Published online Mar 27, 2023. doi: 10.4331/wjbc.v14.i2.52
Peer-review started: October 10, 2022
First decision: January 3, 2023
Revised: January 12, 2023
Accepted: February 13, 2023
Article in press: February 13, 2023
Published online: March 27, 2023
Processing time: 165 Days and 1.1 Hours
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has become a pandemic for the last 2 years. Inflammatory response to the virus leads to organ dysfunction and death. Predicting the severity of inflammatory response helps in managing critical patients using serology tests IgG and IgM.
To investigate the correlation of the serology (IgM and IgG) with reverse tran
We conducted a longitudinal study to correlate serum SARS-CoV-2 immunoglobulin M (IgM) and immunoglobulin G (IgG) serology with clinical outcomes in coronavirus disease 2019 (COVID-19) patients. We analyzed patient data from March to December 2020 for those who were admitted at All India Institute of Medical Sciences Rishikesh. Clinical and laboratory data of these patients were collected from the e-hospital portal and analyzed. A correlation was seen with clinical outcomes and was assessed using MS Excel 2010 and SPSS software.
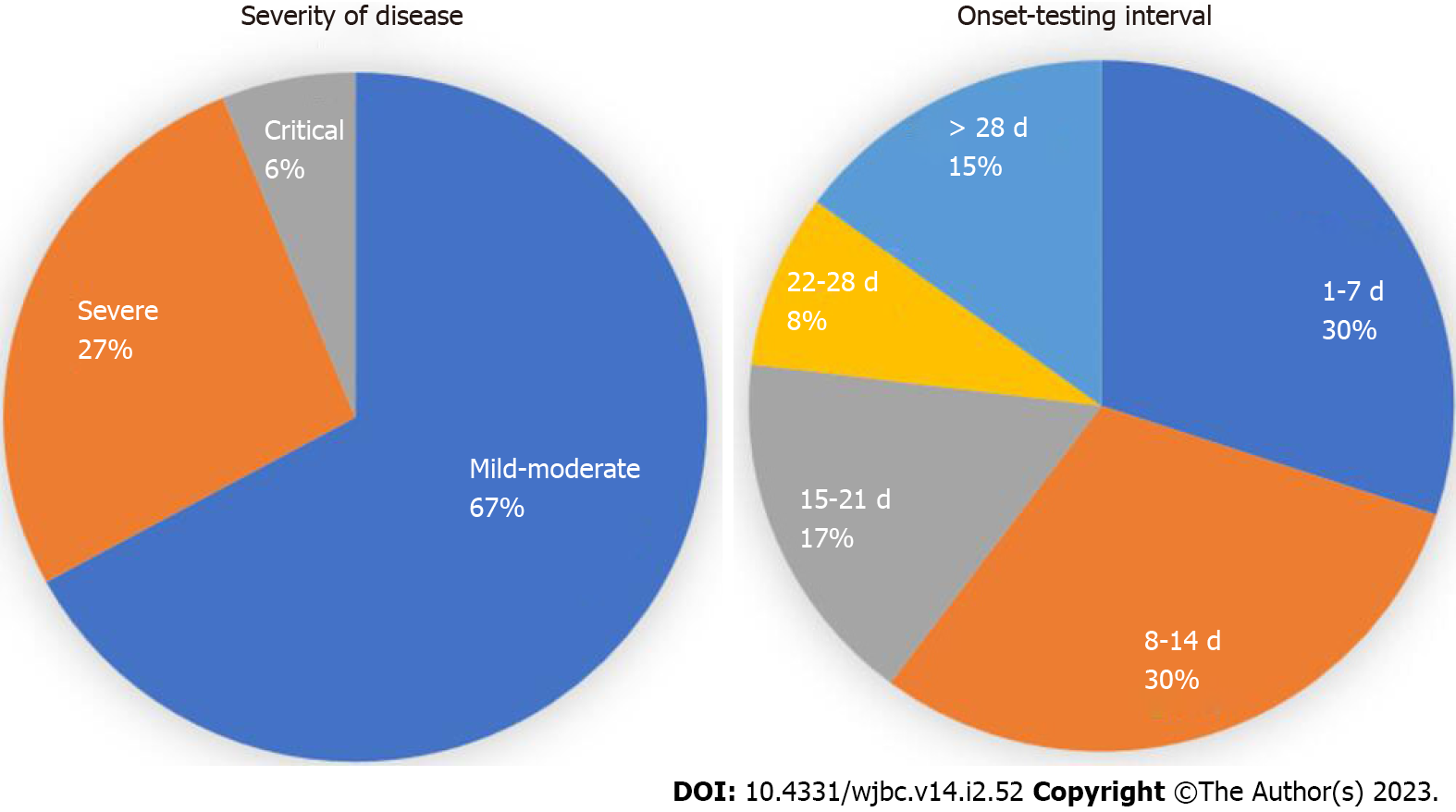
Out of 494 patients, the mean age of patients was 48.95 ± 16.40 years and there were more male patients in the study (66.0%). The patients were classified as mild-moderate 328 (67.1%), severe 131 (26.8%), and critical 30 (6.1%). The mean duration from symptom onset to serology testing was 19.87 ± 30.53 d. In-hospital mortality was observed in 25.1% of patients. The seropositivity rate (i.e., either IgG or IgM > 10 AU) was 50%. IgM levels (AU/mL) (W = 33428.000, P ≤ 0.001) and IgG levels (AU/mL) (W = 39256.500, P ≤ 0.001), with the median IgM/ IgG levels (AU/mL), were highest in the RT-PCR-Positive group compared to RT-PCR-Negative clinical COVID-19. There was no significant difference between the two groups in terms of all other clinical outcomes (disease severity, septic shock, ICU admission, mechanical ventilation, and mortality).
The study showed that serology levels are high in RT-PCR positive group compared to clinical COVID-19. However, serology cannot be useful for the prediction of disease outcomes. The study also highlights the importance of doing serology at a particular time as antibody titers vary with the duration of the disease. In week intervals there was a significant correlation between clinical outcomes and serology on week 3.
Core Tip: Coronavirus disease 2019 (COVID-19) serology levels are high in reverse transcriptase polymerase chain reaction positive group compared to clinical COVID-19. However, serology cannot be useful for the prediction of disease outcomes. The study also highlights the importance of doing serology at a particular time as antibody titres vary with the duration of the disease. In week interval there were significant correlation with clinical outcomes and serology on week 3.
- Citation: Suresh M, Kumar P, Panda PK, Jain V, Raina R, Saha S, Vivekanandhan S, Omar BJ. Correlation of serum SARS-CoV-2 IgM and IgG serology and clinical outcomes in COVID-19 patients: Experience from a tertiary care centre. World J Biol Chem 2023; 14(2): 52-61
- URL: https://www.wjgnet.com/1949-8454/full/v14/i2/52.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v14.i2.52
Coronavirus disease 2019 (COVID-19) has affected almost 581 million people with around 6.4 million deaths as of July 2022 [World Health Organization (WHO)][1]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can infect individuals from different age groups and causes a wide spectrum of disease manifestations ranging from asymptomatic, to mild, moderate to severe symptoms with possible fatal outcomes[2]. Age, sex, pre-existing comorbidities, host genetics as well as host immune response are the key factors determining the outcomes[3]. The reverse transcriptase polymerase chain reaction (RT-PCR) assay is the right method to diagnose SARS-CoV-2. Unfortunately, the sensitivity of the RNA test in the real world is not satisfactory and, false-negative and false-positive cases have also been reported owing to several factors[4]. According to recent WHO case definitions, the RT-PCR negative patients who meet clinical and epidemiological criteria or patients with severe acute respiratory illness who have typical chest imaging features or unexplained anosmia or ageusia are termed as probable COVID-19 patients, better term would be RT-PCR-negative clinical COVID-19[5,6].
Serological tests are increasingly applied for the diagnosis of SARS-CoV-2 infection, though not evidenced by various guidelines. Blood levels of immunoglobulin SARS-CoV-2 immunoglobulin G (IgG) & immunoglobulin M (IgM) are also deployed for evaluating immune responses and confirming the diagnosis in symptomatic patients presenting outside the window of positivity for RT-PCR-based SARS-CoV-2 testing[7]. Few studies have assessed the utility of seroconversion profiles to predict infection severity or outcomes following SARS-CoV-2 infection. A strong association was observed between the magnitude of antibody response and patient survival, disease severity, and fatal outcomes[8]. Furthermore, several studies have documented discrepancies in findings related to the timing of SARS-CoV-2 antibody seroconversion and the onset of symptoms[9-11]. More information about the dynamics of the early humoral immune response is needed to realize the full potential of serological testing for SARS-CoV-2. The dynamics of antibody responses, in COVID-19 patients with different clinical presentations, are still not well-characterized. Such information can help our understanding of the nature of COVID-19 infection and guide patient management.
Here, we studied the seropositivity and kinetics of SARS-CoV-2 IgM and IgG antibodies in blood samples collected between 2 to 85 d post-symptoms onset from a cohort of 493 COVID-19 patients. The objectivity was the correlation of the serology (IgM and IgG) with RT-PCR status, disease severity (mild to critical), intensive care unit (ICU) admission, septic shock, acute kidney injury (AKI), and in-hospital mortality.
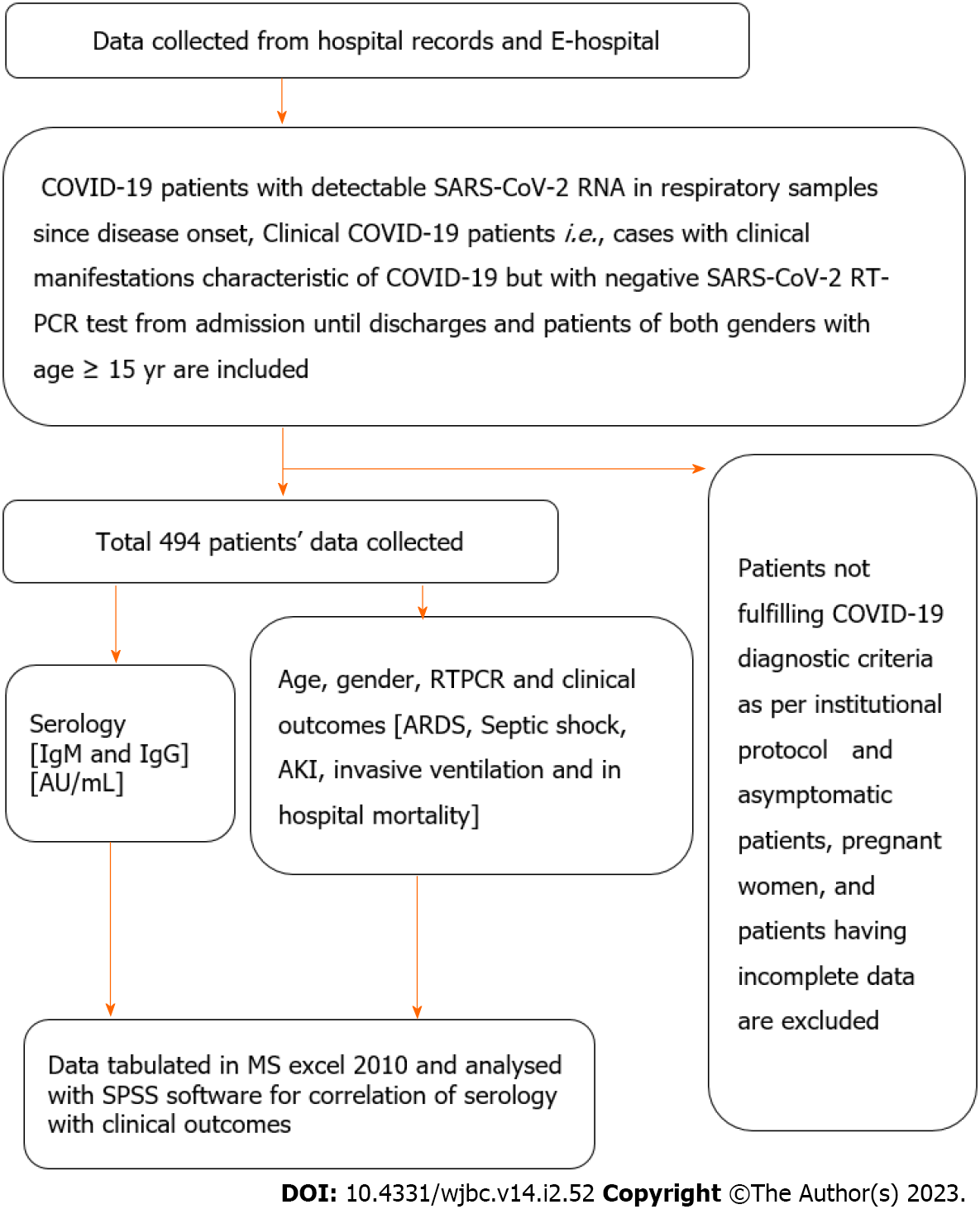
The study was an observational longitudinal study conducted on COVID-19 patients admitted to a tertiary care hospital, All India Institute of Medical Sciences (AIIMS), Rishikesh, India from August 2020 to November 2020. The study was designed according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
COVID-19 patients with detectable SARS-CoV-2 RNA in respiratory samples since disease onset. Clinical COVID-19 patients i.e. cases with clinical manifestations characteristic of COVID-19 but with negative SARS-CoV-2 RT-PCR test from admission until discharge[1,2]. Patients of both genders with age ≥ 15 years. Patients with complete data on serological results available in files.
Patients not fulfilling COVID-19 diagnostic criteria as per institutional protocol. Asymptomatic patients, pregnant women, and patients having incomplete data.
COVID-19 Severity classification: Patients were classified as mild, moderate, severe, and critical according to the WHO guidelines[1].
iFlash-SARS-CoV-2 (Shenzhen Yhlo Biotech Co. Ltd.), a paramagnetic particle-based chemiluminescent immunoassay (CLIA) was used for the determination of IgM and IgG antibodies against SARS-CoV-2 nucleocapsid protein and spike protein. According to the manufacturer's inserts [V1.0 English Fd. 2020–02-20], the IgM and IgG cut-off is 10 AU/mL, i.e., an antibody titer above titer over 10 AU/mL was regarded as positive.
Patients were treated uniformly as per institutional guidelines.
All COVID-19 admitted patients at All India Institute of Medical Sciences, Rishikesh during the above period.
Full information regarding demographic characteristics, the time course of symptoms, time of presentation and testing, presenting symptoms, final diagnosis, treatments received [i.e. oxygen therapy, corticosteroids, ICU admission, invasive ventilation requirement, and dialysis] were collected in master excel. The medical records were further critically reviewed for important missed data.
All consecutive patients during the above period.
The Approval for this study was obtained from the institute ethics committee of AIIMS Rishikesh with approval no CTRI/2020/08/027169.
All the statistical analyses were performed using the statistical package for social sciences (SPSS), Windows version 23 software package (SPSS, CHICAGO, IL, United States). Non-normally distributed continuous variables were presented as medians [interquartile ranges (IQR)]. Differences between non-normally distributed continuous variables were assessed using the Mann–Whitney U test. Categorical variables were presented as counts (%). Differences between categorical variables were assessed using the χ2 or Fisher’s exact tests. A two-sided value of P < 0.05 was considered statistically significant.
As all patients sampling for IgG and IgM was conducted only once, and time to sampling may be an important variable that can confound the study results, we analyzed the association between different clinical outcomes and its association with IgG and IgM levels in a time-dependent manner based on the time interval between symptom onset and IgM and IgG testing. We used Bayesian latent class modeling for the evaluation of the diagnostic performance of RT-PCR, IgM, and IgG tests in COVID-19.
A total of 494 hospitalized patients were enrolled in the study, among them 199 were RT-PCR positive and 294 were clinically diagnosed COVID-19 patients (Table 1, Figures 1 and 2).
| Features | Median [Q1-Q3] or frequency [%] |
| Age (yr) | 50.00 [36.00-61.00] |
| Gender | |
| Male | 326 [66.0] |
| Female | 168 [34.0] |
| IgG (AU/mL) | 7.82 [0.63-57.07] |
| IgG | |
| < 10 AU/mL | 231[50.99] |
| > 10 AU/mL | 224 [49.01] |
| IgM (AU/mL) | 0.96 [0.48-7.68] |
| IgM | |
| < 10 AU/mL | 352 [77.37] |
| > 10 AU/mL | 103 [22.8] |
| RT-PCR | |
| Positive | 199 [40.4] |
| Negative | 294 [59.6] |
| Onset-Testing Interval (d) | 12.00 [7.00-21.00] |
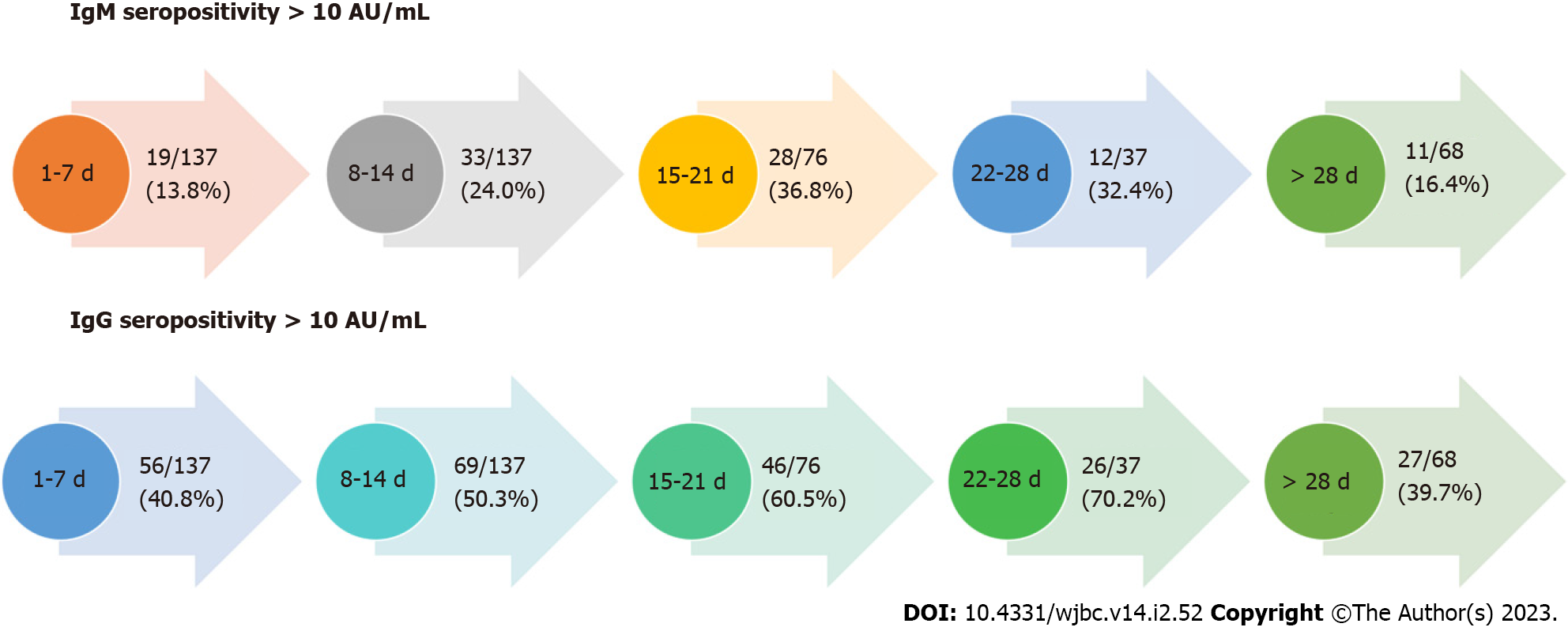
In this cohort of 494 data on seropositivity was available for 455 patients, and the seropositivity rate (i.e. either IgM or IgG > 10 AU) was 247 (54%). Out of these IgM seropositivity was observed in 103/455 (22.63%) and for IgG 224/455 (49.01%). IgM or IgG seropositivity increased to a peak at week 4 and then decreases after 4 wk (> 28 d, Figure 3).
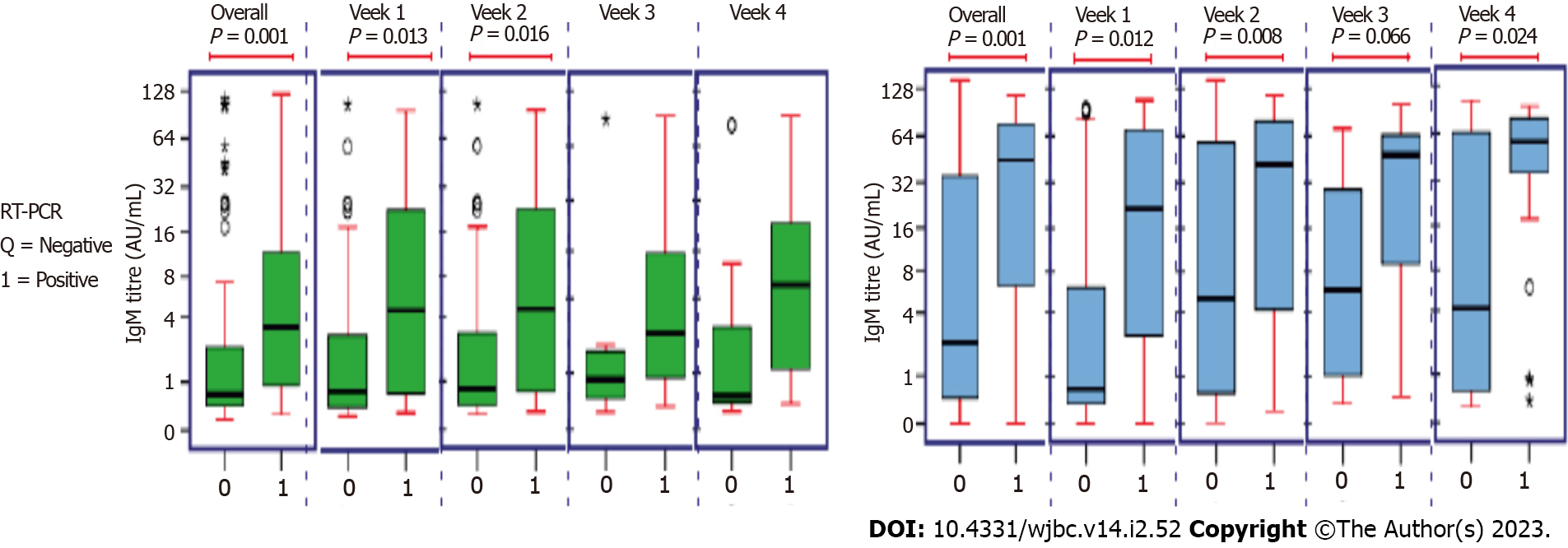
There was a significant difference between the 2 groups in terms of IgM levels (AU/mL) (W = 33428.000, P ≤ 0.001) and IgG levels (AU/mL) (W = 39256.500, P ≤ 0.001), with the median IgM/ IgG levels (AU/mL) being highest in the RT-PCR-Positive group. In all weeks, there was a significant difference between the 2 groups except for week 4 (22-28 Days) there was no significant difference in terms of IgM and IgG levels (AU/mL) (Figure 4).
There was no significant difference between the groups in terms of IgM levels (AU/mL) (χ2 = 2.975, P = 0.395) and IgG levels (χ2 = 2.463, P = 0.482). In week 3, there was a significant difference between the groups in terms of IgM Levels (AU/mL) (χ2 = 7.732, P = 0.021) and IgG levels (AU/mL) (χ2 = 7.707, P = 0.021), with the median IgM and IgG levels (AU/mL) being highest in the critical group. In all the other weeks, there was a significant difference between the 2 groups in terms of IgM and IgG levels (AU/mL) (Supplementary Figure 1).
There was a significant difference between the 4 groups in terms of IgM Levels (AU/mL) (χ2 = 7.985, P = 0.046) and IgG levels (AU/mL) (χ2 = 8.501, P = 0.037). The median IgM levels (AU/mL) were highest in the mild acute respiratory distress syndrome (ARDS) group and median IgG levels (AU/mL) were highest in the Moderate ARDS group.
In all weeks no significant difference between the groups in terms of IgM levels and IgG levels. However, in week 3 there was a significant difference between the 4 groups in terms of IgM levels (AU/mL) (χ2 = 10.837, P = 0.013) and IgG of IgG levels (AU/mL) (χ2 = 9.682, P = 0.021). The median IgM levels (AU/mL) were highest in the Mild ARDS group and the median IgG levels (AU/mL) were highest in the severe ARDS group.
There was a significant difference between the 3 groups in terms of IgM levels (AU/mL) (χ2 = 6.795, P = 0.033), with the median IgM levels (AU/mL) being highest in the Oxygen Therapy: < 6 L/min group. There was no significant difference between the groups in terms of IgG Levels (AU/mL) (χ2 = 4.532, P = 0.104).
There was a significant difference between the 3 groups in terms of IgM levels (AU/mL) in week 1 (χ2 = 6.053, P = 0.048), with the median IgM levels (AU/mL) being highest in the Oxygen Therapy: < 6 L/min group, week 2 (χ2 = 6.392, P = 0.041), with the median IgM levels (AU/mL) being highest in the Oxygen Therapy: > 6 L/min group and Week 3 (χ2 = 6.283, P = 0.043), with the median IgM levels (AU/mL) being highest in the Oxygen Therapy: < 6 L/min group. There was a significant difference between the 3 groups in terms of IgG levels (AU/mL) (χ2 = 8.629, P = 0.013), with the median IgG levels (AU/mL) being highest in the Oxygen Therapy: > 6 L/min group. In all other weeks no significant difference between the groups in terms of IgM levels and IgG levels (Supplementary Figure 2).
There was no significant difference between the groups in terms of IgM Levels (AU/mL) (W = 1191.500, P = 0.168) and IgG levels (AU/mL) (W = 19537.500, P = 0.261).
In all weeks no significant difference between the groups in terms of IgM levels and IgG levels. However, there was a significant difference between the 2 groups in terms of IgM levels AU/mL (W = 1827.000, P = 0.035), with the median IgM levels (AU/mL) being highest in the no Septic Shock group. In week 3 IgG levels (AU/mL) (W = 317.000, P = 0.022), with the median IgG levels (AU/mL) being highest in the Septic Shock group and in > 4 wk (W = 366.000, P = 0.042), with the median IgG levels (AU/mL) being highest in the no Septic Shock group (Supplementary Figure 3).
There was no significant difference between the groups in terms of IgM levels (AU/mL) (W = 23685.000, P = 0.668) and IgG (W = 25763.500, P = 0.157).
In all weeks no significant difference between the groups in terms of IgM levels and IgG levels. However, there was a significant difference between the 2 groups in terms of IgM levels (AU/mL) on week 3 (W = 403.500, P = 0.031) and IgG (W = 460.000, P = 0.038) with the median IgM levels (AU/mL) being highest in the group requiring ICU admission (Supplementary Figure 4).
There was no significant difference between the groups in terms of IgM levels (AU/mL) (W = 20744.500, P = 0.099) and IgG levels (AU/mL) (W = 23067.000, P = 0.460).
In all weeks no significant difference between the groups in terms of IgM levels and IgG levels. However, there was a significant difference between the 2 groups in terms of IgM levels (AU/mL) on week 2 (W = 2070.000, P = 0.035) and > 4 wk (> 28 d) (W = 358.500, P = 0.033), with the median IgM levels (AU/mL) being highest in the no Invasive Ventilation group (Supplementary Figure 5).
There was no significant difference between the groups in terms of IgM Levels (AU/mL) (W = 23261.500, P = 0.425) and IgG levels (AU/mL) (W = 26023.500, P = 0.767).
In all weeks no significant difference between the groups in terms of IgM levels and IgG levels. However, there was a significant difference between the 2 groups in terms of IgM levels (AU/mL) on week 2 (W = 2473.000, P = 0.008), and IgG levels (AU/mL) (W = 2755.500, P = 0.043) with the median IgM/ IgG levels (AU/mL) being highest in the no Acute Kidney Injury group.
There was a significant difference between the 2 groups in terms of IgM levels (AU/mL) (W = 14962.000, P ≤ 0.001), with the median IgM levels (AU/mL) being highest in the no Dialysis group. However, there was no significant difference between the groups in terms of IgG levels (AU/mL) (W = 14553.000, P = 0.206). In all weeks no significant difference between the groups in terms of IgM levels and IgG levels (Supplementary Figure 6).
There was no significant difference between the groups in terms of IgM levels (AU/mL) (W = 21870.000, P = 0.058) and IgG levels (AU/mL) (W = 23088.500, P = 0.738).
In all the weeks there was no significant difference between the groups in terms of IgM levels and IgG levels. However, there was a significant difference between the 2 groups in terms of IgM levels (AU/mL) on week 4 (W = 136.500, P = 0.032) and > 4 wk (> 28 d) (W = 575.500, P = 0.003) with the median IgM levels (AU/mL) being highest in the survival group (Supplementary Figure 7).
The COVID-19 RT-PCR test is the most commonly used molecular test for the diagnosis of COVID-19 infection and is considered the gold standard test[12]. COVID-19 serology has emerged as one of the alternatives for diagnosing the COVID-19 disease. One of the meta-analyses by Chen et al[13] showed that the panel of IgG+ or IgM+ had a sensitivity of almost 79%, followed by IgG+ IgM+/- (73%), IgG+/- IgM+ (68%). Pooled specificities of these tests ranged from 98% to 100%. In our study also, in patients who had clinical COVID-19, almost 50% of patients were seropositive (IgM+ or IgG+).
Various studies have revealed that certain biochemical markers like IL-6 can be used as a prognostic marker for COVID-19[14]. The role of COVID serology in this aspect is less investigated upon. One of the retrospective studies done by Yan et al[15] showed that patients who had severe COVID-19 disease had higher COVID-19 IgG antibodies after 1 year. In this study also patients who were RT-PCR positive had statistically significant COVID-19 antibody serology. Also, Seropositivity for IgG increases as disease severity increases as shown in this study.
In one of the cross-sectional studies done in Iran, the study suggested that the patients who were IgG and IgM-positive had more severe symptoms compared to patients who had negative serology[16]. If we see the relationship between COVID-19 serology and complications, not many studies had been done in the past. This study had shown that patients who had higher COVID-19 IgG levels at three weeks had more severe ARDS and oxygen requirements compared to other patients. We also observed that there was a statistically significant difference in IgG antibody titers between the presence or absence of septic shock at three weeks. A similar trend was seen for ICU admissions and the need for mechanical ventilation. Also, in patients, who developed AKI there was more IgG seropositivity than IgM.
Previous studies by Liu et al[18], 2020, Zhang et al[19] showed that higher antibody (IgM and IgG) levels are seen in patients with severe and critical patients compared to mild-moderate patients[17-19]. Chen et al[20], 2021 study shows similar results as the above studies. However, the study showed antibody titer levels may vary and higher antibody titers were present in some mild-moderate category patients than in severe and critical patients. These findings are due to variations in serology to symptom onset interval[11,20,21]. The study also did not find a statistically significant correlation between antibody tires with AKI, mechanical ventilation, ICU requirement, septic shock, and mortality.
This study shows that higher body titers are associated with poor outcomes at a particular time serology to symptom onset interval. There are some limitations in this study first, it is a retrospective study, most of the patients in the study were not vaccinated and dynamic observation variation in antibody tires with the outcomes studied in a single patient. Second, there are limited patients in severe and critical patients compared to mild and moderate which may lead to biases in the results.
Serology (IgM and IgG) levels are high in RT-PCR positive group compared to clinical COVID-19. However, serology cannot be useful for the prediction of disease outcomes. The study also highlights the importance of doing serology at a particular time as antibody titers vary with the duration of the disease. In week intervals there was a significant correlation between clinical outcomes and serology on week 3.
Predicting the severity of inflammatory response helps in managing critical patients using serology tests immunoglobulin G (IgG) and immunoglobulin M (IgM).
The importance of doing coronavirus disease (COVID) serology at a particular time as antibody titers may vary with the duration of the disease.
The objectivity was the correlation of the serology (IgM and IgG) with reverse transcriptase polymerase chain reaction (RT-PCR) status, disease severity (mild to critical), intensive care unit (ICU) admission, septic shock, acute kidney injury, and in-hospital mortality.
This was a longitudinal study to correlate serum SARS-CoV-2 IgM and IgG serology with clinical outcomes in COVID-19 patients. We analyzed patient data from March to December 2020 for those who were admitted at All India Institute of Medical Sciences Rishikesh. Clinical and laboratory data of these patients were collected from the e-hospital portal and analyzed. A correlation was seen with clinical outcomes and was assessed using SPSS software.
Out of 494 patients, the mean age of patients was 48.95 ± 16.40 years and there were more male patients in the study (66.0%). The patients were classified as mild-moderate 328 (67.1%), severe 131 (26.8%), and critical 30 (6.1%). The mean duration from symptom onset to serology testing was 19.87 ± 30.53 d. In-hospital mortality was observed in 25.1% of patients. The seropositivity rate (i.e., either IgG or IgM > 10 AU) was 50%. IgM levels (AU/mL) (W = 33428.000, P ≤ 0.001) and IgG levels (AU/mL) (W = 39256.500, P ≤ 0.001), with the median IgM/IgG levels (AU/mL), were highest in the RT-PCR-Positive group compared to RT-PCR-Negative clinical COVID-19. There was no significant difference between the two groups in terms of all other clinical outcomes (disease severity, septic shock, ICU admission, mechanical ventilation, and mortality).
The study showed that serology levels are high in RT-PCR positive group compared to clinical COVID-19. The study also highlights the importance of doing serology at a particular time as antibody titers vary with the duration of the disease.
The serology cannot be useful for the prediction of disease outcomes. In week intervals there is a significant correlation between clinical outcomes and serology on week 3.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ait Addi R, Morocco; Hasan A, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | COVID-19 Treatment Guidelines Panel. National Institutes of Health; 2022. Coronavirus Disease 2019 [COVID-19] Treatment Guidelines; August 8, 2022 [cited August 16, 2022]. Available from: https://www.COVID-1919treatmentguidelines.nih.gov/. |
| 2. | COVID-19 [Internet]. CDC; 2020. Assessing Risk Factors; November 30, 2020 [Cited August 16, 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/COVID-19-data/investigations-discovery/ as-sessing-risk-factors.html. |
| 3. | Udwadia ZF, Tripathi AR, Nanda VJ, Joshi SR. Prognostic Factors for Adverse Outcomes in COVID-19 Infection. J Assoc Physicians India. 2020;68:62-66. [PubMed] |
| 4. | Sule WF, Oluwayelu DO. Real-time RT-PCR for COVID-19 diagnosis: challenges and prospects. Pan Afr Med J. 2020;35:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 5. | Lekpa FK, Njonnou SRS, Balti E, Luma HN, Choukem SP; University of Dschang Taskforce for the Elimination of COVID-19 (UNITED#COVID-19). Negative antigen RDT and RT-PCR results do not rule out COVID-19 if clinical suspicion is strong. Lancet Infect Dis. 2021;21:1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Gupta-Wright A, Macleod CK, Barrett J, Filson SA, Corrah T, Parris V, Sandhu G, Harris M, Tennant R, Vaid N, Takata J, Duraisingham S, Gandy N, Chana H, Whittington A, McGregor A, Papineni P. False-negative RT-PCR for COVID-19 and a diagnostic risk score: a retrospective cohort study among patients admitted to hospital. BMJ Open. 2021;11:e047110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Gong F, Wei HX, Li Q, Liu L, Li B. Evaluation and Comparison of Serological Methods for COVID-19 Diagnosis. Front Mol Biosci. 2021;8:682405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Rabaan AA, Al-Ahmed SH, Garout MA, Al-Qaaneh AM, Sule AA, Tirupathi R, Mutair AA, Alhumaid S, Hasan A, Dhawan M, Tiwari R, Sharun K, Mohapatra RK, Mitra S, Emran TB, Bilal M, Singh R, Alyami SA, Moni MA, Dhama K. Diverse Immunological Factors Influencing Pathogenesis in Patients with COVID-19: A Review on Viral Dissemination, Immunotherapeutic Options to Counter Cytokine Storm and Inflammatory Responses. Pathogens. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 9. | Masiá M, Telenti G, Fernández M, García JA, Agulló V, Padilla S, García-Abellán J, Guillén L, Mascarell P, Asenjo JC, Gutiérrez F. SARS-CoV-2 Seroconversion and Viral Clearance in Patients Hospitalized With COVID-19: Viral Load Predicts Antibody Response. Open Forum Infect Dis. 2021;8:ofab005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | O Murchu E, Byrne P, Walsh KA, Carty PG, Connolly M, De Gascun C, Jordan K, Keoghan M, O'Brien KK, O'Neill M, Smith SM, Teljeur C, Ryan M, Harrington P. Immune response following infection with SARS-CoV-2 and other coronaviruses: A rapid review. Rev Med Virol. 2021;31:e2162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 11. | Phipps WS, SoRelle JA, Li QZ, Mahimainathan L, Araj E, Markantonis J, Lacelle C, Balani J, Parikh H, Solow EB, Karp DR, Sarode R, Muthukumar A. SARS-CoV-2 Antibody Responses Do Not Predict COVID-19 Disease Severity. Am J Clin Pathol. 2020;154:459-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | WHO Coronavirus Disease [COVID-19] Technical Guidance: Laboratory Testing for 2019-nCoV in Humans. 2020. Available from: https://www.who.int/emergencies/diseases/novelcoronavirus-2019/technical-guidance/Laboratory-guidance. |
| 13. | Chen M, Qin R, Jiang M, Yang Z, Wen W, Li J. Clinical applications of detecting IgG, IgM or IgA antibody for the diagnosis of COVID-19: A meta-analysis and systematic review. Int J Infect Dis. 2021;104:415-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Jain V, Kumar P, Panda PK, Suresh M, Kaushal K, Mirza AA, Raina R, Saha S, Omar BJ, Subbiah V. Utility of IL-6 in the Diagnosis, Treatment and Prognosis of COVID-19 Patients: A Longitudinal Study. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Yan X, Chen G, Jin Z, Zhang Z, Zhang B, He J, Yin S, Huang J, Fan M, Li Z, Chen F, Zeng Y, Han X, Zhu Y. Anti-SARS-CoV-2 IgG levels in relation to disease severity of COVID-19. J Med Virol. 2022;94:380-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Haghi Ashtiani MT, Sadeghi Rad P, Asnaashari K, Shahhosseini A, Berenji F, Mamishi S. Role of serology tests in COVID-19 non-hospitalized patients: A cross-sectional study. PLoS One. 2022;17:e0266923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30097] [Article Influence: 6019.4] [Reference Citation Analysis (3)] |
| 18. | Liu X, Zheng X, Liu B, Wu M, Zhang Z, Zhang G, Su X. Serum IgM against SARS-CoV-2 correlates with in-hospital mortality in severe/critical patients with COVID-19 in Wuhan, China. Aging (Albany NY). 2020;12:12432-12440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Zhang B, Zhou X, Zhu C, Song Y, Feng F, Qiu Y, Feng J, Jia Q, Song Q, Zhu B, Wang J. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front Mol Biosci. 2020;7:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 342] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 20. | Chen H, Qin R, Huang Z, He L, Luo W, Zheng P, Huang H, Wang H, Sun B. Characteristics of COVID-19 Patients Based on the Results of Nucleic Acid and Specific Antibodies and the Clinical Relevance of Antibody Levels. Front Mol Biosci. 2020;7:605862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Yates JL, Ehrbar DJ, Hunt DT, Girardin RC, Dupuis AP 2nd, Payne AF, Sowizral M, Varney S, Kulas KE, Demarest VL, Howard KM, Carson K, Hales M, Ejemel M, Li Q, Wang Y, Peredo-Wende R, Ramani A, Singh G, Strle K, Mantis NJ, McDonough KA, Lee WT. Serological analysis reveals an imbalanced IgG subclass composition associated with COVID-19 disease severity. Cell Rep Med. 2021;2:100329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |