Published online Nov 27, 2021. doi: 10.4331/wjbc.v12.i6.114
Peer-review started: March 30, 2021
First decision: May 12, 2021
Revised: May 21, 2021
Accepted: November 26, 2021
Article in press: November 26, 2021
Published online: November 27, 2021
Processing time: 258 Days and 17.8 Hours
Hematopoietic stem cell (HSC) transplantation (HSCT) is being accepted as a standard of care in various inflammatory diseases. The treatment of rheumatoid arthritis (RA) has been closely evolving with the understanding of disease pathogenesis. With the rising resistance to the traditional disease-modifying anti-rheumatic drugs and targeted biological therapy, researchers are in pursuit of other methods for disease management. Since the ultimate goal of the ideal treatment of RA is to restore immune tolerance, HSCT attracts much attention considering its reparative, paracrine, and anti-inflammatory effects. However, a systematic review of studies on HSCT in RA is lacking.
To investigate the role of HSCT in the management of RA.
A detailed search of PubMed, Scopus, EMBASE, Cochrane, and the Web of Science databases was made to identify the relevant articles till September 2020 following Cochrane and PRISMA guidelines. We extracted data including the number of patients, source of hematopoietic stem cells, their mobilization and conditioning regimens, results, and complications from the eligible studies. Results were dichotomized into success (ACR 50/70) and failure (ACR 20) based on the improvement from baseline characteristics. The methodological quality of the included studies was also assessed. Analysis was performed using OpenMeta[Analysis] software.
We included 17 studies (1 randomized controlled trial, 11 prospective, and 5 retro
Although the available literature is encouraging towards the use of HSCT in refr
Core Tip: With the rising resistance to the traditional disease-modifying anti-rheumatic drugs and targeted biological therapy, we performed this systematic review and meta-analysis to evaluate the role of hematopoietic stem cell therapy in the management of rheumatoid arthritis. Literature on the effectiveness of the intervention is encouraging with significant improvement till 2 years post-therapy. We have explored the ambiguity in the current treatment methods in hematopoietic stem cell therapy that needs further exploration to optimize the results out of this treatment modality.
- Citation: Muthu S, Jeyaraman M, Ranjan R, Jha SK. Remission is not maintained over 2 years with hematopoietic stem cell transplantation for rheumatoid arthritis: A systematic review with meta-analysis. World J Biol Chem 2021; 12(6): 114-130
- URL: https://www.wjgnet.com/1949-8454/full/v12/i6/114.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v12.i6.114
Rheumatoid arthritis (RA) is an autoimmune disorder essentially triggered by the activation of fibroblast like synoviocytes which in turn triggers a series of inflammatory reactions leading to the disease process[1,2]. The treatment of this disease has been closely evolving with an understanding of its pathogenesis. The key principle guidelines recommended in their routine management include: Disease-modifying anti-rheumatic drugs (DMARDs) is started as soon as possible after diagnosis, meth
HSCT as a treatment option in the management of RA has been tried with cont
While many reviews are available evaluating the role of HSCT in various inflammatory disorders[6-8,16], this is the first systematic review article to analyze the effectiveness of HSCT in RA. In this review, we intend to summarize the available evidence on the role of HSCT in the management of RA and analyze whether it holds a future in the treatment spectrum, and discuss some of the potential queries that need further exploration for the applicability in the current scenario of disease management.
We followed Cochrane guidelines and Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines[17,18] for the conduction and reporting of this systematic review. We included studies that satisfied the below mentioned PICOTS criteria: (1) Population: Patients with RA; (2) Intervention: HSCT; (3) Comparator: Placebo; (4) Outcome: American College of Rheumatology (ACR) 20/50/70 criteria of improvement from baseline; (5) Timeline: Minimum 6 mo follow-up period; and (6) Study design: Any study design satisfying PICOT.
In September 2020, two reviewers (SM and MJ) performed an extensive independent search of electronic databases - PubMed, Scopus, Embase, Cochrane, and the Web of Science - to identify all the relevant studies using keywords: “Rheumatoid arthritis”, “RA”, “Inflammatory Arthritis”, “Stem Cell”, and “Hematopoietic Stem Cell”. The search strategy used for PubMed has been provided in Supplementary File 1. The reference list of the selected articles was also searched to identify studies not identified in the primary search. As per the inclusion and exclusion criteria, eligible studies were included in the systematic review. We utilised kappa statistics to analyse the level of agreement of the reviewers for the inclusion of studies in the review and any discrepancy between the authors was resolved through discussion until a consensus was obtained.
Two reviewers (SM and MJ) retrieved independently relevant data from articles included for analysis. The following data were extracted: (1) Study characteristics: Year of publication, authors, nature of the study, and number of patients involved; (2) Baseline characteristics: Age, source of HSC (autologous/allogenic), HSC mobilization regimen, HSC manipulation methods utilized, HSC characterization, HSC conditioning regimen, and follow-up period; (3) Main outcome: ACR 20/50/70 criteria of improvement from baseline; and (4) Secondary outcome: Complications.
We expected heterogeneity in the scales and scores utilized for reporting the functional outcome of HSCT in the included studies. Hence, we utilized the standard ACR 20/50/70 criteria to categorize the outcome of the patients undergoing HSCT for RA which was commonly used in the studies[19]. In case of studies not reporting their outcome based on the ACR criteria, we utilized the description of recovery of the patient to categorize them under the ACR 20/50/70 criteria and if sufficient information was not available from the study, the corresponding authors were contacted for further information to categorize the patient into appropriate categories.
For ease of analysis, we dichotomized the results of HSCT into treatment success if the patients achieved a minimum of ACR50 criteria of improvement from the baseline as used by Nikolov et al[20]. Moreover, we also expected the included studies to have a variable follow-up period. Hence, we grouped the studies based on their follow-up period to analyze the results of the studies on HSCT for RA at various time points following the procedure. We utilised kappa statistics to analyse the level of agreement of the reviewers in data extraction and any disagreements were resolved by discussion until a consensus was achieved.
The methodological quality of the included studies was assessed independently by two reviewers using the risk of bias tool for case series and case reports given by Murad et al[21]. Risk of bias of the randomized controlled trials was estimated using the RoB 2 tool of Cochrane Collaboration[22]. To evaluate the methodological index of the prospective non-randomized studies, we utilized MINORS criteria[23].
Meta-analysis of the pooled data was performed in the R platform using the OpenMeta[Analyst] software[24]. For dichotomous variables, we utilized proportions with 95% confidence intervals (CIs). We evaluated the heterogeneity of the pooled data using I2 statistics. If I2 < 50% and P > 0.1, a fixed-effects model was employed in meta-analysis and if I2 > 50% and P < 0.1, a random-effects model was utilised. A P value < 0.01 was considered significant. We performed sensitivity analysis and subgroup analysis to explore the source of heterogeneity when it existed.
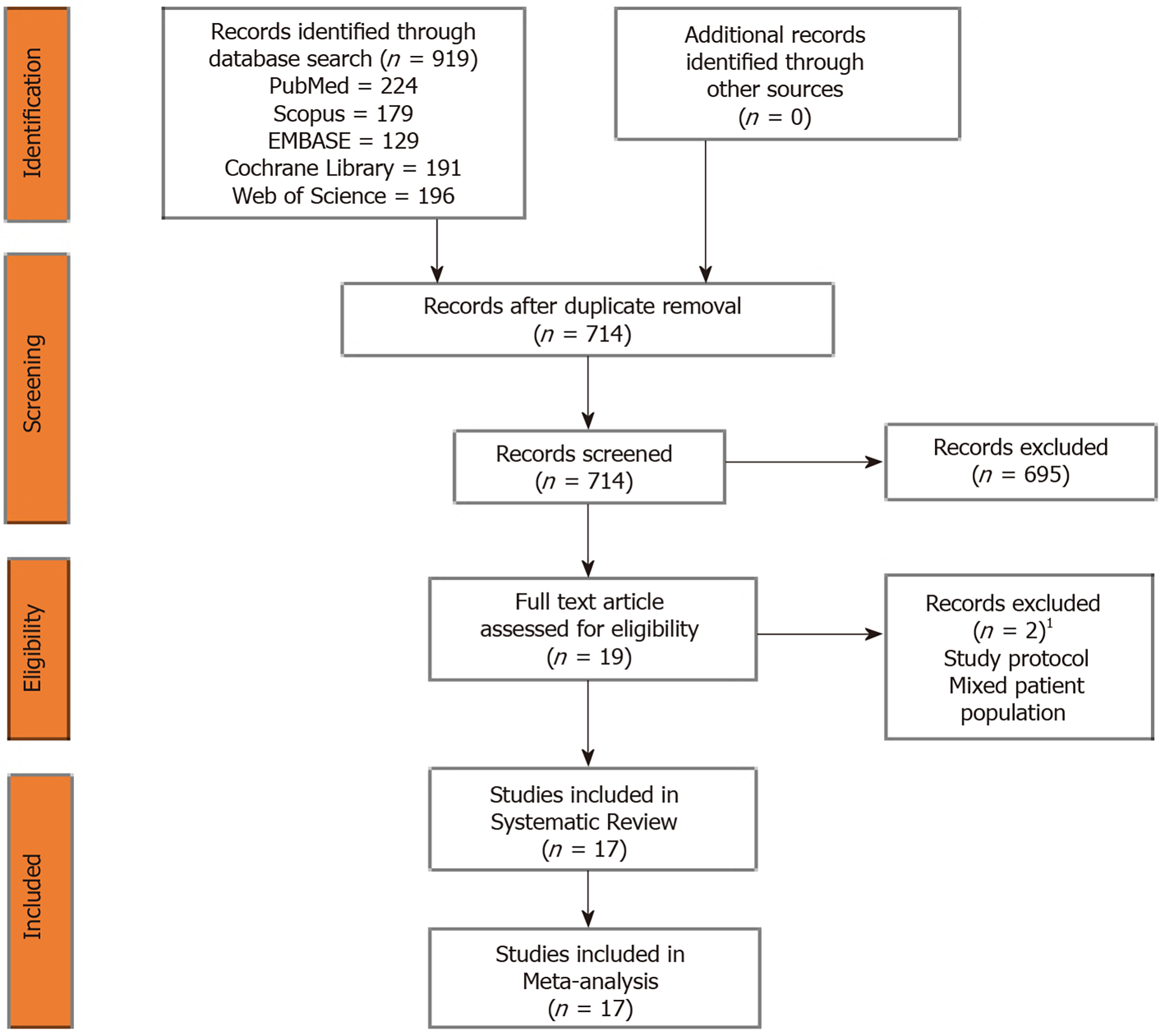
The electronic database search resulted in 919 articles which after initial screening for duplicate removal gave a total of 714 articles. Title and abstract screening were done in those articles and 195 of them were excluded. Nineteen articles were qualified for full-text review. We noted that none of the studies utilized a dual-arm study design to compare the effectiveness of the therapy against control as intended. Instead, we found 17 single-arm studies which analyzed the results of HSCT for RA. Hence, we included those 17 studies into the systematic review and performed a single-arm meta-analysis of the reported results stratified based on their study design. PRISMA flow diagram of the study selection is given in Figure 1. The list of studies excluded from full-text screening with the reason for their exclusion is provided in Supplementary File 2. The inter-reviewer kappa agreement was strong in both study selection and data extraction process with kappa values 0.84 and 0.89, respectively.
The methodological quality of the included studies was given in Table 1. The included studies did not show a high risk of bias to warrant exclusion. The included case reports satisfied all the criteria laid down by Murad et al[21] to be eligible for consideration in systematic review and analysis. The range of MINORS score achieved by the prospective studies was from 12-15, which is acceptable for analysis. The randomised controlled trial (RCT) by Moore et al[13] showed a low risk of bias among all five domains of assessment for inclusion into the analysis based on the RoB2 tool of Cochrane Collaboration.
| Randomized controlled trial | |||||||||||
| Ref. | Randomization process | Deviation from the intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result | Overall Bias | |||||
| Moore et al[13] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | |||||
| Prospective studies | |||||||||||
| Ref. | A clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate to the aim of the study | Unbiased assessment of the study endpoint | Follow-up period appropriate to the aim of the study | Loss to follow up less than 5% | Prospective calculation of the study size | |||
| Tyndall et al[29] | 1 | 1 | 2 | 2 | 2 | 1 | 2 | 1 | |||
| Burt et al[26] | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 1 | |||
| Burt et al[25] | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 1 | |||
| Verburg et al[30] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | |||
| Snowden et al[27] | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 1 | |||
| van Laar et al[28] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | |||
| Snowden et al[15] | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | |||
| Bingham et al[11] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | |||
| Teng et al[9] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | |||
| Pavletic et al[31] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | |||
| Verburg et al[32] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | |||
| Case reports | |||||||||||
| Ref. | Selection score | Ascertainment score | Causality score | Reporting score | Total score | ||||||
| Silva et al[12] | 1 | 2 | 1 | 1 | 5 | ||||||
| Joske et al[33] | 1 | 1 | 1 | 1 | 4 | ||||||
| Kim et al[35] | 1 | 2 | 1 | 1 | 5 | ||||||
| Durez et al[34] | 1 | 1 | 1 | 1 | 4 | ||||||
| Burt et al[36] | 1 | 2 | 1 | 1 | 5 | ||||||
Seventeen studies including one RCT[13], eleven prospective studies[9,11,15,25-32], and five retrospective studies[12,33-36] involving 233 patients were qualified for this systematic review. The baseline characteristics of the included studies are shown in Table 2. Although the publication timeline showed a steady increase in the total number of publications since 1997 to 2005, it was followed by an abrupt cessation of studies owing to the introduction of biological therapy in the management spectrum of RA. However, the common indication for HSCT in RA in the included studies was patients who failed to respond to the traditional lines of management with classical DMARDs or biological therapy. The age of the population included for analysis ranged from 18–65 years. Of the 17 included studies, 14 utilized autologous HSCT, and 3 utilized allogeneic HSCT from compatible donors.
| Sl. No | Ref. | Year | Study design | Indication | Sample size | Age (yr) | Source (Autologous/allogenic) | Mean follow-up (mo) |
| 1 | Joske et al[33] | 1997 | Case report | Failed DMARDs | 1 | 46 | Autologous | 6 |
| 2 | Durez et al[34] | 1998 | Case report | Failed DMARDs | 1 | 22 | Autologous | 10 |
| 3 | Burt et al[26] | 1998 | Prospective study | Failed DMARDs | 2 | 44 | Autologous | 12 |
| 4 | Snowden et al[27] | 1999 | Prospective study | Failed DMARDs | 8 | 18-65 | Autologous | 18 |
| 5 | Burt et al[25] | 1999 | Prospective study | Failed DMARDs | 4 | 46.2 | Autologous | 12 |
| 6 | Kim et al[35] | 2002 | Case report | Failed DMARDs | 1 | 54 | Autologous | 6 |
| 7 | Tyndall et al[29] | 2001 | Prospective study | Primary treatment | 43 | NR | Autologous | 11 |
| 8 | van Laar et al[28] | 2001 | Prospective study | Failed DMARDs | 8 | 18-60 | Autologous | 18 |
| 9 | Verburg et al[30] | 2001 | Prospective study | Failed DMARDs | 14 | 43 | Autologous | 12 |
| 10 | Bingham et al[11] | 2001 | Prospective study | Failed DMARDs | 6 | 37.33 | Autologous | 20 |
| 11 | Pavletic et al[31] | 2001 | Prospective study | Failed DMARDs | 6 | 42.5 | Autologous | 26.5 |
| 12 | Moore et al[13] | 2001 | RCT | Failed DMARDs | 33 | 18-65 | Autologous | 12 |
| 13 | Burt et al[36] | 2004 | Case report | Failed DMARDs | 1 | 52 | Allogenic | 12 |
| 14 | Snowden et al[15] | 2004 | Prospective study | Failed DMARDs | 73 | 42 | Autologous | 18 |
| 15 | Verburg et al[32] | 2005 | Prospective study | Failed DMARDs | 8 | 35-55 years | Autologous | 24 |
| 16 | Teng et al[9] | 2005 | Prospective study | Failed DMARDs | 8 | 43 | Allogenic | 60 |
| 17 | Silva et al[12] | 2018 | Retrospective study | Failed DMARDs (10), failed autologous HSCT (1), secondary haemophagocytic lymphohistiocytosis (5) | 16 | 12 | Allogenic | 29 |
Of the 14 studies that utilized autologous HSCT for RA, all utilized granulocyte colony stimulating factor (G-CSF) for progenitor cell mobilization at a dosage ranging from 5–10 µg/kg and cyclophosphamide (CYC) at a variable non-myeloablative dosage ranging from 1.5 g/m2 to 4 g/m2. Etoposide was also used along with CYC by Durez et al[34] Similarly, all the studies utilized leukapheresis to remove the autoimmune inflammatory cells from the circulation. All the included studies manipulated the cells mobilized by selective isolation of CD 34+ cells. Two of them compared the effect of this selective manipulation in their study and did not find any substantial benefit out of the process[10,15]. The complete HSCT protocol including the mobilization protocol followed by the individual studies is given in Table 3.
| Sl. No | Ref. | Mobilization regimen | Graft manipulation | HSC selection | Conditioning regimen |
| 1 | Joske et al[33] | CYC 4 g/m2, G-CSF 10 µg/kg | Leukapheresis | CD 34 +ve selection | CYC 200mg/kg |
| 2 | Durez et al[34] | CYC 1.5 g/m2, etoposide 300 mg/m2, G-CSF 5 µg/kg | Leukapheresis | CD 34 +ve selection | CYC 60 mg daily and busulfan 4 mg daily |
| 3 | Burt et al[26] | CYC, G-CSF | Leukapheresis | CD 34 +ve selection | CYC 200 mg/kg, ATG 90 mg/kg |
| 4 | Snowden et al[27] | CYC 100-200 mg/kg, G-CSF 5 µg/kg | Leukapheresis | CD34 +ve selection | CYC 100 mg/kg or 200 mg/kg |
| 5 | Burt et al[25] | CYC 2 g/m2, G-CSF | Leukapheresis | CD34 +ve selection | CYC 200 mg/kg, ATG 90 mg/kg |
| 6 | Kim et al[35] | CYC 4 g/m², G-CSF 5 µg/kg | Leukapheresis | CD 34 +ve selection | CYC 200 mg/kg, ATG 90 mg/kg |
| 7 | Tyndall et al[29] | CYC, G-CSF | Leukapheresis | NR | CYC 200 mg/kg, ± ATG 90 mg/kg, ± Busulfan |
| 8 | van Laar et al[28] | CYC 4 g/m², G-CSF 10 µg/kg | Leukapheresis | CD34 +ve selection | CYC 200 mg/kg |
| 9 | Verburg et al[30] | CYC 4 g/m², G-CSF 10 µg/kg | Leukapheresis | CD 34 +ve selection | CYC 200 mg/kg |
| 10 | Bingham et al[11] | CYC 2 g/m2, G-CSF | Leukapheresis | CD 34 +ve selection | CYC 200 mg/kg |
| 11 | Pavletic et al[31] | CYC 2 g/m2, G-CSF | Leukapheresis | CD34 +ve selection | CYC 200 mg/kg, ATG 90 mg/kg |
| 12 | Moore et al[13] | CYC 200 mg/kg, G-CSF 10 µg/kg | Leukapheresis | CD34 +ve selection (18) / No selection (15) | CYC 200 mg/kg |
| 13 | Burt et al[36] | NA | NA | CD 34 +ve selection | CYC 150 mg/kg, fludarabine 125 mg/m2, alemtuzumab 20 mg |
| 14 | Snowden et al[15] | CYC 200 mg/kg, G-CSF 5- 10 µg/kg | Leukapheresis | CD 34 +ve selection (45) / No selection (28) | CYC 200 mg/kg |
| 15 | Verburg et al[32] | CYC 200 mg/kg, G-CSF | Leukapheresis | CD 34 +ve selection | CYC 200 mg/kg |
| 16 | Teng et al[9] | NA | NA | CD 34 +ve selection | CYC 200 mg/kg |
| 17 | Silva et al[12] | NA | NA | CD 34 +ve selection | Fludarabine 30 mg/m²/d, melphalan 140 mg/m²/d, alemtuzumab 0.2 mg/kg/d or fludarabine 30 mg/m²/d, treosulfan 14 mg/m²/d, alemtuzumab 0.2 mg/kg/d |
The commonly employed drug in the conditioning regimen of the included studies to avoid rejection of HSCT in the RA patients was CYC at a dosage ranging from 100-200 mg/kg. In addition to CYC, anti-thymocyte globulin (ATG) was used in 5/17 included studies at a constant dose of 90 mg/kg[25,26,29,35], and busulfan in one of them at 4 mg/d dosage[34]. Two studies utilized fludarabine and alemtuzumab in their condi
We noted significant heterogeneity among the scales used for the assessment of the functional improvement in the included studies such as ACR outcome improvement criteria, Visual Analog Scale, Health Assessment Questionnaire, Disease Activity Score, Larsen Score, C-reactive protein level, Erythrocyte Sedimentation Rate, and Rheumatoid Factor. However, ACR was the most commonly employed outcome measure in HSCT to assess the functional outcome post-procedure. Hence, we converted the outcome of all the studies included under ACR criteria based on the outcome characteristics reported. Significant heterogeneity existed in the ACR results among the included studies (I2 = 81.86%, P < 0.001). Hence, a random-effects model was utilized for analysis.
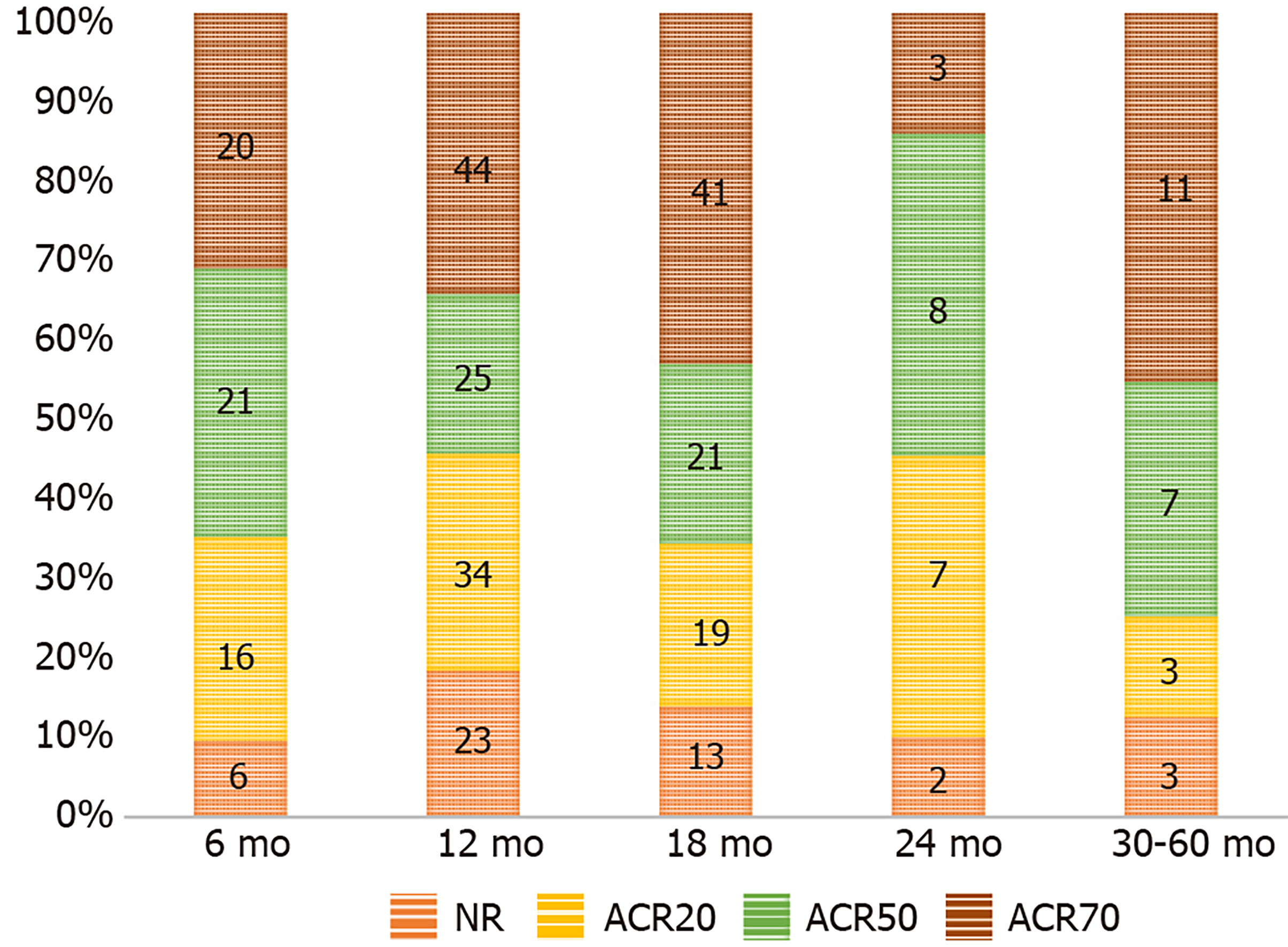
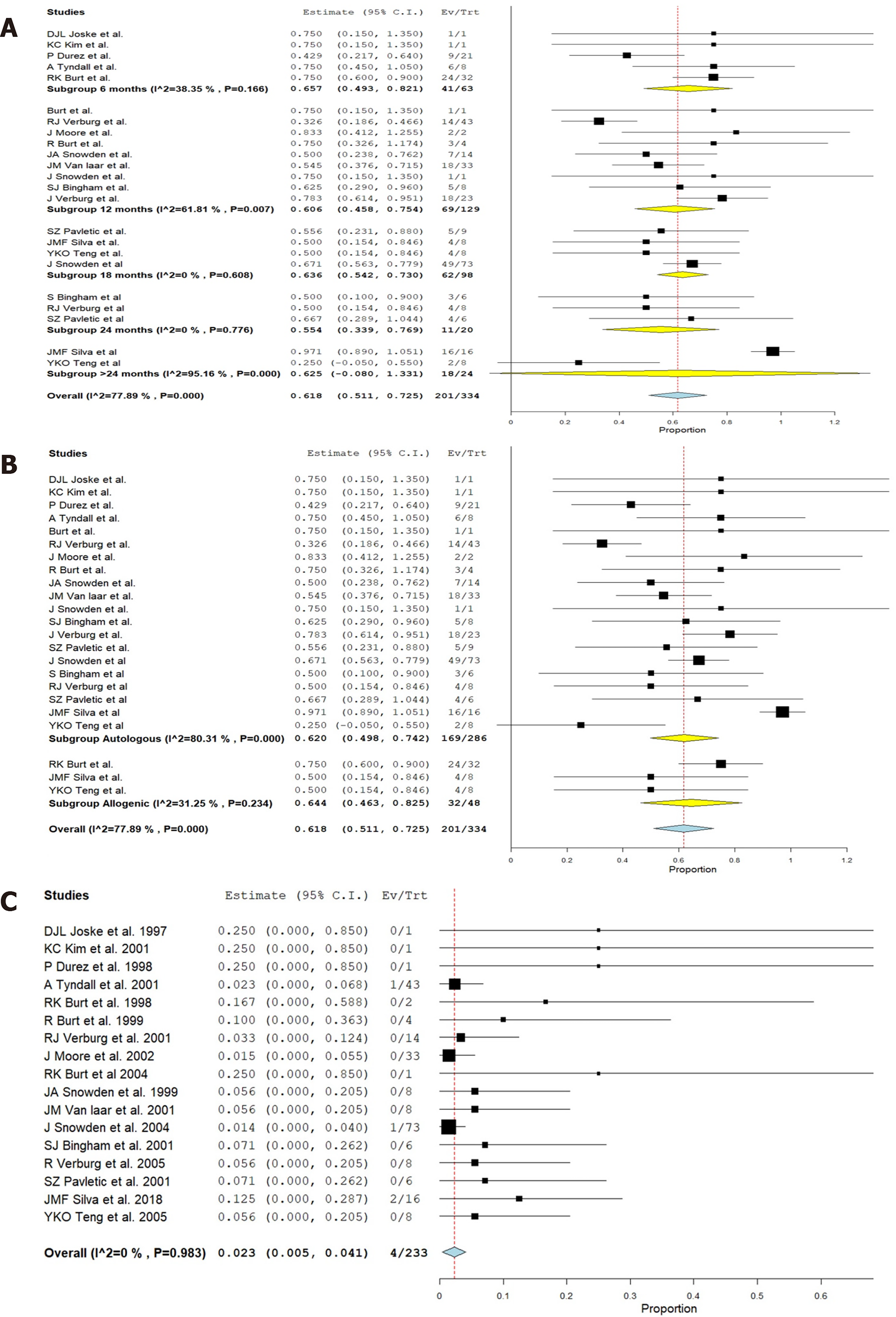
We also found the follow-up period of the included studies to range from 6-60 mo. Hence, we grouped the studies based on their follow-up period to analyze the results at various time points following the procedure. Figure 2 shows the change in the grades of ACR criteria at various time points among the included studies using HSCT for RA. Figure 3A shows the forest plot of analysis of results of studies at various time points following HSCT in comparison to their pre-operative status of RA using a random binary effects model. HSCT provided a significantly beneficial overall improvement in the clinical grades of ACR criteria (Z = 11.309, P < 0.001). A significant difference in the preoperative state of ACR was noted till 24 mo and later on the significance of the result was lost (Z = 1.737, P = 0.082) as shown in Figure 3A.
We explored the heterogeneity among the included studies through subgroup analysis of the results based on the nature of HSCT (i.e., autologous and allogeneic types) and presented the results in Figure 3B. It was noted that the source of HSCT did not play a role in altering the functional outcome and both autologous (Z = 9.972, P < 0.001) and allogenic (Z = 6.978, P < 0.001) sources produced significant improvement in the outcome compared to the pre-operative state despite having a significant heterogeneity among the studies reporting them (I2 = 99.4, P < 0.001). On exploring the heterogeneity, variability was noted in the follow-up period of the included studies despite maintaining the significance of the outcome results.
Despite using a non-myeloablative regimen in the HSCT protocol, the patients tended to undergo a spectrum of side effects. The routine side effects of chemotherapy such as nausea, vomiting, hair loss, skin rash, and fever were noted in most of the patients. We took into account the procedure-related mortality, drug-related major toxicities, and grade III/IV graft-vs-host reaction (GVHD) as significant complications due to the procedure and analyzed their prevalence among the included studies. One transplant-related death was noted by Tyndall et al[29] and death due to sepsis was noted in a study by Snowden et al[15]. We noted a < 1% (2/233) procedure-related mortality from the included studies. No major drug-related toxicities were noted in any of the included studies.
All patients who underwent allogeneic HSCT received immunosuppression in the conditioning regimen to counteract the GVHD which made them vulnerable to infections. High-grade GVHD was noted in patients undergoing allogeneic HSCT by Silva et al[12] along with a higher prevalence of viral infections noted in them. It was noted from the forest plot that HSCT was not associated with a significant increase in the listed major complications (P = 0.015, 95% CI: 0.005-0.041) as shown in Figure 3C. However, it should be prudent to consider on a case-by-case basis whether these risks outweigh the benefits from the therapy.
A sensitivity analysis was performed in each analysis. The results of the outcomes analysed were not significantly altered by sequentially omitting each study in the meta-analysis within each study design. On the other hand, the consistency of the results was maintained after reanalysis by changing the random-effects model.
Despite the usage of both conventional DMARDs and newer biologicals, 40% of patients with RA continue to have frequent relapses with active and progressive disease[20,37]. Autologous HSCT has been considered as an alternative modality of management of such resistant candidates[38]. Although HSCs are multipotent stem cells with the potential to give rise to blood, endothelial cells, and immune cells, in the context of their role in autoimmune diseases they are viewed as immune stem cells[39]. The major complication from the HSCT arises not from the HSC transfer itself but from the immunosuppressive conditioning regimens utilized to inhibit the autoreactive immune cells before the transfer[8]. The rationale of using the immunosuppressive conditioning regimens is not to myeloablate the host immune system but to lymphoablate the autoimmune cells so that immune regeneration starts from the transferred HSCs[20]. These non-myeloablative regimens used in the included studies commonly employed CYC as shown in Table 2. Special attention should be given to the regimen-related side effects particularly from the high dose CYC which forms the backbone of these regimens[40].
Response to the HSCT was shown by the reduction in the serum auto-antibody titers noted in the included studies[10-12]. This shows a temporal relationship between the immune balance restoration and clinical response outcomes as a precondition to get immune tolerance in RA patients[41]. However, to obtain optimal results from the HSCT patient selection is of key importance. Although HSCT is recommended for patients who failed conventional spectrum of management, good results from HSCT are obtained from patients presenting with an early aggressive disease with poor prognostic factors who also have enough residual functional capacity to benefit out of the procedure[14,42].
HSCT is also associated with considerable morbidity and treatment-related mor
The risk of TRM and toxicity depends not only on the HSCT protocol used but also on the source of the donor cells[46]. Allogenic HSCT is associated with a higher risk of complications especially due to the GVHD associated with them. Most of the adverse events are associated with the conditioning regimens utilized following allogeneic HSCT[45]. To clear the autoreactive inflammatory cells causing GVHD an array of conditioning regimens including drugs such as fludarabine, melphalan, alemtuzumab, and treosulfan along with CYC have been utilized in the included studies[9,12]. To optimize the safety of the procedure, the treatment must be offered after preliminary screening for comorbidities and cardiopulmonary ailments and administration of the regimens in dedicated centres with appropriate supportive care to make the procedure successful and safe.
With due consideration to the selected group of patients who is eligible for HSCT, the impact of the disease on society is far from negligible[47]. Although they are small in proportion, consumption of the health care services by these seriously ill patients remains significant[48]. Compared to the lifetime costs incurred in the management of such resistant cases of RA utilizing biologically targeted therapies which are required in the long term without any guaranteed universal effectiveness[49], HSCT appears a promising cost-effective strategy although it is also an expensive treatment by itself. A complete remission out of HSCT would lead to significant cost savings in the long run[50,51]. Apart from the economic benefits, complications of chronic immunosuppressive therapies with targeted biologicals could be avoided with the use of HSCT[50]. So far, no cost-effectiveness analysis has been made for HCST in RA.
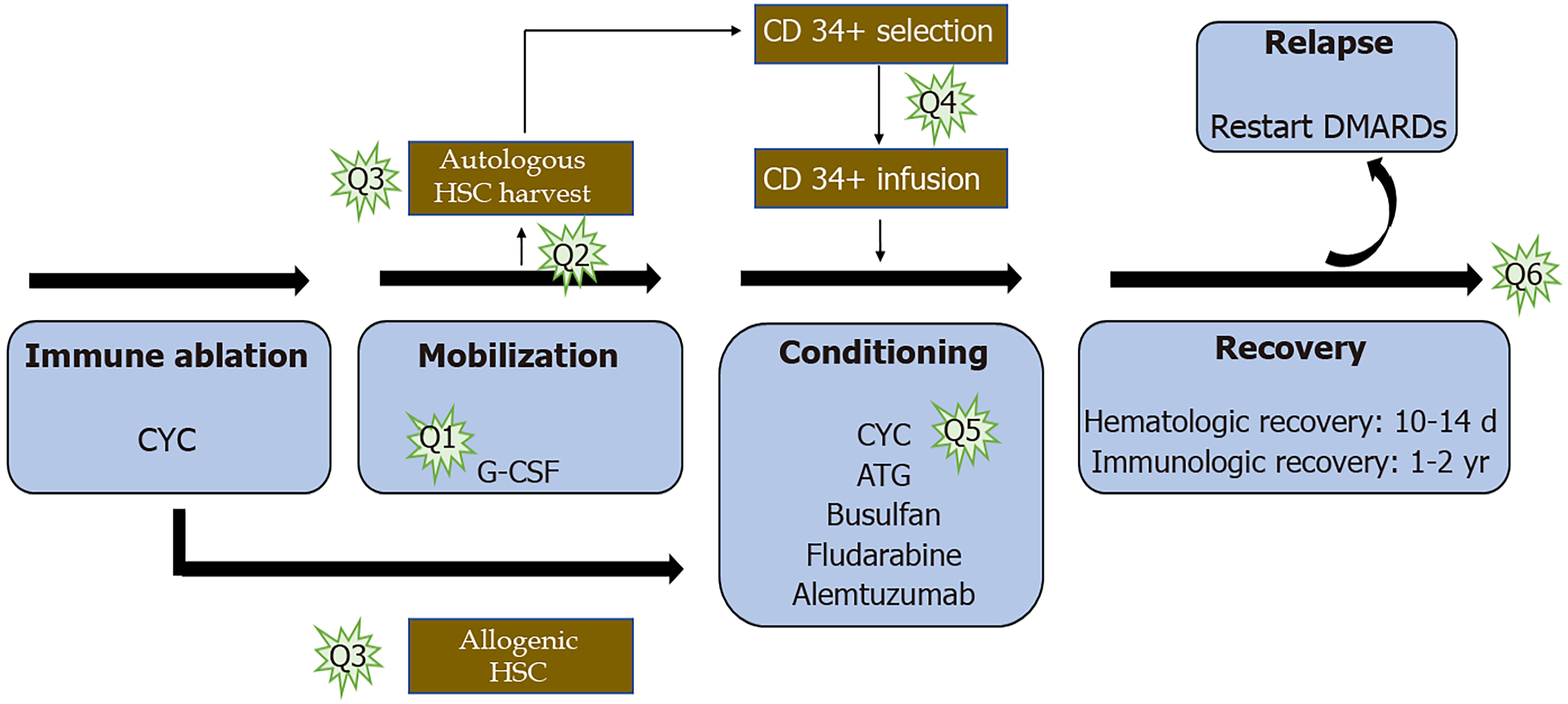
Before the inclusion of HSCT into the routine management protocol for RA, certain questions need further exploration to standardize the treatment protocol to harness maximum benefits out of the procedure. The potential questions that need answering in the various stages of HSCT are enumerated in Figure 4.
With the studies reporting complete remission of severe cases of RA after a myeloablative dose of CYC without being followed by HSCT[52], a question arises as to whether the procedure needs firsthand. Regeneration of the marrow function similar to HSCT was noted but at a slower pace. Introducing the auto-immune lymphocytes into the host following a high dose of CYC may be a reason for noted failure in some of the cases of autologous HSCT[52]. Although the concept appears appealing, whether it could be qualified to be investigated under a clinical trial poses ethical considerations. However, one could plan for a trial with and without immediate stem cell rescue following high dose CYC therapy for RA patients[53].
There has been a shift in the source of autologous HSCT from bone marrow (BM) to peripheral blood stem cells (PBSC) because of the rapid haematological recovery especially platelet and neutrophil counts following reinfusion when PBSC are used as a source of HSCT[54]. It also makes the procedure more cost-effective[55]. But it is also noted that, when PBSC is used as a source of HSCT, an 11-fold increase in T cells and an 8-fold increase in B-cells were noted, thereby making them less likely to provide any sustained benefit compared to the BM source which has a less cellular load on re-infusion[56]. It is also not evident whether the peripheral T cell counts have any temporal association with the damage caused by the disease. One other finding in allogenic HSCT is that patients who undergone HCST with PBSC source did not document any proportional increase in GVHD compared to BM source[57]. Hence, comparative long-term clinical trials to explore the ideal source of HSCs are needed to further explore this issue.
There is a theoretical concern in allogenic HSCT that the patient's immune cells could not be able to continue the disease process following intensive immunosuppressive therapy since the reconstituted immune progenitors belong to the donor. Moreover, the donor T cell elicits a GVHD which enables elimination or suppression of the residual autoimmune clones in the body. We do not have any evidence on this “graft-versus-autoimmune disease” effect, to state a correlation between the degree of GVHD and the resolution of the disease process to weigh one over the other.
Although phase I and phase II clinical studies have established the therapeutic potential, clinical safety, and efficacy of HSCT therapy[20], there is a paucity of liter
Most of the included studies utilized CYC based regimens to minimize the effect of residual autoreactive clones in the body. The effect of the conditioning regimen used on the results of the transplant remains unexplored although the intensity of the conditioning might play a role. Most of the included studies utilized CYC at a high dose of 200 mg/kg administered for 4 d. Such high dose chemotherapy has its side effects such as hemorrhagic cystitis. Further research to identify alternative condi
The major challenge in utilizing HSCT for RA is the timing of initiation of the treatment in the course of the disease. If the patient is considered for HSCT after a trial of response to immunosuppressive therapy with DMARDs, the disease could have evolved beyond the point of maximum benefit from HSCT since they are less effective in patients with advanced organ damage and immune dysregulation. Since there are no specific guidelines to the timing and patient profile selection for enrolling into HSCT, the decision largely lies in the hands of the patients and their treating physicians. Clinicians should help the patients choose the right treatment by weighing their pros and cons together and provide clear information to aid in the decision-making process considering the prognostic factors associated with the disease process in the individual patients[59]. Although the treatment seems promising, the guidelines drafted by the European League against Rheumatism (EULAR) and EMBT for patient selection for optimal response need further improvement on the above-mentioned areas.
The small sample size of the included studies with heterogeneity in their patient selection methods, HSCT protocols utilized, the reported results, and their definition of remission limits their utility in decision making. In the absence of large clinical trials, a Markov clinical decision analysis to compare the conventional therapy with HSCT could be utilized. The model predicted HSCT to be superior to conventional therapy if the TRM could be maintained < 3.3% or if the treatment results are sustainable for 5 years. Having done in the early era of biological therapy, these analyses emphasize that a subset of RA patients could also benefit from HSCT. The differences in the Quality Adjusted Life Years between the two groups involved in the model reinstate the role of the patients in the decision-making process[9]. With the improvement in the treatment methods, the safety of the procedure has largely been improved. In selected cases, HSCT may remain the only effective method available making these risks acceptable. Yet, the decision lies in the hands of the patient, hence it needs careful discussion before making the treatment choice. With the rise in the resistance to traditional therapy for RA, earlier identification of those non-responders based on clinic-serological profile and prognostic markers remains a key element to reap the maximum benefit out of this modality.
Although the available literature is encouraging towards the use of HSCT in refractory cases with significant improvement from baseline till 2 years, the inclusion of HSCT therapy into the standard of care of RA needs further exploration. With the rising proportion of non-responders to conventional DMARDs and biologic therapy, HSCT therapy would find a place in the treatment spectrum of RA provided that large clinical trials with longer follow-up are conducted to establish the ideal treatment strategy to get optimal results out of this treatment modality.
Hematopoietic stem cell (HSC) transplantation (HSCT) has been accepted as a treatment method in the management of various inflammatory diseases. With the evolution in the management of rheumatoid arthritis (RA), and the rising resistance to the traditional disease-modifying anti-rheumatic drugs, researchers are in pursuit of alternate methods for disease management. Having the ultimate goal of achieving systemic immune tolerance, HSCT has now been considered in the management of RA with respect to its reparative, paracrine, and anti-inflammatory properties.
Despite the understanding of the potential of HSCT towards immune reconstitution, considering RA to be an auto-immune disease, a systematic review of studies on utilization of HSCs in RA is lacking. If HSCT proves to be useful in refractory cases of RA, future studies to strengthen the evidence on the same could be recommended.
To investigate the role of HSCT in the management of RA.
A detailed search of PubMed, Scopus, EMBASE, Cochrane, and the Web of Science databases was made to identify the relevant articles till September 2020 following Cochrane and PRISMA guidelines. All the studies included were analyzed to evaluate the role of HSCT in RA by dichotomizing their outcome based on American College of Rheumatology (ACR) criteria for success (ACR 50/70) and failure (ACR 20) based on the improvement from baseline characteristics. The methodological quality of the included studies was also assessed. Analysis was performed using OpenMeta [Analysis] software.
Upon meta-analysis of the 17 included studies on the use of HSCT for refractory cases of RA, it was noted that remission was maintained for 2 years. However, for the imple
Utilization of HSCT in RA cases that are refractory to the conventional line of manag
Before the inclusion of HSCT into the routine management protocol for RA, certain questions need further exploration to standardize the treatment protocol to harness maximum benefits out of the procedure.
Provenance and peer review: Invited manuscript; Externally peer reviewed.
Specialty type: Rheumatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Tung TH, Wang G, Xiao T S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3395] [Cited by in RCA: 3859] [Article Influence: 275.6] [Reference Citation Analysis (0)] |
| 2. | Cooles FA, Isaacs JD. Pathophysiology of rheumatoid arthritis. Curr Opin Rheumatol. 2011;23:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | Mian A, Ibrahim F, Scott DL. A systematic review of guidelines for managing rheumatoid arthritis. BMC Rheumatol. 2019;3:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Khaddour K, Hana CK, Mewawalla P. Hematopoietic Stem Cell Transplantation. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2020. |
| 5. | Tyndall A, Gratwohl A. Immune ablation and stem-cell therapy in autoimmune disease. Clinical experience. Arthritis Res. 2000;2:276-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Gratwohl A, Passweg J, Bocelli-Tyndall C, Fassas A, van Laar JM, Farge D, Andolina M, Arnold R, Carreras E, Finke J, Kötter I, Kozak T, Lisukov I, Löwenberg B, Marmont A, Moore J, Saccardi R, Snowden JA, van den Hoogen F, Wulffraat NM, Zhao XW, Tyndall A; Autoimmune Diseases Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Autologous hematopoietic stem cell transplantation for autoimmune diseases. Bone Marrow Transplant. 2005;35:869-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 7. | Tyndall A, van Laar JM. Stem cells in the treatment of inflammatory arthritis. Best Pract Res Clin Rheumatol. 2010;24:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Spierings J, van Laar JM. Is There a Place for Hematopoietic Stem Cell Transplantation in Rheumatology? Rheum Dis Clin North Am. 2019;45:399-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Teng YK, Verburg RJ, Sont JK, van den Hout WB, Breedveld FC, van Laar JM. Long-term followup of health status in patients with severe rheumatoid arthritis after high-dose chemotherapy followed by autologous hematopoietic stem cell transplantation. Arthritis Rheum. 2005;52:2272-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Sonoda Y. Immunophenotype and functional characteristics of human primitive CD34-negative hematopoietic stem cells: the significance of the intra-bone marrow injection. J Autoimmun. 2008;30:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Bingham SJ, Snowden J, McGonagle D, Richards S, Isaacs J, Morgan G, Emery P. Autologous stem cell transplantation for rheumatoid arthritis--interim report of 6 patients. J Rheumatol Suppl. 2001;64:21-24. [PubMed] |
| 12. | M F Silva J, Ladomenou F, Carpenter B, Chandra S, Sedlacek P, Formankova R, Grandage V, Friswell M, Cant AJ, Nademi Z, Slatter MA, Gennery AR, Hambleton S, Flood TJ, Lucchini G, Chiesa R, Rao K, Amrolia PJ, Brogan P, Wedderburn LR, Glanville JM, Hough R, Marsh R, Abinun M, Veys P. Allogeneic hematopoietic stem cell transplantation for severe, refractory juvenile idiopathic arthritis. Blood Adv. 2018;2:777-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Moore J, Brooks P, Milliken S, Biggs J, Ma D, Handel M, Cannell P, Will R, Rule S, Joske D, Langlands B, Taylor K, O'Callaghan J, Szer J, Wicks I, McColl G, Passeullo F, Snowden J. A pilot randomized trial comparing CD34-selected versus unmanipulated hemopoietic stem cell transplantation for severe, refractory rheumatoid arthritis. Arthritis Rheum. 2002;46:2301-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Swart JF, Delemarre EM, van Wijk F, Boelens JJ, Kuball J, van Laar JM, Wulffraat NM. Haematopoietic stem cell transplantation for autoimmune diseases. Nat Rev Rheumatol. 2017;13:244-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 15. | Snowden JA, Passweg J, Moore JJ, Milliken S, Cannell P, Van Laar J, Verburg R, Szer J, Taylor K, Joske D, Rule S, Bingham SJ, Emery P, Burt RK, Lowenthal RM, Durez P, McKendry RJ, Pavletic SZ, Espigado I, Jantunen E, Kashyap A, Rabusin M, Brooks P, Bredeson C, Tyndall A. Autologous hemopoietic stem cell transplantation in severe rheumatoid arthritis: a report from the EBMT and ABMTR. J Rheumatol. 2004;31:482-488. [PubMed] |
| 16. | Alexander T, Farge D, Badoglio M, Lindsay JO, Muraro PA, Snowden JA; Autoimmune Diseases Working Party (ADWP) of the European Society for Blood and Marrow Transplantation (EBMT). Hematopoietic stem cell therapy for autoimmune diseases - Clinical experience and mechanisms. J Autoimmun. 2018;92:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Higgins JP, Green S, editor. Cochrane Handbook for Systematic Reviews of Interventions. Publisher: John Wiley & Sons, 2008: 674. |
| 18. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47073] [Article Influence: 2942.1] [Reference Citation Analysis (0)] |
| 19. | Ward MM, Guthrie LC, Alba MI. Brief report: rheumatoid arthritis response criteria and patient-reported improvement in arthritis activity: is an American College of Rheumatology twenty percent response meaningful to patients? Arthritis Rheumatol. 2014;66:2339-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Nikolov NP, Pavletic SZ. Technology Insight: hematopoietic stem cell transplantation for systemic rheumatic disease. Nat Clin Pract Rheumatol. 2008;4:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1008] [Cited by in RCA: 1533] [Article Influence: 219.0] [Reference Citation Analysis (0)] |
| 22. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15079] [Article Influence: 2513.2] [Reference Citation Analysis (0)] |
| 23. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3743] [Cited by in RCA: 5610] [Article Influence: 255.0] [Reference Citation Analysis (0)] |
| 24. | Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J Stat Softw. 2012;49:1-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 511] [Article Influence: 39.3] [Reference Citation Analysis (1)] |
| 25. | Burt RK, Georganas C, Schroeder J, Traynor A, Stefka J, Schuening F, Graziano F, Mineishi S, Brush M, Fishman M, Welles C, Rosen S, Pope R. Autologous hematopoietic stem cell transplantation in refractory rheumatoid arthritis: sustained response in two of four patients. Arthritis Rheum. 1999;42:2281-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Burt RK, Traynor AE, Pope R, Schroeder J, Cohen B, Karlin KH, Lobeck L, Goolsby C, Rowlings P, Davis FA, Stefoski D, Terry C, Keever-Taylor C, Rosen S, Vesole D, Fishman M, Brush M, Mujias S, Villa M, Burns WH. Treatment of autoimmune disease by intense immunosuppressive conditioning and autologous hematopoietic stem cell transplantation. Blood. 1998;92:3505-3514. [PubMed] |
| 27. | Snowden JA, Biggs JC, Milliken ST, Fuller A, Brooks PM. A phase I/II dose escalation study of intensified cyclophosphamide and autologous blood stem cell rescue in severe, active rheumatoid arthritis. Arthritis Rheum. 1999;42:2286-2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | van Laar JM, Verburg RJ, Fibbe WE, Breedveld FC. Intensive immunosuppression and autologous stem cell transplantation for patients with severe rheumatoid arthritis: the Leiden experience. J Rheumatol Suppl. 2001;64:25-27. [PubMed] |
| 29. | Tyndall A, EBMT/EULAR International Data Base. European Group for Blood and Marow Transplantation and European League Against Rheumatism. Autologous hematopoietic stem cell transplantation for severe autoimmune disease with special reference to rheumatoid arthritis. J Rheumatol Suppl. 2001;64:5-7. [PubMed] |
| 30. | Verburg RJ, Kruize AA, van den Hoogen FH, Fibbe WE, Petersen EJ, Preijers F, Sont JK, Barge RM, Bijlsma JW, van de Putte LB, Breedveld FC, van Laar JM. High-dose chemotherapy and autologous hematopoietic stem cell transplantation in patients with rheumatoid arthritis: results of an open study to assess feasibility, safety, and efficacy. Arthritis Rheum. 2001;44:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Pavletic SZ, Klassen LW, Pope R, O'Dell JR, Traynor AE, Haire CE, Graziano F, Oyama Y, Barr W, Burt RK. Treatment of relapse after autologous blood stem cell transplantation for severe rheumatoid arthritis. J Rheumatol Suppl. 2001;64:28-31. [PubMed] |
| 32. | Verburg RJ, Sont JK, van Laar JM. Reduction of joint damage in severe rheumatoid arthritis by high-dose chemotherapy and autologous stem cell transplantation. Arthritis Rheum. 2005;52:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Joske DJ, Ma DT, Langlands DR, Owen ET. Autologous bone-marrow transplantation for rheumatoid arthritis. Lancet. 1997;350:337-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Durez P, Toungouz M, Schandené L, Lambermont M, Goldman M. Remission and immune reconstitution after T-cell-depleted stem-cell transplantation for rheumatoid arthritis. Lancet. 1998;352:881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Kim KC, Lee IH, Choi JH, Oh MR, Ahn MJ, Kim SY. Autologous stem cell transplantation in the treatment of refractory rheumatoid arthritis. J Korean Med Sci. 2002;17:129-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Burt RK, Oyama Y, Verda L, Quigley K, Brush M, Yaung K, Statkute L, Traynor A, Barr WG. Induction of remission of severe and refractory rheumatoid arthritis by allogeneic mixed chimerism. Arthritis Rheum. 2004;50:2466-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Sokka T, Envalds M, Pincus T. Treatment of rheumatoid arthritis: a global perspective on the use of antirheumatic drugs. Mod Rheumatol. 2008;18:228-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Lowenthal RM, Graham SR. Does hemopoietic stem cell transplantation have a role in treatment of severe rheumatoid arthritis? J Clin Immunol. 2000;20:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Morgan RA, Gray D, Lomova A, Kohn DB. Hematopoietic Stem Cell Gene Therapy: Progress and Lessons Learned. Cell Stem Cell. 2017;21:574-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 40. | Openshaw H, Nash RA, McSweeney PA. High-dose immunosuppression and hematopoietic stem cell transplantation in autoimmune disease: clinical review. Biol Blood Marrow Transplant. 2002;8:233-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Petrovská N, Prajzlerová K, Vencovský J, Šenolt L, Filková M. The pre-clinical phase of rheumatoid arthritis: From risk factors to prevention of arthritis. Autoimmun Rev. 2021;20:102797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 42. | Hügle T, van Laar JM. Stem cell transplantation for rheumatic autoimmune diseases. Arthritis Res Ther. 2008;10:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Farge D, Labopin M, Tyndall A, Fassas A, Mancardi GL, Van Laar J, Ouyang J, Kozak T, Moore J, Kötter I, Chesnel V, Marmont A, Gratwohl A, Saccardi R. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years' experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica. 2010;95:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 259] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 44. | Daikeler T, Tichelli A, Passweg J. Complications of autologous hematopoietic stem cell transplantation for patients with autoimmune diseases. Pediatr Res. 2012;71:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, Denton C, Hawkey C, Labopin M, Mancardi G, Martin R, Moore JJ, Passweg J, Peters C, Rabusin M, Rovira M, van Laar JM, Farge D; EBMT Autoimmune Disease Working Party (ADWP); Paediatric Diseases Working Party (PDWP). Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012;47:770-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 46. | Snowden JA, Badoglio M, Labopin M, Giebel S, McGrath E, Marjanovic Z, Burman J, Moore J, Rovira M, Wulffraat NM, Kazmi M, Greco R, Snarski E, Kozak T, Kirgizov K, Alexander T, Bader P, Saccardi R, Farge D; European Society for Blood and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party (ADWP); EBMT Paediatric Working Party (PWP); Joint Accreditation Committee of the International Society for Cellular Therapy (ISCT); EBMT (JACIE). Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv. 2017;1:2742-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 47. | Chaplin H, Carpenter L, Raz A, Nikiphorou E, Lempp H, Norton S. Summarizing current refractory disease definitions in rheumatoid arthritis and polyarticular juvenile idiopathic arthritis: systematic review. Rheumatology (Oxford). 2021;60:3540-3552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Mahmood S, van Oosterhout M, de Jong S, Landewé R, van Riel P, van Tuyl LHD. Evaluating quality of care in rheumatoid arthritis: the patient perspective. RMD Open. 2017;3:e000411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Santos-Moreno P, Valencia O. Experience of biological therapy units in rheumatoid arthritis and other autoimmune diseases. Reumatol Clin (Engl Ed). 2019;15:61-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Bouffi C, Djouad F, Mathieu M, Noël D, Jorgensen C. Multipotent mesenchymal stromal cells and rheumatoid arthritis: risk or benefit? Rheumatology (Oxford). 2009;48:1185-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Gabriel SE, Crowson CS, Luthra HS, Wagner JL, O'Fallon WM. Modeling the lifetime costs of rheumatoid arthritis. J Rheumatol. 1999;26:1269-1274. [PubMed] |
| 52. | Brodsky RA, Petri M, Smith BD, Seifter EJ, Spivak JL, Styler M, Dang CV, Brodsky I, Jones RJ. Immunoablative high-dose cyclophosphamide without stem-cell rescue for refractory, severe autoimmune disease. Ann Intern Med. 1998;129:1031-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 171] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Tyndall A, Millikan S. Bone marrow transplantation. Baillieres Best Pract Res Clin Rheumatol. 1999;13:719-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A, Demuynck HM, Link H, Zander A, Barge A. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996;347:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 397] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 55. | Smith TJ, Hillner BE, Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A, Link H, Zander A, Yanovich S, Kitchin R, Erder MH. Economic analysis of a randomized clinical trial to compare filgrastim-mobilized peripheral-blood progenitor-cell transplantation and autologous bone marrow transplantation in patients with Hodgkin's and non-Hodgkin's lymphoma. J Clin Oncol. 1997;15:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Snowden JA, Nink V, Cooley M, Zaunders J, Keir M, Wright L, Milliken ST, Brooks PM, Biggs JC. Composition and function of peripheral blood stem and progenitor cell harvests from patients with severe active rheumatoid arthritis. Br J Haematol. 1998;103:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Ringdén O, Remberger M, Runde V, Bornhäuser M, Blau IW, Basara N, Hölig K, Beelen DW, Hägglund H, Basu O, Ehninger G, Fauser AA. Peripheral blood stem cell transplantation from unrelated donors: a comparison with marrow transplantation. Blood. 1999;94:455-464. [PubMed] |
| 58. | Snowden JA, Biggs JC, Milliken ST, Fuller A, Staniforth D, Passuello F, Renwick J, Brooks PM. A randomised, blinded, placebo-controlled, dose escalation study of the tolerability and efficacy of filgrastim for haemopoietic stem cell mobilisation in patients with severe active rheumatoid arthritis. Bone Marrow Transplant. 1998;22:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Sullivan KM, Horwitz M, Osunkwo I, Shah N, Strouse JJ. Shared Decision-Making in Hematopoietic Stem Cell Transplantation for Sickle Cell Disease. Biol Blood Marrow Transplant. 2018;24:883-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |