Published online Mar 27, 2023. doi: 10.4331/wjbc.v14.i2.40
Peer-review started: August 28, 2022
First decision: November 30, 2022
Revised: December 8, 2022
Accepted: February 2, 2023
Article in press: February 2, 2023
Published online: March 27, 2023
Processing time: 205 Days and 11.2 Hours
Understanding the humoral response pattern of coronavirus disease 2019 (COVID-19) is one of the essential factors to better characterize the immune memory of patients, which allows understanding the temporality of reinfection, provides answers about the efficacy and durability of protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and consequently helps in global public health and vaccination strategy. Among the patients who became infected with SARS-CoV-2, the majority who did not progress to death were those who developed the mild COVID-19, so understanding the pattern and tempo
To investigate the temporal pattern of humoral response of specific immunoglobulin G (IgG) in mild cases of COVID-19.
Blood samples from 191 COVID-19 real-time reverse transcriptase-polymerase chain reaction (RT-qPCR)-positive volunteers from the municipality of Toledo/ Paraná/Brazil, underwent two distinct serological tests, enzyme-linked immunosorbent assay, and detection of anti-nucleocapsid IgG. Blood samples and clinicoepidemiological data of the volunteers were collected between November 2020 and February 2021. All assays were performed in duplicate and the manufacturers' recommendations were strictly followed. The data were statistically analyzed using multiple logistic regression; the variables were selected by applying the P < 0.05 criterion.
Serological tests to detect specific IgG were performed on serum samples from volunteers who were diagnosed as being positive by RT-qPCR for COVID-19 or had disease onset in the time interval from less than 1 mo to 7 mo. The time periods when the highest number of participants with detectable IgG was observed were 1, 2 and 3 mo. It was observed that 9.42% of participants no longer had detectable IgG antibodies 1 mo only after being infected with SARS-CoV-2 and 1.57% were also IgG negative at less than 1 mo. At 5 mo, 3.14% of volunteers were IgG negative, and at 6 or 7 mo, 1 volunteer (0.52%) had no detectable IgG. During the period between diagnosis by RT-qPCR/symptoms onset and the date of collection for the study, no statistical significance was observed for any association analyzed. Moreover, considering the age category between 31 and 59 years as the exposed group, the P value was 0.11 for the category 31 to 59 years and 0.32 for the category 60 years or older, showing that in both age categories there was no association between the pair of variables analyzed. Regarding chronic disease, the exposure group consisted of the participants without any comorbidity, so the P value of 0.07 for the category of those with at least one chronic disease showed no association between the two variables.
A temporal pattern of IgG response was not observed, but it is suggested that immunological memory is weak and there is no association between IgG production and age or chronic disease in mild COVID-19.
Core Tip: This study suggests that no precise temporal pattern of humoral immunoglobulin G (IgG) response could be established. This fact suggests the absence of a robust immunological memory in mild cases of coronavirus 2019 disease, and furthermore, due to the lack of association between IgG response and age group, in mild cases of the disease the elderly do not appear to be a risk group for infection.
- Citation: Pilati Campos IM, Marques M, Peiter GC, Brandalize APC, dos Santos MB, de Melo FF, Teixeira KN. Temporal pattern of humoral immune response in mild cases of COVID-19. World J Biol Chem 2023; 14(2): 40-51
- URL: https://www.wjgnet.com/1949-8454/full/v14/i2/40.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v14.i2.40
Coronavirus disease 2019 (COVID-19), a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in Wuhan, China, in December 2019 and spread worldwidevery quickly, causing an unprecedented pandemic that impacted healthcare systems, the economy, politics, and social organization. Patients with COVID-19 may be asymptomatic or present with critical illness, and with symptoms including fever, cough, sore throat, malaise and myalgia. Some patients may experience gastrointestinal symptoms such as anorexia, nausea, and diarrhea[1].
Asymptomatic patients can be assumed to be uninfected, and thus they can be the focus of new outbreaks of the infection by transmitting the virus to healthcare workers or individuals with risk factors[1,2]. According to some studies, risk factors for complications of COVID-19 include advanced age, cardiovascular disease, chronic lung disease, diabetes, obesity and immunosuppression[3-7].
Therefore, the influence of comorbidities on the immune response profile and disease susceptibility has been widely discussed. The main comorbidities involved in this study are diseases of the immune system, such as asthma, rheumatoid arthritis (RA) and autoimmune gastritis (AIG). Although patients with severe asthma have been associated with a higher risk of COVID-19-related death[8], studies have indicated that the disease was not statistically associated with increased risk of infection or being hospitalized due to the disease[9,10].
Regarding the gastrointestinal system diseases, specifically AIG, the data in the literature indicate no relationship between autoimmunity and increased susceptibility to SARS-CoV-2[11]. On the other hand, in relation to RA, a study of patients with rheumatic diseases (91.89% were autoimmune with 72.97% of RA, and 18.92% of systemic lupus erythematosus) showed that cardiovascular manifestations caused by COVID-19 appeared more frequently in patients with rheumatic diseases[12].
According to the Chinese data, 81% of individuals infected with SARS-CoV-2 developed mild or moderate COVID-19, 14% developed severe disease, and only 5% developed critical disease[13]. Therefore, it is critical to study asymptomatic cases and those with mild symptoms, as they represent a risk of COVID-19 spreading. One example is the infection of 71 individuals originated from an asymptomatic case in China[14]. Furthermore, it has been evidenced that mild cases of COVID-19 also cause an increase in the need for primary health care, potentially lasting up to 3 mo[15], which can further burden the health care system.
Another point that emphasizes the importance of studying mild cases is the sequelae left by the virus. SARS-CoV-2 uses the angiotensin-converting enzyme 2 (ACE2) as a functional receptor to invade host cells. This enzyme's main function is the regulation of angiotensin 2 and is highly expressed in the lungs, intestine and kidneys[16]. This gives the pathogen a high capacity of dissemination[16,17]. Thus, although the virus mainly infects the respiratory system, its systemic dissemination can occur, affecting several organs[17].
As respiratory sequelae, besides lung damage, the main consequences observed were alterations in taste and smell, such as anosmia, hyposmia, ageusia or dysgeusia[17,18]. Such symptoms were mainly associated with the appearance of symptomatic manifestations of COVID-19, since the gateway of the virus into the body are both the oral and nasal cavities, the sites whose epithelial tissue presents receptors for these senses[17].
Furthermore, one study demonstrated the high presence of ACE2 in biopsies of the olfactory mucosa, and the enzyme is mainly present in Bowman's glands[16]. In addition, other reported sequelae involve the cardiovascular system, such as heart attack and pulmonary thromboembolism, as well as kidney disease, liver damage, and even neurological changes[17].
As for the differences in immune response between mild and severe cases of COVID-19, it was observed that patients with critical illnesses showed a higher and earlier immunoglobulin G (IgG) and immunoglobulin A (IgA) response against SARS-CoV-2, as well as high viral neutralization. On the other hand, 75% of patients with mild symptoms developed antibodies and these showed low or even no viral neutralization rate[19,20].
Accordingly, this study set out to evaluate the temporal pattern of IgG anti-SARS-CoV-2 response in mild cases of COVID-19. To this end, the study analyzed data from patients presenting with the disease between September 2020 and January 2021, prior to the introduction of vaccines.
This study was approved by the Ethics Committee for research with humans of the Setor de Ciências da Saúde-Universidade Federal do Paraná (UFPR)/Brazil (Protocol no. 35872520.8.0000.0102) and all participants signed an informed consent form. Volunteers older than 18 years (n=393) were tested for COVID-19 by real-time reverse transcriptase-polymerase chain reaction (RT-qPCR) and 191 were positive. Blood samples and clinicoepidemiological data from the volunteers were collected from November 2020 to February 2021. The clinicoepidemiological data were collected by means of a semi-structured questionnaire. Venous blood was collected from each volunteer in a tube without anticoagulant and the serum was separated by centrifugation at 2000 rpm for 2 min. At this time, COVID-19 vaccines were not yet being applied in Brazil. The 191 individuals diagnosed as being positive for COVID-19 by RT-qPCR were included in this study; all were residents of the municipality of Toledo/Paraná/Brazil. Serum samples were subjected to two different serological tests by enzyme-linked immunosorbent assay, a commercial test-Allserum EIA COVID19 IgG (MBiolog Diagnostics) and a test developed by UFPR/Setor Litoral[21]. Both tests detected anti-nucleocapsid IgG, the secondary antibody was bound to Horseradish peroxidase, chromogenic substrate was Tetramethylbenzidine and absorbance reading was performed at 450 nm. All assays were performed in duplicate and the manufacturers' recommendations were strictly followed; assays were analyzed by UV/Vis Multiskan Sky spectrometer (Thermo Fisher Scientific Inc.).
A database containing clinicoepidemiological characteristics and serological results was prepared. To perform multiple logistic regression, variables were selected by applying the P < 0.05 adjusted odds ratio criterion and using the Maximum likelihood estimation. The final model was obtained after testing for all possible multiple interactions with subsequent verification of model fit by the Hosmer & Lemeshow method. A receiver operating characteristic (ROC) curve was done to evaluate the ability of the model created to represent reality.
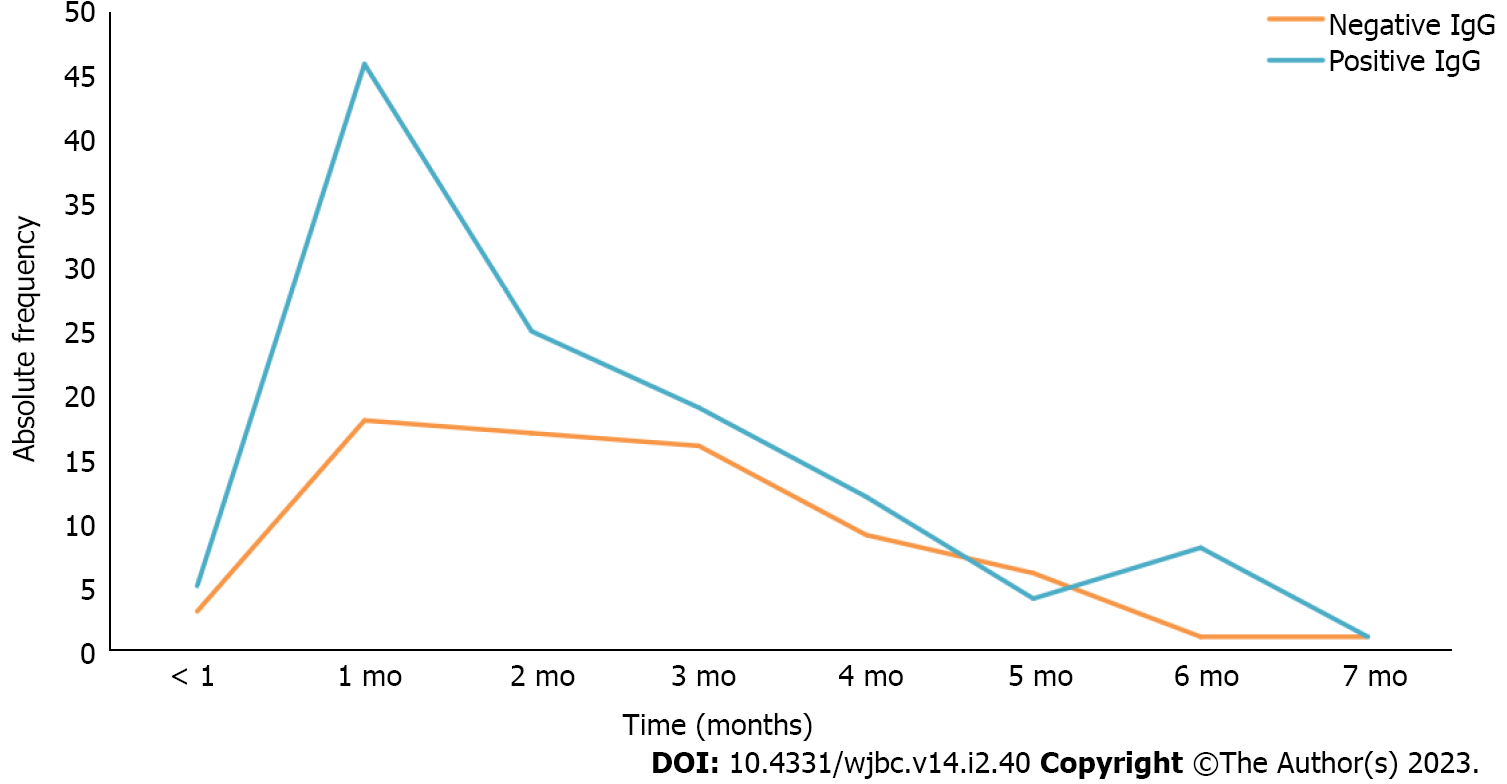
Serum samples from the volunteers spanned a time interval of less than 1 mo up to 7 mo between positive diagnosis for COVID-19/symptoms onset and serological testing for specific IgG. The majority of study participants (62.83%) had detectable IgG for SARS-CoV-2, of which 37.17% had no antibodies at the time of blood collection. Furthermore, as shown in Figure 1, regarding the residence time of IgG detectable by serological testing, only 1 volunteer (0.52%) was IgG positive for 7 mo. The time periods where the highest number of participants with detectable IgG was observed were 1, 2 and 3 mo, with 46 (24%), 25 (13.08%) and 19 (9.94%), respectively. Twelve participants (6.28%) had IgG antibodies at the end of 4 mo, 4 participants (2.09%) remained IgG positive at the end of 5 mo, 8 (4.19%) had IgG for 6 mo, and 5 (2.61%) were IgG positive for less than 1 mo.
Figure 1 also shows that 18 individuals (9.42%) no longer had detectable anti-SARS-CoV-2 IgG antibodies one month after being infected with SARS-CoV-2 and 3 (1.57%) were negative at less than 1 mo. Seventeen volunteers (8.90%) had no detectable anti-SARS-CoV-2 IgG after 2 mo; the same was observed with 16 volunteers (8.37%) at the end of 3 mo and 9 participants (4.71%) at the end of 4 mo. At 5 mo, 6 volunteers (3.14%) had negative anti-SARS-CoV-2 IgG, and at 6 or 7 mo 1 volunteer (0.52%) had no detectable IgG.
As shown in Table 1, regarding the period of time elapsed between diagnosis by RT-qPCR/symptoms onset and the date of blood collection for the study, no statistical significance was observed between the variables.
| Variable | n | % | P value | OR adjusted | 95%CI | |
| IgG | ||||||
| Negative | 71 | 37.17 | ||||
| Positive | 120 | 62.83 | ||||
| Age | ||||||
| 18 to 30 yr | 57 | 29.84 | ||||
| 31 to 59 yr | 115 | 60.21 | 0.11 | 1.26 | (0.89-3.48) | |
| 60 yr or + | 19 | 9.95 | 0.32 | 1.92 | (0.60-6.60) | |
| Time | ||||||
| < 1 mo | 8 | 4.19 | 0.09 | 0.79 | (0.16-3.83) | |
| 1 mo | 64 | 33.51 | ||||
| 2 mo | 42 | 21.99 | 0.18 | 0.56 | (0.24-1.30) | |
| 3 mo | 35 | 18.32 | 0.13 | 0.50 | (0.20-1.22) | |
| 4 mo | 21 | 10.99 | 0.36 | 0.61 | (0.21-1.75) | |
| 5 mo | 10 | 5.24 | 0.09 | 0.29 | (0.07-1.21) | |
| 6 mo | 9 | 4.71 | 0.25 | 3.55 | (0.41-30.69) | |
| 7 mo | 2 | 1.05 | 0.39 | 0.27 | (0.01-5.14) | |
| Chronic disease | ||||||
| Not | 135 | 70.68 | ||||
| Yes | 56 | 29.32 | 0.07 | 2.03 | (0.94-4.40) | |
| Number of chronic diseases | ||||||
| 0 | 135 | 70.68 | ||||
| 1 | 45 | 23.56 | ||||
| 2 | 10 | 5.24 | ||||
| 3 | 1 | 0.52 | ||||
Regarding age, most participants (115 or 60.21%) were between 31 and 59 years old; 57 (29.84%) were between 18 and 30 years old, and 19 (9.95%) participants were 60 years old or older. Considering the age group between 31 and 59 years as the exposed group, the P value was 0.11 for the age group of 31 - 59 years and 0.32 for the group of 60 years or older, showing that there was no association between the pair of variables analyzed.
About the presence of chronic diseases, 135 (70.68%) participants had no comorbidity. On the other hand, 45 (23.56%) individuals had one chronic disease, while 10 (5.24%) had two and one (0.52%) participant had three. Regarding this variable, the exposure group considered was the participants without any comorbidity, so that the P value of 0.07 for those with at least one chronic disease showed no association between the two variables studied.
The statistical model showed adequate fit evaluated by the Hosmer & Lemeshow method (χ2 = 3.656; GL = 8; P = 0.887). The area under the ROC curve showed that the estimated probability model could predict approximately 67.59% of the factors associated with the outcome.
The viral proteins play a striking role in diagnosing COVID-19 and monitoring the production of antibodies against the coronavirus by serological tests. In this regard, tests for COVID-19 can be either nucleic acid amplification tests (RT-qPCR) or serological tests. While RT-qPCR is recommended for active coronavirus infection, serological tests are recommended for antibody response [immunoglobulin M (IgM) and IgG][22]. Thus, serologic testing and antibody analysis are useful to verify whether there has been previous exposure to the virus and to quatify the patient's humoral immunity levels and types of antibodies produced[23].
Therefore, the production of specific antibodies is essential, since they are responsible for effective protection against the severe forms of the disease, even though there are other cells, such as TCD4+ and TCD8+, that act in the immunity process[24]. In addition, IgM antibodies provide the first line of defense during infections, while IgG production provides immunity and long-term memory[25].
The level of antibody production depends on the elapsed time of infection, the severity of the disease, the viral load to which the patient has been exposed, and individual patient characteristics such as age, sex, and pathogen elimination[26]. There may also be variations in the detection of antibody levels according to the sensitivity of the serological test used.
Regarding the elapsed time of infection, while IgM antibodies can be detected at about five days of infection and reach higher rates between two and three weeks of illness, IgG antibodies begin to be produced about 14 d after the symptoms onset and individuals with more severe disease have higher antibody levels[27,28]. Regarding COVID-19, the observation period for most studies on the production of specific anti-SARS-CoV-2 antibodies is 12 wk, and it is still unclear how antibody titers may change in subsequent periods[29].
In a study conducted in Wuhan, China, after confirmation of coronavirus infection by RT-qPCR, asymptomatic individuals were recruited to detect levels of anti-SARS-CoV-2 antibodies. Of a total of 63 individuals with asymptomatic infections, 38.1% (24 patients) produced no antibodies and 61.9% (39 patients) produced only small titers. Six (11.8%) out of 51 patients with mild symptoms, produced no antibodies and 88.2% (45 patients) produced higher levels of antibodies when compared to asym
In the same study, in asymptomatic individuals, antibody production started seven days after exposure, peaked between 10 and 25 d, and decreased rapidly thereafter. On the other hand, in individuals with mild symptoms, one day after the onset of symptoms, antibodies were already produced, even at a low level, and titration increased persistently up to 22 d, maintaining high levels for at least 65 d[30].
In a study of 164 participants in Singapore, 19 patients (12%) did not develop neutralizing antibodies against SARS-CoV-2; 44 patients (27%) produced antibodies early (approximately 20 d after symptoms onset), but disappeared in less than 180 d; 46 patients (28%) had neutralizing antibodies for more than 180 d after symptoms onset; 52 patients (32%) had minimal decay of neutralizing antibodies; and three patients (2%) who had increased neutralizing antibodies 90 d after symptoms onset[31].
Similarly, 140 patients with COVID-19 positive for RT-qPCR were recruited for a study in France, of whom 44 were admitted to the intensive care unit (ICU), 42 were hospitalized without the need for ICU, and 54 received outpatient treatment only (including eight asymptomatic cases). It was observed that most patients in the different groups produced neutralizing antibodies, but the neutralizing activity was variable, i.e. higher in the group of patients admitted to the ICU, so that only one patient in this group did not develop a neutralizing antibody response at the time of collection. In contrast, 21.9% of hospitalized patients and 25% of outpatients treated did not develop neutralizing antibodies at the time of the study[32].
This study supports the hypothesis that seroconversion is observed more frequently in individuals with severe symptoms and that they have higher antibody titers than mild and asymptomatic cases. This means that plasma titers are approximately eight times higher for severe cases[33].
Therefore, this study focused on understanding humoral immunity in mild cases of COVID-19 and regarding the time period of IgG detection in the serum of patients, similar results were obtained to previous studies. This finding contributes and adds data to the current knowledge about the humoral response against mild COVID-19 as it is a study conducted in a Western country with a larger sample size.
However, the temporal profile of the antibody response raised a concern by other authors[34-36]. Humoral immunity against SARS-CoV-2 does not appear to be durable, especially in individuals with mild symptoms or those who are asymptomatic, which make up the majority of COVID-19 cases. This fact is corroborated by the several doses of the SARS-CoV-2 vaccine, which is currently in its fourth dose in Brazil. On the other hand, it was also found that individuals with low titers or even undetectable levels of neutralizing antibodies can still be protected from subsequent infections, considering that memory B cells are still present in recovered patients[37,38].
The effect of age on immunity against SARS-CoV-2 has been widely discussed. A cohort study developed by the University of Virginia analyzed the antibody responses in individuals who received two doses of the vaccine BNT162b2 (Pfizer®) or mRNA-1273 (Moderna®) and had a blood sample collected seven to 31 d after the second dose. The results showed that participants aged 50 years and older who received BNT162b2 had lower pre-boost IgG levels than participants younger than 50 years who received the same vaccine. Individuals aged 50 years and older who received BNT162b2 had post-boost IgG levels that were also lower than levels found in younger participants[39].
Another study examined the immune response in elderly participants and younger healthcare professionals following immunization with the BNT162b. The results showed that after the first dose of the vaccine, IgG or IgA levels were lower in older individuals. In addition, elderly participants showed lower interferon-γ and interleukin (IL)-2 production by T cells specific against SARS-CoV-2 when compared to younger individuals[40].
Furthermore, a cohort study conducted in Greece analyzed the IgG response against the protein S of SARS-CoV-2 in a group of individuals after immunization with two doses of the BNT162b2 vaccine. The results revealed that younger patients (21-30 years old) had the highest antibody levels in both periods[41].
On the other hand, some studies have observed that older patients have been related to higher levels of antibodies against SARS-CoV-2. A study from Union Hospital (Huazhong University of Science and Technology, Wuhan, China) conducted with convalescent patients identified the presence of anti-SARS-CoV-2 antibodies one year after infection, in addition to a difference in IgG response according to the age of the patients. It was observed that the mean IgG antibody level was relatively low in younger convalescent patients (aged 21 - 35 years), and gradually increased to about 60% in older patients. The mean anti-SARS-CoV-2 IgG level was significantly higher in patients older than 35 years when compared to those younger than or equal to 35 years[42]. In our study, however, we found no statistical significance in the association between age and anti-SARS-CoV-2 IgG antibody levels. Seroconversion in the group of older individuals was not lower when compared to younger participants, demonstrating that age is possibly not a risk factor when analyzing mild cases of COVID-19.
Regarding the association between COVID-19 and pre-existing comorbidities, those with the highest association are hypertension and obesity, followed by metabolic disease, cardiovascular disease, neurological disease, chronic lung disease, kidney disease, asthma, immunosuppression, gast
It has been found in numerous studies that individuals with DM2 may have higher severity and mortality from COVID-19. This fact is due to the existing inflammatory condition in these patients, with higher levels of pro-inflammatory molecules, such as cytokines, especially IL-6. In addition, the presence of DM2 causes the response to SARS-CoV-2 to show a large amount of interferon and a delayed Th1 and Th17 response, which contributes to a more intense inflammatory response[45]. An example of this is a study showing that increased viral replication and production of pro-inflammatory cytokines may be related to high glucose concentration[46].
Coronaviruses bind to ACE2, reducing the activity of this receptor and increasing vascular permeability[47]. However, in individuals with systemic arterial hypertension (SAH) and DM, there is a higher number of these receptors when compared to the general population, which may explain the more severe cases of COVID-19 in these patients. Furthermore, SARS-CoV-2 produces endothelial injury, causing an inflammatory vascular state, pro-coagulant state, and a cellular infiltrate, also clarifying the more severe symptoms in individuals with these chronic diseases[48,49].
In addition, the presence of SAH also determines a pro-inflammatory state, arising from the endothelial dysfunction caused by this disease, leading to excessive activation of coagulation and platelets, in addition to the production of cytokines, antimicrobial peptides, and reactive oxygen species. This excessive activation may not only cause damage to the respiratory epithelium, but also reduce lung function and increase the local inflammatory response, contributing to further occurrence of complications from COVID-19[50,51].
An important question of the study is how COVID-19 affects patients with autoimmune diseases, such as RA. In a study of 11 122 individuals with COVID-19, patients with RA were found to have a higher chance of hospitalization or death than healthy individuals, and the study used an unadjusted model. However, when adjusting for age, sex, and comorbidities, no greater chance of unfavorable outcomes was observed[51]. Thus, AR is associated with a higher risk of infection and death in patients with COVID-19 when taking into account active AR, the presence of other diseases and the use of medications such as Rituximab, sulfasalazine or other immunosuppressive drugs[52].
Regarding the association between AIG and COVID-19, a study conducted at the Foundation of San Matteo Hospital, Italy, analyzed the susceptibility to COVID-19 in 400 drug-free immunosuppressive patients with autoimmune diseases, 100 of whom had AIG. The findings showed that among the individuals with AIG, seven (7%) had already tested positive for COVID-19, one (1%) required hospitalization for COVID-19, and 43 (43%) were vaccinated for SARS-CoV-2.
Furthermore, considering all investigated autoimmune diseases, molecular nasopharyngeal swabs and/or serology for SARS-CoV-2 testing showed that 33 (8.2%) tested positive[53]. These data are similar to those reported in the general population in the same geographical area in Italy[53], suggesting that the risk of COVID-19 in individuals with autoimmune diseases appears to be the same as in the general population.
Asthma is still being studied as a risk factor for COVID-19. The proposed hypothesis that the occurrence of more severe complications caused by COVID-19 in patients with asthma is due to a possible interaction between the pathobiology of SARS-CoV-2 and asthma. Thus, since the virus causes an intense inflammatory response and asthmatic individuals already have narrowed airways with high mucus production, pneumonia caused by the virus can lead to severe complications[54]. However, asthma can also lead to favorable outcomes, since it induces a negative regulation of ACE2, an enzyme that assists in the process of viral entry of SARS-CoV-2 into lung tissue[55]. On the other hand, a meta-analysis study did not identify a statistically significant increase in mortality and a worse prognosis for COVI-19 in asthmatic individuals[9].
Thus, although there are studies indicating the need for more intensive treatment in individuals with DM2, SAH, and other comorbidities[56], in patients with mild symptoms, this association does not seem to materialize, as the presence of previous diseases was not statistically significant for seroconversion in our study participants.
This study suggests that in mild cases of COVID-19, it is not possible to establish a temporal relationship of specific IgG production, raising the hypothesis that such a relationship may, in fact, not exist or that perhaps there is more than one type of relationship since there is interference from several factors, such as age, sex, presence of comorbidities, viral elimination, viral load, among others. It is also suggested that the virus generates a weak and non-lasting immune response in mild cases. Furthermore, a lower production of IgG antibodies was not observed in the elderly and in individuals with previous chronic diseases, leading to the conclusion that in mild cases of COVID-19, these patients may not be a risk group for unfavorable outcomes when analyzing the humoral response.
The molecular test used in the diagnosis of coronavirus disease 2019 is very specific and sensitive, however, it is not able to detect previous exposure to the virus nor to assess immunological memory. Therefore, serological tests that have this capability are used as tools for understanding the course of the humoral immune response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The motivation for this study arose from the serological test developed at the Federal University of Paraná, which in the validation process showed better sensitivity than commercial tests. A more sensitive test allows specific antibodies to be detected even at low titers, and thus to effectively assess whether there is still a protective antibody response in individuals who have been infected by the virus.
The aim of this study was to identify if a pattern of SARS-CoV-2 specific immunoglobulin G (IgG) production can be determined according to the time elapsed since diagnosis of the disease/onset of symptoms. The data could indicate, for example, the interval between vaccination doses.
This study was initiated after approval by the ethics committee. The participants were tested by real-time reverse transcriptase-polymerase chain reaction, the municipal government provided us with the data. Only positive cases were included in the study. Blood collection was performed by our research team and the method used for specific IgG antibodies was the indirect enzyme-linked immunosorbent assay. Statistical analyses were performed by the statistician of the research group, one of the authors of the manuscript.
The results of the study showed that there is no time pattern for the production of specific IgG. Less than one month after infection, some participants no longer have detectable IgG in the serum, while others have the antibodies seven months after infection.
In addition to the impossibility of establishing a temporal pattern of IgG response, the data indicate that SARS-CoV-2 does not appear to induce a long-lasting humoral response.
The study perspective is to analyze the immunoglobulin M (IgM) response of the same volunteers and determine the titers of both IgG and IgM to better understand seroconversion and the robustness of the anti-SARS-CoV-2 antibody response.
The authors would like to thank the Universidade Federal do Paraná, Municipal Health Secretariat of Toledo/Paraná/Brazil and Professor Ph.D. Luciano Fernandes Huergo for support.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arunachalam J, India; Bharara T, India S-Editor: Fan JR L-Editor: Ma JY-MedE P-Editor: Fan JR
| 1. | Di Marzo F, Sartelli M, Cennamo R, Toccafondi G, Coccolini F, La Torre G, Tulli G, Lombardi M, Cardi M. Recommendations for general surgery activities in a pandemic scenario (SARS-CoV-2). Br J Surg. 2020;107:1104-1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 2. | Mowbray NG, Ansell J, Horwood J, Cornish J, Rizkallah P, Parker A, Wall P, Spinelli A, Torkington J. Safe management of surgical smoke in the age of COVID-19. Br J Surg. 2020;107:1406-1413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 3. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30097] [Article Influence: 6019.4] [Reference Citation Analysis (3)] |
| 4. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5515] [Article Influence: 1103.0] [Reference Citation Analysis (1)] |
| 5. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18189] [Article Influence: 3637.8] [Reference Citation Analysis (0)] |
| 6. | CDC COVID-19 Response Team. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 878] [Cited by in RCA: 1052] [Article Influence: 210.4] [Reference Citation Analysis (0)] |
| 7. | Cai Q, Chen J, Xu L. Response to Comment on Cai et al. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes Care 2020;43:1392-1398. Diabetes Care. 2020;43:e162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4205] [Article Influence: 841.0] [Reference Citation Analysis (0)] |
| 9. | Bhattarai A, Dhakal G, Shah S, Subedi A, Sah SK, Mishra SK. Effect of Preexisting Asthma on the Risk of ICU Admission, Intubation, and Death from COVID-19: A Systematic Review and Meta-Analysis. Interdiscip Perspect Infect Dis. 2022;2022:8508489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Sunjaya AP, Allida SM, Di Tanna GL, Jenkins CR. Asthma and COVID-19 risk: a systematic review and meta-analysis. Eur Respir J. 2022;59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Ruscitti P, Conforti A, Cipriani P, Giacomelli R, Tasso M, Costa L, Caso F. Pathogenic implications, incidence, and outcomes of COVID-19 in autoimmune inflammatory joint diseases and autoinflammatory disorders. Adv Rheumatol. 2021;61:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Medina GA, Pino M, Geffner J, Perrotta NA, Barrios CV. [Clinical evolution and levels of anti-S SARS-CoV-2 IgG in rheumatic disease and COVID-19]. Medicina (B Aires). 2021;81:902-907. [PubMed] |
| 13. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11503] [Article Influence: 2300.6] [Reference Citation Analysis (0)] |
| 14. | Liu J, Huang J, Xiang D. Large SARS-CoV-2 Outbreak Caused by Asymptomatic Traveler, China. Emerg Infect Dis. 2020;26:2260-2263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Skyrud KD, Hernæs KH, Telle KE, Magnusson K. Impacts of mild COVID-19 on elevated use of primary and specialist health care services: A nationwide register study from Norway. PLoS One. 2021;16:e0257926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Chen M, Shen W, Rowan NR, Kulaga H, Hillel A, Ramanathan M Jr, Lane AP. Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Respir J. 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 17. | Azizi SA, Azizi SA. Neurological injuries in COVID-19 patients: direct viral invasion or a bystander injury after infection of epithelial/endothelial cells. J Neurovirol. 2020;26:631-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Hajikhani B, Calcagno T, Nasiri MJ, Jamshidi P, Dadashi M, Goudarzi M, Eshraghi AA; FACS, Mirsaeidi M. Olfactory and gustatory dysfunction in COVID-19 patients: A meta-analysis study. Physiol Rep. 2020;8:e14578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Rijkers G, Murk JL, Wintermans B, van Looy B, van den Berge M, Veenemans J, Stohr J, Reusken C, van der Pol P, Reimerink J. Differences in Antibody Kinetics and Functionality Between Severe and Mild Severe Acute Respiratory Syndrome Coronavirus 2 Infections. J Infect Dis. 2020;222:1265-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 20. | Liu ZL, Liu Y, Wan LG, Xiang TX, Le AP, Liu P, Peiris M, Poon LLM, Zhang W. Antibody Profiles in Mild and Severe Cases of COVID-19. Clin Chem. 2020;66:1102-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Conzentino MS, Forchhammer K, Souza EM, Pedrosa FO, Nogueira MB, Raboni SM, Rego FGM, Zanette DL, Aoki MN, Nardin JM, Fornazari B, Morales HMP, Celedon PAF, Lima CVP, Mattar SB, Lin VH, Morello LG, Marchini FK, Reis RA, Huergo LF. Antigen production and development of an indirect ELISA based on the nucleocapsid protein to detect human SARS-CoV-2 seroconversion. Braz J Microbiol. 2021;52:2069-2073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Esbin MN, Whitney ON, Chong S, Maurer A, Darzacq X, Tjian R. Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection. RNA. 2020;26:771-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 362] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 23. | Damluji AA, Rajan D, Haymond A, deFilippi C. Serological Testing for COVID-19 Disease: Moving the Field of Serological Surveillance Forward. J Appl Lab Med. 2021;6:584-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Glöckner S, Hornung F, Baier M, Weis S, Pletz MW, Deinhardt-Emmer S, Löffler B; The CoNAN Study Group. Robust Neutralizing Antibody Levels Detected after Either SARS-CoV-2 Vaccination or One Year after Infection. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Li K, Huang B, Wu M, Zhong A, Li L, Cai Y, Wang Z, Wu L, Zhu M, Li J, Wu W, Li W, Bosco B, Gan Z, Qiao Q, Wu J, Wang Q, Wang S, Xia X. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat Commun. 2020;11:6044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 26. | Benner SE, Patel EU, Laeyendecker O, Pekosz A, Littlefield K, Eby Y, Fernandez RE, Miller J, Kirby CS, Keruly M, Klock E, Baker OR, Schmidt HA, Shrestha R, Burgess I, Bonny TS, Clarke W, Caturegli P, Sullivan D, Shoham S, Quinn TC, Bloch EM, Casadevall A, Tobian AAR, Redd AD. SARS-CoV-2 Antibody Avidity Responses in COVID-19 Patients and Convalescent Plasma Donors. J Infect Dis. 2020;222:1974-1984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 27. | Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, Dela Cruz CS, Wang Y, Wu C, Xiao Y, Zhang L, Han L, Dang S, Xu Y, Yang QW, Xu SY, Zhu HD, Xu YC, Jin Q, Sharma L, Wang L, Wang J. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis. 2020;71:778-785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1043] [Cited by in RCA: 1084] [Article Influence: 216.8] [Reference Citation Analysis (0)] |
| 28. | Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin Infect Dis. 2020;71:2027-2034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1590] [Cited by in RCA: 1838] [Article Influence: 367.6] [Reference Citation Analysis (0)] |
| 29. | Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, Sun R, Wang Y, Hu B, Chen W, Zhang Y, Wang J, Huang B, Lin Y, Yang J, Cai W, Wang X, Cheng J, Chen Z, Sun K, Pan W, Zhan Z, Chen L, Ye F. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92:1518-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 1089] [Article Influence: 217.8] [Reference Citation Analysis (0)] |
| 30. | Lei Q, Li Y, Hou HY, Wang F, Ouyang ZQ, Zhang Y, Lai DY, Banga Ndzouboukou JL, Xu ZW, Zhang B, Chen H, Xue JB, Lin XS, Zheng YX, Yao ZJ, Wang XN, Yu CZ, Jiang HW, Zhang HN, Qi H, Guo SJ, Huang SH, Sun ZY, Tao SC, Fan XL. Antibody dynamics to SARS-CoV-2 in asymptomatic COVID-19 infections. Allergy. 2021;76:551-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 31. | Chia WN, Zhu F, Ong SWX, Young BE, Fong SW, Le Bert N, Tan CW, Tiu C, Zhang J, Tan SY, Pada S, Chan YH, Tham CYL, Kunasegaran K, Chen MI, Low JGH, Leo YS, Renia L, Bertoletti A, Ng LFP, Lye DC, Wang LF. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240-e249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 271] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 32. | Legros V, Denolly S, Vogrig M, Boson B, Siret E, Rigaill J, Pillet S, Grattard F, Gonzalo S, Verhoeven P, Allatif O, Berthelot P, Pélissier C, Thiery G, Botelho-Nevers E, Millet G, Morel J, Paul S, Walzer T, Cosset FL, Bourlet T, Pozzetto B. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18:318-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 246] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 33. | Wang P, Liu L, Nair MS, Yin MT, Luo Y, Wang Q, Yuan T, Mori K, Solis AG, Yamashita M, Garg A, Purpura LJ, Laracy JC, Yu J, Joshua-Tor L, Sodroski J, Huang Y, Ho DD. SARS-CoV-2 neutralizing antibody responses are more robust in patients with severe disease. Emerg Microbes Infect. 2020;9:2091-2093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 34. | Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, Ferbas KG, Tobin NH, Aldrovandi GM, Yang OO. Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N Engl J Med. 2020;383:1085-1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 819] [Cited by in RCA: 817] [Article Influence: 163.4] [Reference Citation Analysis (0)] |
| 35. | Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, Hu JL, Xu W, Zhang Y, Lv FJ, Su K, Zhang F, Gong J, Wu B, Liu XM, Li JJ, Qiu JF, Chen J, Huang AL. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1878] [Cited by in RCA: 1974] [Article Influence: 394.8] [Reference Citation Analysis (0)] |
| 36. | Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H, Wei P, Ge J, Gou M, Li X, Sun L, Cao T, Wang P, Zhou C, Zhang R, Liang P, Guo H, Wang X, Qin CF, Chen F, Dong C. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity. 2020;52:971-977.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 774] [Cited by in RCA: 814] [Article Influence: 162.8] [Reference Citation Analysis (0)] |
| 37. | Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, Cipolla M, Viant C, Barnes CO, Bram Y, Breton G, Hägglöf T, Mendoza P, Hurley A, Turroja M, Gordon K, Millard KG, Ramos V, Schmidt F, Weisblum Y, Jha D, Tankelevich M, Martinez-Delgado G, Yee J, Patel R, Dizon J, Unson-O'Brien C, Shimeliovich I, Robbiani DF, Zhao Z, Gazumyan A, Schwartz RE, Hatziioannou T, Bjorkman PJ, Mehandru S, Bieniasz PD, Caskey M, Nussenzweig MC. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1247] [Article Influence: 311.8] [Reference Citation Analysis (0)] |
| 38. | Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, Takehara KK, Eggenberger J, Hemann EA, Waterman HR, Fahning ML, Chen Y, Hale M, Rathe J, Stokes C, Wrenn S, Fiala B, Carter L, Hamerman JA, King NP, Gale M Jr, Campbell DJ, Rawlings DJ, Pepper M. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell. 2021;184:169-183.e17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 545] [Cited by in RCA: 510] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 39. | Richards NE, Keshavarz B, Workman LJ, Nelson MR, Platts-Mills TAE, Wilson JM. Comparison of SARS-CoV-2 Antibody Response by Age Among Recipients of the BNT162b2 vs the mRNA-1273 Vaccine. JAMA Netw Open. 2021;4:e2124331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 40. | Collier DA, Ferreira IATM, Kotagiri P, Datir RP, Lim EY, Touizer E, Meng B, Abdullahi A; CITIID-NIHR BioResource COVID-19 Collaboration, Elmer A, Kingston N, Graves B, Le Gresley E, Caputo D, Bergamaschi L, Smith KGC, Bradley JR, Ceron-Gutierrez L, Cortes-Acevedo P, Barcenas-Morales G, Linterman MA, McCoy LE, Davis C, Thomson E, Lyons PA, McKinney E, Doffinger R, Wills M, Gupta RK. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 517] [Cited by in RCA: 520] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 41. | Anastassopoulou C, Antoni D, Manoussopoulos Y, Stefanou P, Argyropoulou S, Vrioni G, Tsakris A. Age and sex associations of SARS-CoV-2 antibody responses post BNT162b2 vaccination in healthcare workers: A mixed effects model across two vaccination periods. PLoS One. 2022;17:e0266958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 42. | Zeng F, Wu M, Wang J, Li J, Hu G, Wang L. Over 1-year duration and age difference of SARS-CoV-2 antibodies in convalescent COVID-19 patients. J Med Virol. 2021;93:6506-6511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 43. | Ullah H, Ullah A, Gul A, Mousavi T, Khan MW. Novel coronavirus 2019 (COVID-19) pandemic outbreak: A comprehensive review of the current literature. Vacunas. 2021;22:106-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Qiu P, Zhou Y, Wang F, Wang H, Zhang M, Pan X, Zhao Q, Liu J. Clinical characteristics, laboratory outcome characteristics, comorbidities, and complications of related COVID-19 deceased: a systematic review and meta-analysis. Aging Clin Exp Res. 2020;32:1869-1878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 45. | Codo AC, Davanzo GG, Monteiro LB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, Prodonoff JS, Carregari VC, de Biagi Junior CAO, Crunfli F, Jimenez Restrepo JL, Vendramini PH, Reis-de-Oliveira G, Bispo Dos Santos K, Toledo-Teixeira DA, Parise PL, Martini MC, Marques RE, Carmo HR, Borin A, Coimbra LD, Boldrini VO, Brunetti NS, Vieira AS, Mansour E, Ulaf RG, Bernardes AF, Nunes TA, Ribeiro LC, Palma AC, Agrela MV, Moretti ML, Sposito AC, Pereira FB, Velloso LA, Vinolo MAR, Damasio A, Proença-Módena JL, Carvalho RF, Mori MA, Martins-de-Souza D, Nakaya HI, Farias AS, Moraes-Vieira PM. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020;32:498-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 46. | Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 502] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 47. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14253] [Article Influence: 2850.6] [Reference Citation Analysis (0)] |
| 48. | Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 426] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 49. | Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, Daßler-Plenker J, Guerci P, Huynh C, Knight JS, Loda M, Looney MR, McAllister F, Rayes R, Renaud S, Rousseau S, Salvatore S, Schwartz RE, Spicer JD, Yost CC, Weber A, Zuo Y, Egeblad M. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1054] [Cited by in RCA: 1102] [Article Influence: 220.4] [Reference Citation Analysis (0)] |
| 50. | Newton AH, Cardani A, Braciale TJ. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin Immunopathol. 2016;38:471-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 324] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 51. | Reilev M, Kristensen KB, Pottegård A, Lund LC, Hallas J, Ernst MT, Christiansen CF, Sørensen HT, Johansen NB, Brun NC, Voldstedlund M, Støvring H, Thomsen MK, Christensen S, Gubbels S, Krause TG, Mølbak K, Thomsen RW. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49:1468-1481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 52. | Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, Mateus EF, Richez C, Santos MJ, Schmajuk G, Scirè CA, Sirotich E, Sparks JA, Sufka P, Thomas T, Trupin L, Wallace ZS, Al-Adely S, Bachiller-Corral J, Bhana S, Cacoub P, Carmona L, Costello R, Costello W, Gossec L, Grainger R, Hachulla E, Hasseli R, Hausmann JS, Hyrich KL, Izadi Z, Jacobsohn L, Katz P, Kearsley-Fleet L, Robinson PC, Yazdany J, Machado PM; COVID-19 Global Rheumatology Alliance. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 497] [Article Influence: 124.3] [Reference Citation Analysis (0)] |
| 53. | Santacroce G, Lenti MV, Aronico N, Miceli E, Lovati E, Lucotti PC, Coppola L, Gentile A, Latorre MA, Di Terlizzi F, Soriano S, Frigerio C, Pellegrino I, Pasini A, Ubezio C, Mambella J, Canta R, Fusco A, Rigano G, Di Sabatino A. Impact of COVID-19 in immunosuppressive drug-naïve autoimmune disorders: Autoimmune gastritis, celiac disease, type 1 diabetes, and autoimmune thyroid disease. Pediatr Allergy Immunol. 2022;33 Suppl 27:105-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Parasher A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad Med J. 2021;97:312-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 402] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 55. | Chhapola Shukla S. ACE2 expression in allergic airway disease may decrease the risk and severity of COVID-19. Eur Arch Otorhinolaryngol. 2021;278:2637-2640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen MM, Liu Y, Yan Y, Liu M, Mao W, Zou J, Liu L, Chen G, Luo P, Xiao B, Zhang Z, Lu Z, Wang J, Lu H, Xia X, Wang D, Liao X, Peng G, Ye P, Yang J, Yuan Y, Huang X, Guo J, Zhang BH. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31:1068-1077.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1198] [Cited by in RCA: 1098] [Article Influence: 219.6] [Reference Citation Analysis (0)] |