Published online Feb 26, 2012. doi: 10.4331/wjbc.v3.i2.34
Revised: September 19, 2011
Accepted: September 26, 2011
Published online: February 26, 2012
AIM: To investigate putative biological damage caused by GSM mobile phone frequencies by assessing electromagnetic fields during mobile phone working.
METHODS: Neuron-like cells, obtained by retinoic-acid-induced differentiation of human neuroblastoma SH-SY5Y cells, were exposed for 2 h and 4 h to microwaves at 1800 MHz frequency bands.
RESULTS: Cell stress response was evaluated by MTT assay as well as changes in the heat shock protein expression (Hsp20, Hsp27 and Hsp70) and caspase-3 activity levels, as biomarkers of apoptotic pathway. Under our experimental conditions, neither cell viability nor Hsp27 expression nor caspase-3 activity was significantly changed. Interestingly, a significant decrease in Hsp20 expression was observed at both times of exposure, whereas Hsp70 levels were significantly increased only after 4 h exposure.
CONCLUSION: The modulation of the expression of Hsps in neuronal cells can be an early response to radiofrequency microwaves.
- Citation: Calabrò E, Condello S, Currò M, Ferlazzo N, Caccamo D, Magazù S, Ientile R. Modulation of heat shock protein response in SH-SY5Y by mobile phone microwaves. World J Biol Chem 2012; 3(2): 34-40
- URL: https://www.wjgnet.com/1949-8454/full/v3/i2/34.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v3.i2.34
Several experimental studies on biological models have indicated that mobile phone radiation is not physiologically inert. However, in spite of the results of these studies, our knowledge about the adverse effects of radiofrequency and microwave (RF/MW) radiation on human health, or the biological responses to their exposure, is still limited[1,2].
Several studies have suggested that exposure to RF/MW radiation can damage DNA and gene structures[3,4], and that electromagnetic fields can affect electron distribution and movement in DNA under conditions of oxidative stress[5,6].
Various efficient molecular mechanisms have been developed to protect different organisms against environmental stress, such as exposure to electromagnetic fields. Among these mechanisms, the accumulation of heat-induced chaperones is able to prevent heat damages in native proteins and membranes.
Indeed, when alterations in the cell environment occur, protein folding may be affected. Under these conditions, the activation of specific molecular pathways induces the overexpression and accumulation of the rapidly synthesized heat shock proteins (Hsps), a group of highly conserved proteins that may prevent damaged proteins from immediately precipitating and permanently affecting cell viability[7,8].
There are several different Hsps grouped into different families primarily on the basis of their molecular weights. These include Hsp27, Hsp40, Hsp60, Hsp70, Hsp90 and Hsp110. Hsp70 is one of the major proteins induced by stress in the nervous system, and its neuroprotective roles have been demonstrated both in vivo and in vitro[9]. Hsp27 is a member of the small Hsp family, and has also been shown to have protective effects in response to stress[10,11].
As a result of their high sensitivity to changes in the environment, these proteins are suggested as possible early biomarkers of exposure in ecotoxicological studies. Although many conflicting views have been expressed regarding changes in the Hsp expression induced by electromagnetic fields, no results have been reported in neuronal cells[12].
In this study, we investigated whether exposure to RF/MW radiation may be regarded as an environmental insult by examining the stress response pathway in SH-SY5Y neuroblastoma cells, differentiated to dopaminergic neuron-like cells. In order to better elucidate the relationship between MW radiation and putative changes in cell cultures, we measured the exact frequency values of electromagnetic fields due to mobile phone exposure.
Human neuroblastoma cell line, SH-SY5Y (ATCC CRL-2266), was obtained from American Type Culture Collections (ATCC; Rockville, MD, United States). Fetal bovine serum (FBS), antibiotics, Eagle’s Minimum Essential Medium (MEM), Nutrient Mixture F-12 Ham, all-trans retinoic acid (RA), MTT [3-(4, 5-methylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide], glutamine, sodium pyruvate, PBS, caspase-3 substrate Ac-Ala-Ala-Val-Ala-Leu-Leu-Pro-Ala-Val-Leu-Leu-Ala-Leu-Leu-Ala-Pro-Asp-Glu-Val-Asp-p-nitro-aniline (DEVDpNa), and other chemicals of analytical grade as well as mouse monoclonal antibodies against α-tubulin and Hsp70, and rabbit polyclonal antibodies against Hsp27 and Hsp20 were from Sigma (Milan, Italy). Rabbit polyclonal antibody against caspase-3 was from Chemicon (Millipore, Billerica, MA, United States). Rabbit polyclonal IgG against Ser-78 phosphorylated Hsp27 was from SantaCruz (DBA, Milan, Italy). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG and ECL Chemiluminescence detection kit were from GE Healthcare (Milan, Italy).
SH-SY5Y human neuroblastoma cells were grown in a mixture of two media, MEM and F12, supplemented with 10% heat-inactivated FBS, penicillin (50 U/mL), streptomycin (50 μg/mL), L-glutamine (2 mmol/L) and sodium pyruvate (1 mmol/L) in a 5% CO2/95% air humidified incubator at a fixed temperature of 37 °C. Sub-confluent cells were differentiated for 7 d with 10 μmol/L all-trans-RA (10 mmol/L in DMSO stock solution) in MEM + F12 containing 1% FBS. The medium was renewed every 2 d.
Cultured cells in dishes were placed inside an incubator (37 °C, 5% CO2) for a minimum of 2 h before exposure to allow temperature stabilization.
In order that the experimental setup was similar to the normal conditions of exposure during a conversation by mobile phone, we generated the MW signal by an operational cell phone. The signal provider was Wind-mobile and the exposure source was a mobile phone Samsung E1130, to which a sound was transmitted from another one, as described previously[13] regarding exposure of proteins in D2O solution to mobile phone MWs.
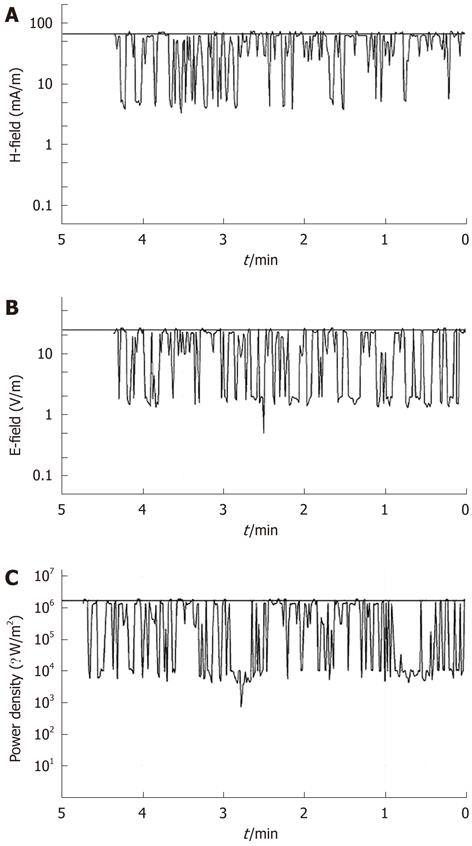
A SRM-3000 device (Selective Radiation Meter) of Narda Safety Test Solutions was used to measure the electromagnetic field due to the mobile phone exposure. It was linked through a Narda cable to a Narda three-axis antenna, covering the frequency range from 75 MHz to 3 GHz, which determined the three spatial components of the electromagnetic field being measured. A spectrum analysis was performed to find the exact frequency values where the highest peaks occurred during MW exposure, and a time analysis was successively conducted to monitor the electromagnetic field during exposure, as shown in Figure 1. Cell cultures were placed at 3 cm from the antenna of the mobile phone, and exposed to MWs. The average specific absorption rate (SAR) that was computed during exposure was 0.086 W/kg.
According to previous studies[14-17], we investigated the effects of continuous exposure to mobile phone MWs. The operating conditions for 2 and 4 h were chosen such that the overall average SAR was < 0.2 W/kg. This value is well within limits specified by ANSI C95.1 and DIN VDE0848 standards, thus, exposure conditions could safely be assumed to be non-thermal.
However, inside the culture medium, temperatures were monitored with accurate Pt100 probes, using a hand-held thermometer (model CTH 6200; Wika Wiegand GmbH and Co., Klingenberg, Germany); no increase of temperature was measured around the cultures during exposure.
To assess RF/MW adverse effects on cell viability, an MTT reduction assay was performed. After electromagnetic field treatment, 10 μmol/L RA differentiated SH-SY5Y cells, grown in 96-well culture plates at a density of 5 × 104 cells/well, were incubated for 4 h with fresh medium containing MTT (0.5 mg/mL) at 37.5 °C. Then, insoluble formazan crystals were dissolved in 100 μL of a 10% (w/v) SDS solution in HCl 0.01 mol/L for 10 min. The OD in each well was evaluated by spectrophotometry. Absorbance was determined at 570 nm using a microplate reader (Tecan Italia, Cologno Monzese, Italy). All experiments were performed in triplicate.
For the preparation of cell lysates, cells were plated in 25-cm2 flasks at a density of 5 × 105 cells/mL. Cells were harvested at 2 h and 4 h following exposure to electromagnetic fields, and homogenized in ice-cold lysis buffer. Protein concentration was evaluated by Bradford method and equal protein aliquots (20 μg) were separated by 8.5% SDS-PAGE and transferred to nitrocellulose membranes. After protein transfer, the membranes were blocked for 1 h with 5% non-fat dried milk. Then, the membranes were probed with mouse monoclonal antibodies against α-tubulin and Hsp70 [respectively diluted 1:10 000 and 1:1000 in Tris-buffered saline-Tween 20 (TBS-T)], rabbit monoclonal antibody against Hsp27 and Hsp20 (diluted both 1:2500 in TBS-T), rabbit polyclonal antibody against p-Hsp27 (diluted 1:1000 in TBS-T) and rabbit polyclonal antibody against caspase-3 (diluted 1:300) followed by incubation with horseradish-peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies (respectively diluted 1:5000, 1:2500, 1:2500, 1:2500 and 1:20 000 in TBS-T). Immunoblots were developed by chemiluminescence according to manufacturer’s instructions.
The bands were scanned and quantified by densitometric analysis with AlphaImager 1200 System (Alpha Innotech; San Leandro, CA, United States), after normalization against α-tubulin.
All values are presented as mean ± SE. Statistical analysis was performed using one-way ANOVA, followed by Newman-Keuls post-hoc test.
Under our experimental conditions, the frequency bands resulted in the MW range related to the mobile phone.
Time analysis mode was carried out to monitor the electromagnetic field strength at the selected channel of 1760 MHz. The resolution bandwidth was chosen as 5 MHz. Time analysis results were transferred to a PC and monitored during the entire exposures.
In line with the guidelines for general public exposure limits to electromagnetic fields of the I.C.N.I.R.P.[18], it was verified that the magnetic field component was < 159 mA/m, resulting values from the expression H < 0.0037 f ½. Time analysis showed that magnetic and electric field components did not reach the values of 66 mA/m and 25 V/m, respectively, during exposure (Figure 1A and B). The relative power density was < 1.7 W/m2 (Figure 1C).
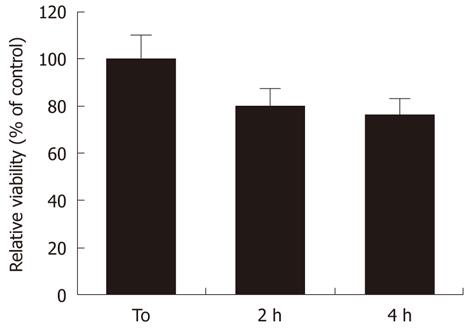
MW radiation affected the cell viability of RA-differentiated SH-SY5Y neuroblastoma cells. We found that MW radiation induced a time-dependent decrease in cell viability as demonstrated by MTT assay. Indeed, MW radiation caused a reduction in cell viability to 79% ± 9.8% and 74% ± 11.1% of sham samples, in the absence of stimulation, at 2 and 4 h, respectively (Figure 2).
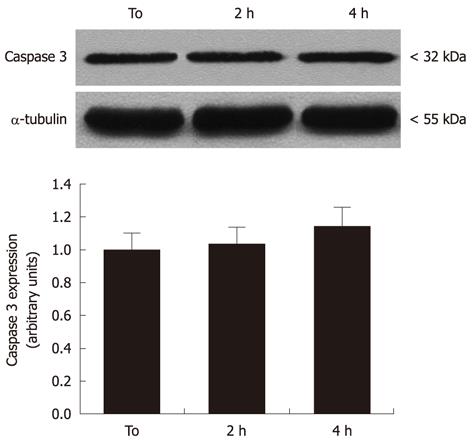
To evaluate the effects of MW radiation on apoptosis induction in neural cells, caspase-3 expression and activity was measured. As shown in Figure 3, there was no evidence for significant pro-caspase-3 increases or pro-caspase-3 cleavage in cell cultures exposed to 2 and 4 h MW radiation, when compared to controls.
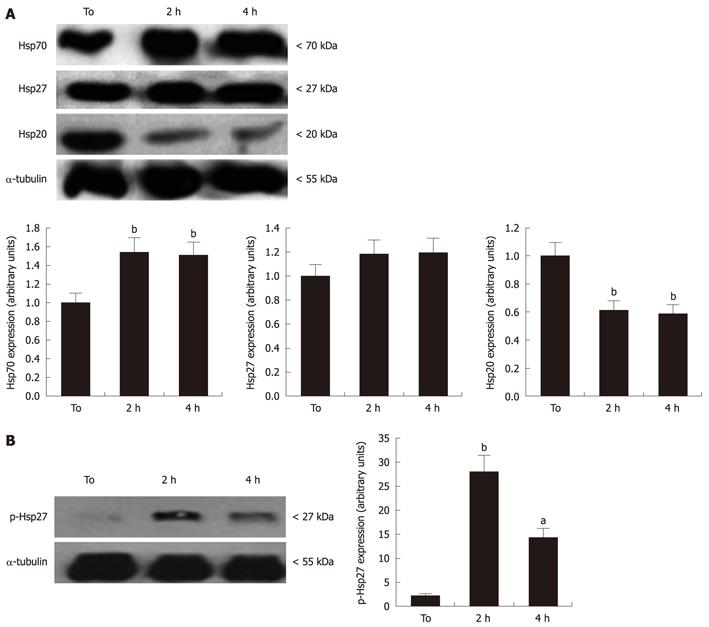
Given the reported ability of Hsps to suppress apoptosis onset in response to several stimuli[10], four independent experiments were carried out to evaluate changes in Hsp20, Hsp27 and Hsp70 expression, by Western blotting assay. As shown in Figure 4A, RA-differentiated SH-SY5Y expressed high basal levels of these Hsps that were affected by exposure to MW radiation. In particular, a 40% and 50% elevation of Hsp70 levels was observed after 2 and 4 h MW radiation exposure, respectively, in comparison with sham cultures (P < 0.05). In contrast, MW radiation exposure strongly decreased the basal levels of Hsp20, achieving about 40% reduction at 4 h that was significantly different in comparison to controls (P < 0.001) (Figure 4A). The expression of Hsp27 was not significantly affected by MW radiation exposure. However, a significant increase in Hsp27 phosphorylation levels, detected by antibody specifically directed against phosphoSer-78, was observed in treated vs untreated cells (Figure 4B). This suggest that, despite the total Hsp27 levels remaining unchanged, cell exposure to MW radiation triggered the activation of Hsp27 through phosphorylation.
Although MW radiation can result in thermal damage in association with high rates of energy absorption, it has been shown that MW toxicity relies on non-thermal effects induced by lower intensities of exposure. Moreover, the cytotoxic effect of MW irradiation differs by cell type and irradiation conditions[19].
In the present study, exposure to MW radiation did not significantly affect viability of neuron-like cells, obtained by RA-driven differentiation of human SH-SY5Y neuroblastoma cells.
Moreover, our results indicate that short-term MW exposure did not upregulate activation of apoptosis executioners, such as caspase-3. These results agree with previous studies that were unable to demonstrate any difference in apoptotic markers such as caspase-3 activity between the RF/MW exposed and unexposed cells[20,21].
Indeed, several in vitro studies have failed to demonstrate that effects of exposure to electromagnetic fields are responsible for cell damage, and results are still variable[22]. Exposure to a GSM-type signal may result in minor effects on brain activity, but such changes have never been found to relate to any adverse health effects. However, the data of the currently available literature still do not allow us to exclude with certainty the possibility of biological effects[23].
In this regard, we here show that the exposure to MW radiation was able to trigger either changes in the expression levels of Hsp20 and Hsp70, or activation of Hsp27 that might be related to non-thermal effects of the electromagnetic field produced by the MW source.
To date, several different observations have been reported showing that non-thermal RF energy induces the heat shock response in various cellular targets. In vitro studies, carried out on different cell systems, have yielded different results, indicating that cell sensitivity to electromagnetic fields may differ according to the cell type. For example, Hsp expression is not changed in fibroblasts and keratinocytes[24], peripheral blood mononuclear cells[25] and neuroblastoma cell line SK-N-SH exposed to RF/MW at low SAR levels[5]. Howeer, Hsp expression has been reported to be upregulated by RF/electromagnetic field exposure in KB cells[26], and levels of the inducible Hsp70C transcript were significantly enhanced after 24 h exposure to GSM signals in human trophoblast cells[27].
In addition, previous observations have demonstrated that RF radiation from mobile phones could be associated with alterations in cancer, via the chronic activation of the heat shock response[28].
Other than involvement in protein misfolding, the altered expression of Hsps plays a critical role in the regulation of different cell processes in response to internal and external stimuli, including apoptotic mechanisms[9,29].
Hsp70, one of the most studied Hsp proteins, has been shown to protect cells against a variety of stresses, including heat shock, anoxia, and heavy metals[30].
The increase in expression of Hsp70 and Hsp27 can be modulated by different components involved in the cellular response against MW exposure. Our data suggest a correlation between constitutive basal levels of Hsp70, Hsp27 and a potential role in protection against apoptotic effectors.
Hsp20 has received special attention because of its localization in muscle and heart[31,32]. Moreover, it has been demonstrated that Hsp20 is also localized in different brain areas and its expression changes in response to hypoxic stress[33].
To the best of our knowledge, this is the first report of a significant relation between Hsp20 levels and MW exposure in in vitro models. On the basis of these results, we hypothesize that, dependent on cell types and different stimuli, the expression levels of Hsp20 could be modulated with progression of cell damage as well in different stress conditions including hypoxic status[34-36]. Our study shows that the reduction in Hsp20 expression could be an early biomarker of cell stress in neuron-like SH-SY5Y cells exposed to MWs.
To summarize, most of the studies on the RF/MW effects on mammalian cells have failed to reach any firm conclusion. Differences from many studies can be ascribed to different cell responses. Indeed, if a cell is subjected to a number of subthreshold stimuli, its threshold also increases, which is an example of accommodation. In most cases, a continuous stimulus produces adaptation, so the number of action potentials elicited by a constant stimulus is usually limited. Some cells accommodate rapidly and only generate a few action potentials in response to a stimulus; others accommodate more slowly; and some do not accommodate at all. The observed changes in Hsp expression can be part of the cell response, however, large well-coordinated multidisciplinary investigations are needed to reach any robust conclusions.
Several experimental studies indicate that exposure to mobile phone radiation can produce effects on organic systems. However, in spite of the significant achieved results, the knowledge about the adverse effects of mobile phone radiation on human health is still limited. Hence the aim of this paper was to investigate some biological responses to exposure to microwaves (MWs) radiation.
When alterations of cell environment occur the protein folding may be affected. Under these conditions, the activation of specific molecular pathways induces the over-expression and accumulation of the rapidly synthesized heat shock proteins (Hsps), proving that mobile phone radiation can alter cellular morphology. The research hotspot is to understand how radiofrequency (RF) and MW radiations can interact with organic biochemical system, and to investigate if they can really produce effects on human health.
Most of the studies on the RF/MW effects on mammalian cells failed to reach any firm conclusion. Differences from many studies can be ascribed to different cell responses. Interestingly, a significant decrease of Hsp20 expression was observed at both indicated times of exposure while Hsp70 levels were significantly increased only after 4h exposure. The modulation of the expression of heat-shock proteins (Hsps) in neuronal cells can be an early response to RF-MWs. Furthermore, MW radiation induced a time-dependent decrease in cell viability as demonstrated by MTT assay. The observed changes in Hsp expression can be part of cell response. However, large well-coordinated multidisciplinary investigations are needed in order to reach any robust conclusion.
When alterations of cell environment occur the protein folding may be affected. Under these conditions, the activation of specific molecular pathways induces the over-expression and accumulation of the rapidly synthesized Hsps. The different behaviour of Hsp20 with respect to other Hsps after exposure of neuronal-like cells to mobile phone radiation lead us to consider it as early biomarker of MW radiation effects in neuronal cells.
RF-MW: RF radiation is a rate of oscillation of electromagnetic field in the range of about 3 kHz to 300 GHz; MW radiation are radio waves with frequencies between 300 MHz and 300 GHz. The energy in an RF/MW current can radiate off a conductor into space as electromagnetic waves (radio-waves); this is the basis of radio technology. Hsps: Hsps are a class of functionally related proteins involved in the folding and unfolding of other proteins. Their expression is increased when cells are exposed to elevated temperatures or other stress. The MTT assay is a colorimetric assay for measuring the activity of enzymes that reduce MTT or close dyes (XTT, MTS, WSTs) to formazan dyes, giving a purple color. A main application allows to assess the viability (cell counting) and the proliferation of cells (cell culture assays).
The studied the effects of mobile phone microwaves on the viability of retinoic acid (RA)-differentiated SH-SY5Y cells and the expression levels of heat shock proteins. The study is relevant to the public concerns of the potential health risk imposed by prolonged usage of mobile phones. However, the experimental designs are largely not faulty, and further clarifications including data analysis are required in order to make the conclusion to be tenable.
Peer reviewers: Lin-Hua Jiang, PhD, Institute of Membrane and Systems Biology, Garstang Building, Faculty of Biological Sciences, University of Leeds, Leeds LS2 9JT, United Kingdom; Yongchang Chang, Associate Professor, Department of Neurobiology, Barrow Neurological Institute/St. Joseph’s Hospital and Medical Center, 350 West Thomas Road, Phoenix, AZ 85013, United States
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
| 1. | O'Carroll MJ, Henshaw DL. Aggregating disparate epidemiological evidence: comparing two seminal EMF reviews. Risk Anal. 2008;28:225-234. [PubMed] |
| 2. | Makker K, Varghese A, Desai NR, Mouradi R, Agarwal A. Cell phones: modern man’s nemesis? Reprod Biomed Online. 2009;18:148-157. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Tice RR, Hook GG, Donner M, McRee DI, Guy AW. Genotoxicity of radiofrequency signals. I. Investigation of DNA damage and micronuclei induction in cultured human blood cells. Bioelectromagnetics. 2002;23:113-126. [PubMed] |
| 4. | Diem E, Schwarz C, Adlkofer F, Jahn O, Rüdiger H. Non-thermal DNA breakage by mobile-phone radiation (1800 MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mutat Res. 2005;583:178-183. [PubMed] |
| 6. | Porath D, Bezryadin A, de Vries S, Dekker C. Direct measurement of electrical transport through DNA molecules. Nature. 2000;403:635-638. [PubMed] |
| 7. | Christians ES, Yan LJ, Benjamin IJ. Heat shock factor 1 and heat shock proteins: critical partners in protection against acute cell injury. Crit Care Med. 2002;30:S43-S50. [RCA] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Rylander MN, Feng Y, Bass J, Diller KR. Thermally induced injury and heat-shock protein expression in cells and tissues. Ann N Y Acad Sci. 2005;1066:222-242. [PubMed] |
| 9. | Brown IR. Heat shock proteins and protection of the nervous system. Ann N Y Acad Sci. 2007;1113:147-158. [PubMed] |
| 10. | Beere HM. Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Invest. 2005;115:2633-2639. [PubMed] |
| 11. | Stetler RA, Gao Y, Signore AP, Cao G, Chen J. HSP27: mechanisms of cellular protection against neuronal injury. Curr Mol Med. 2009;9:863-872. [PubMed] |
| 12. | Zhao TY, Zou SP, Knapp PE. Exposure to cell phone radiation up-regulates apoptosis genes in primary cultures of neurons and astrocytes. Neurosci Lett. 2007;412:34-38. [PubMed] |
| 13. | Calabrò E, Magazù S. Inspections of mobile phone microwaves effects on proteins secondary structure by means of Fourier Transform Infrared Spectroscopy. J Electromagn Anal Appl. 2010;2:607-617. |
| 14. | Gurisik E, Warton K, Martin DK, Valenzuela SM. An in vitro study of the effects of exposure to a GSM signal in two human cell lines: monocytic U937 and neuroblastoma SK-N-SH. Cell Biol Int. 2006;30:793-799. [PubMed] |
| 15. | de Pomerai DI, Smith B, Dawe A, North K, Smith T, Archer DB, Duce IR, Jones D, Candido EP. Microwave radiation can alter protein conformation without bulk heating. FEBS Lett. 2003;543:93-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Karinen A, Heinävaara S, Nylund R, Leszczynski D. Mobile phone radiation might alter protein expression in human skin. BMC Genomics. 2008;9:77. [PubMed] |
| 17. | Achudume A, Onibere B, Aina F, Tchokossa P. Induction of oxidative stress in male rats subchronically exposed to electromagnetic fields at non-thermal intensities. J Electromagn Anal Appl. 2010;2:482-487. |
| 18. | Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). International Commission on Non-Ionizing Radiation Protection. Health Phys. 1998;74:494-522. [PubMed] |
| 19. | Belyaev IY. Non-thermal biological effects of microwaves. Microwave Rev. 2005;11:13-29. |
| 20. | Joubert V, Leveque P, Rametti A, Collin A, Bourthoumieu S, Yardin C. Microwave exposure of neuronal cells in vitro: Study of apoptosis. Int J Radiat Biol. 2006;82:267-275. [PubMed] |
| 21. | Joubert V, Leveque P, Cueille M, Bourthoumieu S, Yardin C. No apoptosis is induced in rat cortical neurons exposed to GSM phone fields. Bioelectromagnetics. 2007;28:115-121. [PubMed] |
| 22. | de Gannes FP, Billaudel B, Taxile M, Haro E, Ruffié G, Lévêque P, Veyret B, Lagroye I. Effects of head-only exposure of rats to GSM-900 on blood-brain barrier permeability and neuronal degeneration. Radiat Res. 2009;172:359-367. [PubMed] |
| 23. | Vanderstraeten J. [GSM fields and health: an updated literature review]. Rev Med Brux. 2009;30:416-424. [PubMed] |
| 24. | Sanchez S, Haro E, Ruffié G, Veyret B, Lagroye I. In vitro study of the stress response of human skin cells to GSM-1800 mobile phone signals compared to UVB radiation and heat shock. Radiat Res. 2007;167:572-580. [PubMed] |
| 25. | Capri M, Scarcella E, Bianchi E, Fumelli C, Mesirca P, Agostini C, Remondini D, Schuderer J, Kuster N, Franceschi C. 1800 MHz radiofrequency (mobile phones, different Global System for Mobile communication modulations) does not affect apoptosis and heat shock protein 70 level in peripheral blood mononuclear cells from young and old donors. Int J Radiat Biol. 2004;80:389-397. [PubMed] |
| 26. | Caraglia M, Marra M, Mancinelli F, D'Ambrosio G, Massa R, Giordano A, Budillon A, Abbruzzese A, Bismuto E. Electromagnetic fields at mobile phone frequency induce apoptosis and inactivation of the multi-chaperone complex in human epidermoid cancer cells. J Cell Physiol. 2005;204:539-548. [PubMed] |
| 27. | Franzellitti S, Valbonesi P, Contin A, Biondi C, Fabbri E. HSP70 expression in human trophoblast cells exposed to different 1.8 Ghz mobile phone signals. Radiat Res. 2008;170:488-497. [PubMed] |
| 28. | French PW, Penny R, Laurence JA, McKenzie DR. Mobile phones, heat shock proteins and cancer. Differentiation. 2001;67:93-97. [PubMed] |
| 29. | Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12:743-761. [PubMed] |
| 30. | Santoro MG. Heat shock factors and the control of the stress response. Biochem Pharmacol. 2000;59:55-63. [RCA] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 386] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 31. | Verschuure P, Tatard C, Boelens WC, Grongnet JF, David JC. Expression of small heat shock proteins HspB2, HspB8, Hsp20 and cvHsp in different tissues of the perinatal developing pig. Eur J Cell Biol. 2003;82:523-530. [PubMed] |
| 32. | Golenhofen N, Perng MD, Quinlan RA, Drenckhahn D. Comparison of the small heat shock proteins alphaB-crystallin, MKBP, HSP25, HSP20, and cvHSP in heart and skeletal muscle. Histochem Cell Biol. 2004;122:415-425. [PubMed] |
| 33. | David JC, Boelens WC, Grongnet JF. Up-regulation of heat shock protein HSP 20 in the hippocampus as an early response to hypoxia of the newborn. J Neurochem. 2006;99:570-581. [PubMed] |
| 34. | Zhu YH, Ma TM, Wang X. Gene transfer of heat-shock protein 20 protects against ischemia/reperfusion injury in rat hearts. Acta Pharmacol Sin. 2005;26:1193-1200. [PubMed] |