. Moreover, purified murine CD38 was able to convert NAD to cADPR in an in vitro assay[28]. Later, CD157, a GPI-anchored antigen that shared 30% homology with CD38, was found to have ADP-ribosyl cyclase activity as well[31].
Overall, these ADP-ribosyl cyclases share about 25%-30% sequence identity[32], and this family is likely to grow since researchers have continued to find ADP-ribosyl cyclase activity that is undefined. In addition, it appears that these unknown cyclases function differently in different tissues. For example, an unidentified cardiac ADPR cyclase can be inhibited by micromolar concentrations of Zn2+, which is different from the effects of this cation on CD38 and CD157[33,34]. A similar ADP-ribosyl cyclase that can be inhibited by the divalent cations Zn2+ and Cu2+ has also been found in the disks of bovine retinal rod outer segments[35]. Specific inhibitor based analysis confirmed the existence of a distinct ADP-ribosyl cyclase in the kidney since it responded differently to the inhibitor 4,4’-dihydroxy azobenzene (DHAB) treatment than CD38[36].
So far, CD38 is still considered to be the main mammalian ADP-ribosyl cyclase, as shown by the fact that extracts of tissues from CD38 knockout mice have little if any ADP-ribosyl activity compared to those from wild type mice. When incubated with NAD in vitro, CD38 only produced a small portion of cADPR, while the majority of the product is ADP-ribose; thus CD38 possesses both cyclase and NADase activities. In addition, CD38 can hydrolyze cADPR to ADP-ribose and, other than CD157, it remains the only ADP-ribosyl cyclase that has been identified in mammals[28]. Moreover, CD38 shows another bifunctional character in that it catalyzes the synthesis and hydrolysis of another secondary messenger, NAADP. In this reaction, CD38 catalyzes the exchange of the nicotinamide group of NADP with nicotinic acid under acidic conditions to generate NAADP; furthermore, NAADP can also be hydrolyzed by CD38 to ADPRP (Figure 2)[37,38]. Understanding the structure and function of CD38 is a crucial part of cADPR/Ca2+ signaling research.
STRUCTURE AND ENZYMATIC FUNCTION OF CD38
CD38 is a transmembrane protein, containing a short 21 amino acid residue N-terminal cytoplasmic tail, a 23 amino acid residue hydrophobic transmembrane domain, and a large 256 amino acid residue carboxyl-terminal extracellular domain with four putative glycosylation sites[39]. The extracellular domain of human CD38 with the glycosylation sites removed has been expressed in yeast and purified. Structural analysis of the recombinant CD38 by X-ray crystallography showed that the secondary structure of CD38 is similar to that of the Aplysia cyclase. Overall, both CD38 and the cyclase have similar topology although the cyclase forms dimers in the crystals whereas CD38 does not. The middle cleft of both proteins forms a deep pocket as the active site, with a TLEDTL conserved sequence sitting in the bottom of the pocket[40,41]. Site-directed mutagenesis studies identified Glu226 as the catalytic residue of CD38[42]. Two other residues, Glu146 and Thr221, were found to be essential for the cyclization and hydrolysis activity of CD38, respectively[43]. Upon binding of NAD to the active site, the nicotinamide ring interacts with Trp189 by hydrophobic ring stacking, the 2’ and 3’ hydroxyls of the northern ribose form hydrogen bonds with Glu226, and the ribose diphosphate moiety interacts with amino acids Trp125, Ser126, Arg127, Thr221 and Phe222. Upon cleavage of the nicotinamide ring, the N1 nitrogen of the adenine ring gains access to the anomeric carbon to form a covalent bond and produce cADPR. Alternatively, a water molecule, rather than the adenine ring, attacks the intermediate to form ADP-ribose[44]. In contrast to the formation of cyclic ADP-ribose from NAD, CD38 also catalyzes the formation of NAADP from NADP. Under acidic pH and in the presence of nicotinic acid, the acidic residues in the active site of CD38 are protonated, thereby facilitating the nucleophilic attack of the intermediate of NADP by nicotinic acid to generate NAADP[44].
TOPOLOGY OF CD38
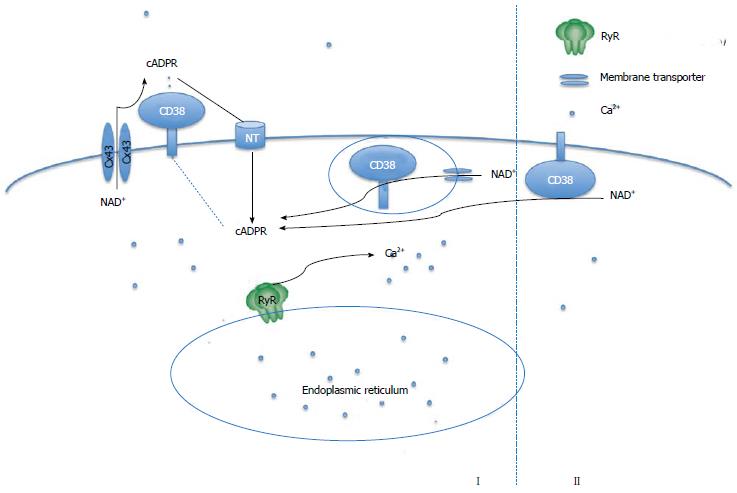
Structurally, CD38 is predicted to be a type-II transmembrane protein with its catalytic C-terminal domain located outside of the cell[39]. This circumstance presents a dilemma because the NAD substrate is located intracellularly whereas the enzyme is positioned extracellularly. If so, cytosolic NAD must be transported out of cells first and then cyclized by CD38 to produce cADPR in the extracellular space. Subsequently, the cADPR product must be transported back into the cytosol to induce Ca2+ release from the ER. This scenario obviously presents a “topological paradox” for the cADPR/Ca2+ signaling cascade. Two general hypotheses have been proposed to solve this puzzle (Figure 3). The first proposal is based on the presence of transporters, such as connexin 43 hemichannels, which allow intracellular NAD to move to the extracellular space so that it is available for access to the catalytic domain of CD38 to be converted to cADPR[45]. The cADPR product is then transferred back to cells via either CD38 or nucleoside transporters[46]. Besides this direct transport model via transporters, Zocchi et al[47] also suggested that CD38 undergoes an extensive internalization through invaginations of the plasma membrane to form endocytotic vesicles, which makes the active site of CD38 intravesicular and able to convert cytosolic NAD into cADPR. CD38 itself is a unidirectional transmembrane transporter of cADPR that mediates the cADPR efflux into the cytoplasm to reach the Ca2+ store, while influx of the cytosolic NAD+ substrate into the endocytotic CD38-containing vesicles is mediated by other transmembrane transporters, such as connexin 43 hemichannels[48]. The internalization of CD38 has been supported by several studies. For example, the internalization of CD38 can be induced by NADP in Chinese hamster ovary (CHO) cells[49] and hemin treatment can induce internalization of CD38 in K562 cells[50]. Rah et al[51] have also demonstrated that association of phospho-nonmuscle myosin heavy chain IIA with tyrosine kinase Lck and CD38 is critical for the internalization and activation of CD38. However, mechanisms regarding the transporter mediated CD38 activation process remain elusive. For example, connexin 43 hemichannels are opened for NAD export only when the cellular Ca2+ is 100 nmol/L; thus this system is unlikely to operate when Ca2+ is elevated above basal levels[45].
Figure 3 Models of CD38 topology.
cADPR: Cyclic adenosine diphosphate ribose; NAD: Nicotinamide adenine dinucleotide; RyR: Ryanodine receptor.
The second proposal offered to explain the topological paradox involves a consideration of the orientation of CD38. Bruzzone and coworkers have shown that treatment of granulocytes with 8-Br-cyclic adenosine monophosphate (cAMP), a cell-permeant analog of cAMP, induced serine phosphorylation of CD38, correlating with a cAMP-dependent intracellular cADPR synthesis[52]. Although the exact location of the phosphorylation sites is unknown, it was predicted to be in the catalytic C-terminal domain that contains multiple serine residues. However, if the catalytic domain of CD38 is phosphorylated by protein kinase A (PKA), this domain should be in the cytosol to directly cyclize NAD, thereby synthesizing cADPR intracellularly. This suggests that although CD38 is believed to be a type-II protein, at least a portion of the total CD38 is expressed as a type-III membrane protein with its C-terminal catalytic domain sitting in the cytosol[53]. Since the number of positive charges that determine the polarity of membrane protein is equal on each side of the CD38 transmembrane segment, studies from protease digestion[54] and electron microscopy[55] showed that the nuclear CD38 might be a type-III membrane protein. Most recently, Zhao et al[56] reported that expression of a cytosolic CD38 protein with deletion of both the N-terminal tail and transmembrane domain results in intact disulfides as well as active enzyme in spite of the cytosolic reductive environment; this result appears to solve the fundamental need of the six disulfides for CD38 enzymatic activity. Based on this finding, they consequently proved the co-expression of type II and type III CD38 on the surface of leukemia HL-60 cells during retinoic acid-induced differentiation and on interferon Υ-activated natural human monocytes and U937 cells[57]. They proposed that the type-III structure may take part in fast cellular responses, while the type-IIstructure may be more suitable for slower and long term responses (Figure 3)[58].
PHYSIOLOGICAL FUNCTIONS OF THE CD38/CADPR/CA2+ PATHWAY
In addition to its role in cADPR production, another function of CD38 is to regulate the NAD level inside cells. It has been well established that NAD plays an essential role in energy metabolism and is involved in diverse signal transduction pathways. A rather surprising finding is that CD38 has a dramatic role in intracellular NAD metabolism. NAD levels in CD38 knockout mice are 10 to 20-fold higher than that in wild-type animals. These results suggest that CD38 is a major regulator of NAD levels in mammalian cells[59].
CD38 was originally identified as a lymphocyte antigen; thus it is not surprising that the CD38/cADPR/Ca2+ pathway plays an important role in inflammatory processes. In an ischemic stroke study, CD38-/- mice produced less monocyte chemoattractant protein-1 (MCP-1) after temporary middle cerebral artery occlusion and had fewer infiltrating macrophages and lymphocytes in the ischemic hemisphere than the wild type mice, whereas the amount of resident microglia was unaltered. The same study also demonstrated that CD38 affected immune cell migration as well as activation, two crucial postischemic inflammatory responses in secondary brain damage, suggesting that CD38 might be a therapeutic target to modulate the inflammatory mechanisms after cerebral ischemia[60]. Recently, Ng et al[61] used intravital multi-photon microscopy to observe the neutrophil granulocyte traffic into the injury site in the dermis of mice and found that the amplification phase, which is the attraction of more neutrophils toward the damage focus after the initial phase of migration by scouting neutrophils, was mediated by cADPR. cADPR and CD38 were also involved in the regulation of leukocyte adhesion and chemotaxis and were required for the deletion of T regulatory cells during inflammation as well[62]. In addition, 8-Br-cADPR, a cADPR antagonist, inhibited the MCP-1 induced Ca2+ increase, reactive oxygen species (ROS) production and apoptosis in human retinal pigment epithelium, suggesting that cADPR is also involved in the inflammatory responses of age-related macular degeneration (AMD)[63].
Recently, we demonstrated that cADPR is important for regulating cell proliferation and neuronal differentiation in PC12 cells. We found that acetylcholine (Ach) activates the CD38/cADPR pathway to induce Ca2+ release and the CD38/cADPR/Ca2+ signaling pathway is required for Ach-stimulated cell proliferation in PC12 cells. Interestingly, inhibition of the cADPR pathway accelerated nerve growth factor (NGF)-induced neuronal differentiation in PC12 cells. On the other hand, CD38 overexpression increased cell proliferation but delayed NGF-induced differentiation. Taken together, we demonstrated that cADPR plays a dichotomic role in regulating proliferation and neuronal differentiation of PC12 cells[64].
Abscisic acid (ABA) is an endogenous stimulator of insulin secretion in human and murine pancreatic beta cells. ABA triggered activation of CD38 and production of cADPR before insulin release, suggesting that CD38 is a regulator of insulin release[65]. Also, CD38 expression and cADPR production induced by ABA were required for ABA-induced upregulation of COX-2 and prostaglandin E2 in human mesenchymal stem cells (MSC) and for chemokinesis of MSC[66].
Since cADPR can activate RyRs for Ca2+ release from ER and can modulate the CICR process, the CD38/cADPR/Ca2+ pathway is predicted to participate in the regulation of cardiac activities, including cardiogenesis and the function of adult cardiac tissue. In fact, ever since the discovery of cADPR, researchers have vigorously explored its role in cardiac tissues. Galione et al[67] showed that application of cADPR through a patch electrode resulted in an increase in Ca2+ transients with a concomitant increase of the magnitude of contraction in guinea-pig cardiac ventricular myocytes, whereas application of the inhibitor 8-amino-cADPR resulted in a significant reduction in contractions and Ca2+ release from the SR. Similarly, in rat cardiac ventricular myocytes, cADPR increased the frequency of Ca2+“sparks”, which may contribute to the increase in subsequent whole-cell Ca2+ transients[68]. In addition, Prakash et al[69] found that microinjection of cADPR into adult rat ventricular myocytes not only induced sustained Ca2+ responses in a concentration dependent manner but also increased the frequency and amplitude of spontaneous Ca2+ waves, which were completely blocked by 8-amino-cADPR, a cADPR antagonist.
Interestingly, cardiac hypertrophy developed only in CD38 knockout male mice. The expression of RyR protein was increased only in female CD38 knockout mice compared with wild type, suggesting that the CD38/cADPR signaling plays an important role in intracellular Ca2+ homeostasis in cardiac myocytes in vivo, although its deficiency was compensated differentially according to gender[70].
cADPR was also shown to be involved in angiotensin II-induced cardiac hypertrophy[71]. In rat cardiomyocytes, angiotensin II evoked a Ca2+ increase via IP3R to activate PKC, which then activated the NAD(P)H oxidase to initiate ROS generation. The ROS together with Ca2+ then activated the ADP-ribose cyclase to synthesize cADPR, which induced a sustained increase of both Ca2+ and ROS and finally led to cardiac hypertrophy[72]. Most recently, Xu et al[73] demonstrated that CD38/cADPR was involved in the regulation of superoxide (O2•-) production in mouse coronary arterial myocytes (CAMs). NAD(P)H oxidase is responsible for O2•- production. Since CD38 can use NAD, an NAD(P)H oxidase product, to produce cADPR and cADPR production can result in an increase in NAD(P)H oxidase activity, the system contains a positive feedback loop. Xu et al[73] found that oxotremorine, a muscarinic type 1 receptor agonist, stimulated intracellular O2•- production in CAMs that was inhibited in CD38 knockout, CD38 knockdown, or nicotinamide-treated (a CD38 inhibitor) cells. On the other hand, direct application of cADPR into CAMs increased intracellular Ca2+ and O2•- production in CD38-/- CAMs. Moreover, CD38 knockout, Nox1 knockdown or Nox4 knockdown blocked oxotremorine-induced contraction in the isolated perfused coronary arteries in mice. Taken together, these data indicate that the CD38/cADPR pathway is an important regulator of Nox-mediated intracellular O2•- production.
The CD38/cADPR/Ca2+ pathway has also been shown to regulate the cardiogenesis process. We recently studied the role of CD38/cADPR/Ca2+ in the cardiomyogenesis of mouse embryonic stem (ES) cells. We found that beating cells appeared earlier and were more abundant in CD38 knockdown embryoid bodies (EBs) than control EBs, and the expression of several cardiac markers was increased significantly in CD38 knockdown EBs than control EBs. Similarly, more cardiomyocytes (CMs) existed in CD38 knockdown or cADPR antagonist-treated EBs compared to control EBs. Conversely, CD38 overexpression in mouse ES cells markedly inhibited CM differentiation. Surprisingly, CD38 knockdown ES cell derived CMs possess the functional properties characteristic of normal ES cell derived CMs. In addition, we found that the CD38/cADPR pathway inhibited the Erk1/2 cascade during CM differentiation of ES cells, and transient inhibition of Erk1/2 blocked the enhancive effects of CD38 knockdown on the differentiation of CM from ES cells. Taken together, we demonstrated that the CD38/cADPR/Ca2+ signaling pathway inhibits the CM differentiation of mouse ES cells[74].
The mechanism underlying cADPR regulation of Ca2+ sparks in cardiomyocyte remains elusive. Zhang et al[19]. showed that cADPR markedly increased the Ca2+ spark frequency in cardiomyocytes isolated from wild type mice, whereas cADPR failed to initiate Ca2+ sparks in cardiomyocytes isolated from FK506 binding protein 12.6 (FKBP12.6) knockout mice. They further demonstrated that cADPR induced FKBP12.6 dissociation from RyRs in a phosphorylation-dependent manner. Yet, another study showed that cAMP signaling is required for the role of cADPR in the beta-adrenergic receptor induced Ca2+increase in rat cardiomyocytes. They found that the isoproterenol-mediated increase of Ca2+ was blocked by pretreatment with 8-Br-cADPR, PKA inhibitor H89 or a high concentration of ryanodine. Moreover, incubation of ventricular lysates with isoproterenol, forskolin or cAMP resulted in activation of ADP-ribosyl cyclase of the ventricular lysates[34]. Interestingly, for comparison, estrogen increased CD38 expression and its cyclase activity, but did not affect its hydrolase activity, while progesterone eliminated the effects of estrogen on CD38 in the rat myometrium[75]. Nevertheless, the mechanism of how the CD38/cADPR is involved in the regulation of cardiac function is still unclear.
CD38/CADPR/CA2+ PATHWAY IN PATHOLOGICAL PROCESSES
The CD38/cADPR/Ca2+ pathway has been suggested to be involved in various pathological processes. For example, CD38 deficiency accelerated diabetes in a non-obese diabetic (NOD) mice model[76]. It has also been shown that both the specific kidney ADP-ribosyl cyclase activity and cADPR production were increased in the kidneys of diabetic mice, suggesting that cADPR plays a role in the renal pathogenesis of diabetes[77]. Down-regulation of CD38 has also been shown to mediate the intermittent hypoxia induced impairment of glucose-induced insulin secretion, suggesting that CD38 plays a role in type 2 diabetes progression[78]. Numerous studies have been attempted to dissect the molecular mechanism of the role of CD38/cADPR/Ca2+ pathway in mediating diabetes in order to identify an alternative therapeutic tool. Tian et al[79] found that the content of cADPR was elevated with concomitant enhanced activity of RyR2 in ventricular myocytes isolated from a type 1 diabetic rat model, suggesting that cADPR mediates type 1 diabetes through regulating the function of RyR2. Chen et al[80] demonstrated that the ATP-gated ion channel P2X7 was required for the acceleration of type 1 diabetes induced by CD38 deficiency. Taken together, knowledge about the role of the CD38/cADPR/Ca2+ pathway in diabetes is accumulating rapidly and there is hope that understanding this pathway will facilitate the development of novel therapeutics for the disease.
The CD38/cADPR/Ca2+ pathway has been associated with inflammatory airway disorders. In human airway smooth muscle (ASM) cells, increased ASM contractility in inflammatory diseases such as asthma was due to enhanced Ca2+ sensitivity to cytokines, which was correlated with the increase of CD38 expression and cADPR level[81]. This increase of CD38 was induced by TNFαvia NFκB and could be inhibited by glucocorticoids[82]. In addition, the CD38/cADPR/Ca2+ pathway also mediated the 2-arachidonoylglycerol induced rapid actin rearrangement during differentiation of HL-60 cells into macrophage-like cells[83], and extracellular NAD+ induced stimulation and recruitment of human granulocytes during the inflammation process[84]. In addition, CD38 was involved in a neuroinflammatory disorder where CD38 expression level was markedly increased in IL-1beta- or HIV-1-activated human astrocytes, whereas CD38 knockdown significantly reduced proinflammatory cytokine and chemokine production in astrocytes[85]. Considering these results, the CD38/cADPR/Ca2+ pathway plays important roles in multiple inflammatory processes.
CONCLUSION
The CD38/cADPR/Ca2+ pathway modulates various processes of cells, including inflammation, insulin secretion, cardiogenesis, cardiac regulation etc. With further investigation, it is likely that other physiological roles of the CD38/cADPR/Ca2+ pathway will be revealed. For example, Yue et al[64] have shown that the CD38/cADPR/Ca2+ pathway delayed the nerve growth factor induced differentiation of PC12 cells; thus it is reasonable to predict that this pathway might also be involved in the regulation of neurogenesis. Using the mouse embryonic stem cell in vitro differentiation model, our preliminary results showed that the CD38/cADPR/Ca2+ pathway does play a role in neural differentiation of mES (unpublished data); however, further research is needed to decipher the underlying mechanism. A comprehensive understanding of the physiological and pathological roles of the CD38/cADPR/Ca2+ pathway in various cellular processes will undoubtedly be helpful for exploiting new molecular therapy targets. In addition, it still remains to be determined whether cADPR binds directly to RyRs or through some unknown proteins. Recently, the long-sought-after store-operated Ca2+ entry proteins were identified using a genome-wide RNAi screen by several groups[86-88]. A similar strategy could be applied to identify novel cADPR-interacting proteins or regulators.
ACKNOWLEDGMENTS
We thank members of the Yue lab for advice on the manuscript.