INTRODUCTION AND EDUCATIONAL EXPERIENCE
Dr. Benfang Lei (Figure 1) is an Associate Professor of Bacteriology in the Department of Veterinary Molecular Biology at Montana State University, USA. He received his Bachelor’s degree in Chemistry from Wuhan University, China in 1982 and Master’s degree in Chemistry from Beijing Institute of Chemical Technology, China in 1985. After performing research at the Institute of Photographic Chemistry, Academia Sinica for 3 years, he went to USA and received his Master’s degree in chemistry from the University of Texas at El Paso in 1989 and PhD. in Biochemistry from the University of Houston in 1993. Prior to receiving his training in bacterial pathogenesis at Rocky Mountain Laboratories, the National Institute of Allergy and Infectious Diseases, NIH, he worked at a biotech company and then at the University of Houston as a Research Assistant Professor. In 2003, Dr. Lei was recruited to the Faculty of Montana State University. His research was supported by grants from The National Institutes of Health, US Department of Agriculture (USDA), and the Montana Board of Research and Commercialization Technology, USDA Animal Health Formula Funds, and the Montana State Agricultural Experiment Station.
Figure 1 Benfang Lei, PhD, Department of Veterinary Molecular Biology, Montana State University, 960 Technology Blvd, PO Box 173610, Bozeman, MT 59717, United States.
ACADEMIC STRATEGY AND GOALS
The primary goals of Benfang Lei’s research are to elucidate molecular mechanisms of Group A Streptococcus (GAS) and Streptococcus equi (S. equi) pathogenesis and to develop vaccines and therapeutic strategies to prevent and treat infections caused by both pathogens. To achieve these goals, Lei group uses multidisciplinary approach to identify novel protective antigens and virulence factors, investigate their functions, regulation, and role in pathogenesis and virulence, and explore applications of novel virulence factors in vaccine and therapeutics. Using this strategy, Lei group has identified a secreted protein as a novel virulence factor that is regulated by the two-component regulatory system CovRS (Control of Virulence), is a protective antigen, is critical for GAS dissemination, and has esterase activity. He is currently examining the role of the esterase in GAS evasion of the innate immunity. His laboratory also conduct detailed mechanistic studies on the GAS and Staphylococcus aureus (S. aureus) heme acquisition systems to understand how Gram-positive pathogens acquire heme from host. They are particularly interested in determining how heme is extracted from hemoglobin by its surface receptors Shr and IsdB and how heme is rapidly transferred from one protein to another. To this end, they will continue to examine the biochemical and/or chemical mechanisms, thermodynamics, and structural basis of the hemoglobin-to-Shr, hemoglobin-to-IsdB, and Shp-to-HtsA heme transfer reactions using biochemical, biophysical, structural, and spectroscopic approaches.
PRE-INDEPENDENT ACADEMIC ACHIEVEMENTS
Flavin-dependent two-component monooxygenase systems with potential application in biodesulfurization of fossil fuels
The flavin-dependent two-component monooxygenase systems are composed of a flavin reductase and a monooxygenase. The flavin reductase generates reduced flavin (FMNH2), which is used as a co-substrate of the monooxygenase to oxidize another substrate. We cloned the Vibrio harveyi (V. harveyi) flavin reductase P (FRP), which reduces FMN using NADPH as the electron donor and provides it as a co-substrate of V. harveyi luciferase[1]. FRP was the first cloned flavin reductase of the two-component flavin monooxygenase systems. Another contribution to the field is that we established Sox/DszC, a component of the Rhodococcus organic sulfur oxidization system, as a FMN-dependent sulfide/sulfoxide monooxygenase[2]. A critical question unique to these systems is how FMNH2 is transferred from the donor to the acceptor to avoid its rapid autooxidation when it is free. We conducted a detained kinetic analysis of FMNH2 transfer in the FRP/ luciferase reaction and found that FMNH2 is directly channeled from FRP to luciferase[3]. This is the first and the most thorough study on the mechanism of FMNH2 transfer in the field. These studies conducted during the early stage of the field are well recognized in the field, which is evident in a recent review[4]. In addition, these studies have had impact on developing biotechnology for biodesulfurization of fossil fuels.
Action and resistance mechanisms of antitubercular isoniazid
Tuberculosis due to Mycobacterium tuberculosis (M. tuberculosis) infection is the leading cause of death worldwide among known infectious diseases. Isoniazid has been the cornerstone in tuberculosis chemotherapy. Isoniazid is a pro-drug and requires in vivo activation by the catalase/peroxidase KatG, and the activated compound inhibits the enoyl reductase InhA, resulting in inhibition of the synthesis of mycolic acid, a long chain fatty acid-containing component of the mycobacterial cell wall. We characterized the KatG-catalyzed isoniazid activation, isolated the resulting InHA inhibitor, and developed an inhibition assay[5]. We subsequently demonstrated that the common KatG mutations present in isoniazid-resistant clinical M. tuberculosis isolates abolish the ability of KatG to activate isoniazid[6]. High citations of these studies indicate that they had significant impact on studies on the mechanisms of isoniazid action and resistance and search for inhibitors of InhA for treating tuberculosis caused by isoniazid-resistant M. tuberculosis.
Identification of dominant antigens, the novel virulence factor Mac, and potential new vaccine candidates of GAS
Using contemporary investigative methods in the post-genomic era[7], we conducted the first systematic analysis of GAS culture supernatant proteins, identifying eight novel dominant extracellular antigens[8]. We subsequently found that one of the novel antigens, Mac, is a novel virulence factor that inhibits opsonophagocytosis of GAS by human neutrophils[9]. We found that there are two major Mac variants[10] that block immunoglobulin recognition by Fc receptors and degrade immunoglobulins, thereby enhancing survival of the pathogen through the inhibition of phagocytosis, endocytosis of IgG-opsonized particles, and antibody-dependent cell-mediated cytotoxicity[11]. These studies advanced the understanding of GAS pathogenesis and interactions with host. In another systematic study using the in silico analysis of the GAS genome, we identified all putative lipoproteins of GAS and then evaluated them for the potential as new vaccine candidates[12]. Further evaluation of these potential new vaccine candidates may develop an efficacious GAS vaccine.
INDEPENDENT ACADEMIC ACHIEVEMENTS
In the past 7 years, Dr. Lei’s laboratory has contributed considerably to the literature in understanding heme acquisition in Gram-positive pathogens at the machinery, pathway, and kinetic and molecular mechanisms and pathogenesis or bacteriology of GAS and S. equi.
Iron acquisition and regulation in GAS
An in silico analysis of a GAS genome sequence identified 19 putative cell surface proteins, and one of them was identified as a novel heme-binding protein (Shp)[13]. The shp gene is co-transcribed with eight downstream genes, including three genes encoding an ATP-binding cassette transporter, HtsABC, and an upstream gene encoding another surface protein, Shr. We subsequently found that Shr and HtsA, the lipoprotein component of the HtsABC transporter also bind heme[14,15]. These studies suggest that Shr, Shp, and HtsABC constitute a heme acquisition machinery in GAS. Shp is the first cell surface heme binding protein identified in Gram-positive pathogens, which indicates that the surface proteins, in addition to ABC transporters, are required for heme acquisition by Gram-positive bacteria. We then found that the ftsABCD locus encodes a ferric ferrichrome transporter[16]. Thus, we contributed to discovery of two of the three known iron transporters in GAS. Interestingly, we found that the metalloregulator MtsR displays a different metal iron specificity in regulating the expression of iron- and manganese-specific MtsABC and heme-specific HtsABC transporters[17].
The molecular mechanism of heme transfer among the components of the GAS heme acquisition machinery
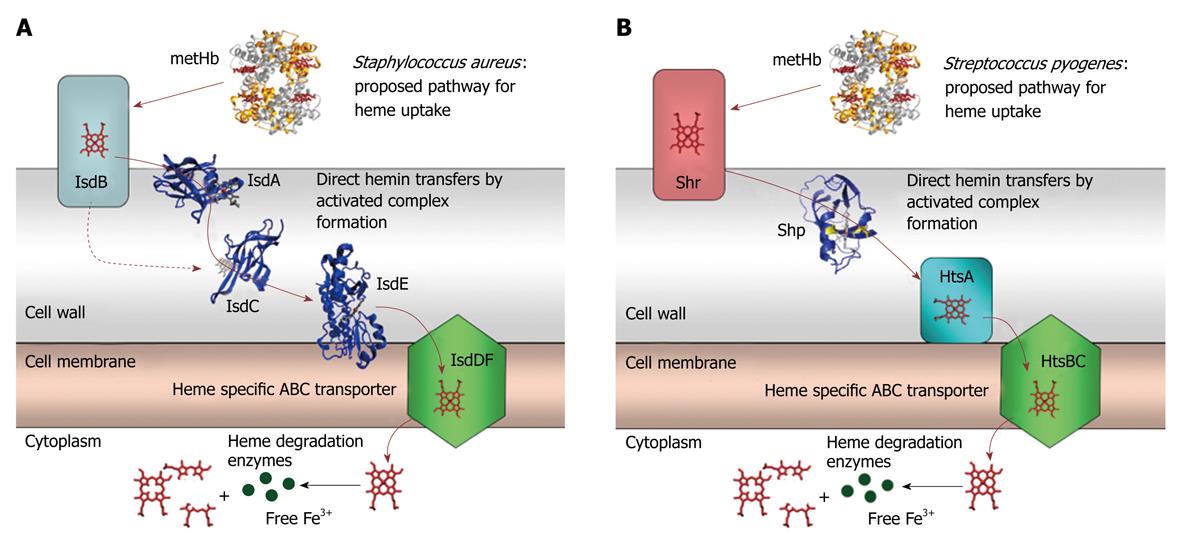
We found that Shp rapidly and efficiently transfers heme to HtsA[18], the first example of heme transfer from a cell surface protein to the lipoprotein component of a heme-specific ABC transporter in Gram-positive pathogens. Subsequently, we found that Shr efficiently transfers its heme to Shp but not to HtsA[15]. These findings led us to propose a model in which Shr acquires heme from methemoglobin and Shp relays heme from Shr to HtsA of HtsABC, which brings heme across the cytoplasmic membrane (Figure 2).
Figure 2 Cartoons for the proposed pathway of heme acquisition from metHb by the Staphylococcus aureus Isd (A) and Group A Streptococcus Shr/Shp/HtsABC (B) systems.
The arrows indicate the direction of direct heme transfer. Heme transfer from IsdB to IsdC represented by the dotted arrow may be prevented in vivo by their physical locations in the cell wall. The structure models of the proteins were from the coordinates 2Q8Q, 2ITF, 2O6P, 2Q7A, and 1HHO. This figure was originally published in J Biol Chem. Zhu H, Xie G, Liu M, Olson JS, Fabian M, Dooley DM, Lei B. Pathway for heme uptake from human methemoglobin by the iron-regulated surface determinants system of Staphylococcus aureus. J Biol Chem 2008; 283: 18450-18460[23].
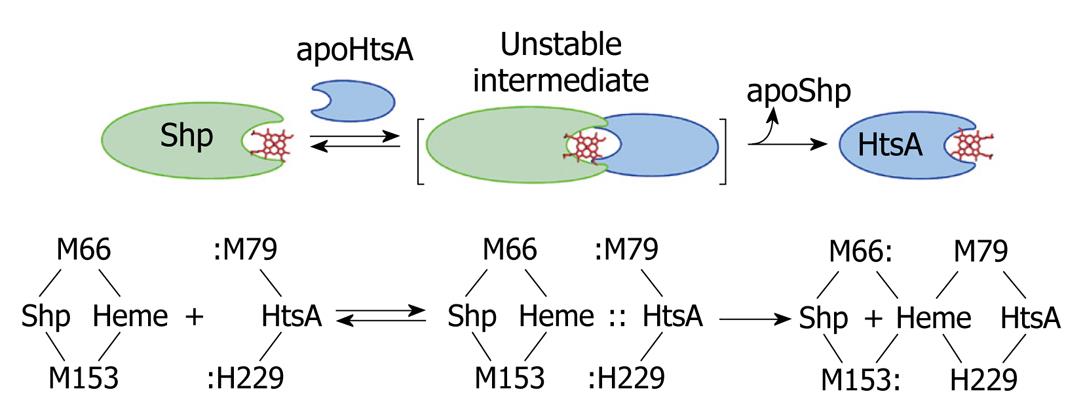
We chose to further study the Shp/HtsA reaction as a model to understand how heme is transferred from one protein to another during heme acquisition. We first demonstrated direct and rapid heme transfer from Shp to HtsA and elucidated the kinetic mechanism of the transfer reaction[19]. This is the first example of direct heme transfer from a surface protein to ABC transporter and the first detailed kinetic mechanism of heme transfer from one protein to another in bacterial heme acquisition processes. More importantly, this study demonstrated an activated heme transfer mechanism, that is, the heme donor Shp and acceptor HtsA form an activated complex to facilitate heme transfer. In addition, we developed a kinetic approach to distinguish direct and indirect heme transfer reactions. In a following publication[20], we demonstrated the unexpected importance of the axial ligands of the Shp heme iron on the transfer reaction and detected donor-heme-acceptor ternary complexes in the reactions of the Shp axial ligand mutants with wild-type HtsA. The significant advancement of this study is that a reaction mechanism model we called “plug-in” mechanism was derived. In this model, the side chains of the axial ligands of the empty heme pocket in apo-HtsA are inserted along the two axial sides of the Shp heme plane and displace the Shp axial residues to pry the cofactor out of Shp (Figure 3). We recently generated and characterized the axial ligand mutants of HtsA[21], which will be used to further test the plug-in model.
Figure 3 A direct heme axial ligand displacement model for the holoShp-holoHtsA reactions.
The side chains of the heme axial ligands, M79 and H229, in apoHtsA are proposed to be inserted into the axial positions of heme in Shp, simultaneously displace M66 and M153 of Shp, and extract heme from the donor. M66/M153 and M79/H229 are the axial ligand residues of the heme iron in Shp and HtsA, respectively.
Heme acquisition pathway in S. aureus
The heme acquisition system of S. aureus consists of the surface proteins IsdA, IsdB, and IsdC and the ABC transporter IsdDEF. Using the expertise we developed from the studies of heme acquisition in GAS, we first examined the reaction of holo-IsdA and apo-IsdC and found that heme transfer from IsdA to IsdC is very efficient[22]. The significance of this work resides that these results provide the first example of heme transfer from one surface protein to another surface protein in Gram-positive bacteria and, perhaps most importantly, indicate that the mechanism of activated heme transfer, which we previously demonstrated in the Shp/HtsA reaction, may apply in general to all bacterial heme transport systems. Next, we found that methemoglobin directly transfers its heme to IsdB, but not to IsdA, IsdC, nor IsdE, that IsdB directly transfers its heme to IsdA and IsdC, and that IsdC, but not IsdB and IsdA, directly donates its heme to IsdE[23]. Taken together, these findings enable us to demonstrate an experimental model for heme acquisition in S. aureus (Figure 2). The most important achievement of this study is the establishment of the pathway for heme uptake from methemoglobin through the Isd surface proteins to the ABC transporter. In addition, this study provides the first example of direct and rapid heme transfer from methemoglobin to a bacterial protein. Furthermore, these findings also suggest that there are parallel functions of the components in the GAS and S. aureus heme uptake systems.
Discovery of the secreted esterase as a novel virulence factor
GAS produces a large number of extracellular proteins to mediate its pathogenesis, and the functions and functional mechanisms of most of these proteins are unknown. A secreted antigenic protein was found to have esterase activity[24]. Immunization with this protein protects mice against subcutaneous infection of GAS strains of more than one serotype[24]. Another significant observation in this publication is that the bacteria failed to spread from the infection site in the immunized mice, suggesting that the esterase is involved in the dissemination of the pathogen in the soft tissue. Consistent with the immunization and challenge results, a mutant defective in this esterase gene is attenuated in virulence and is unable to spread in the skin and disseminate into organs and blood[25]. Furthermore, we found that the esterase gene is regulated by the two-component regulatory system CovRS[25], which regulates many known virulence factors. The S. equi homologue of the GAS esterase has optimal activities against acetyl esters[26]. We are in the process to determine how this protein contributes to GAS pathogenesis and virulence.
Studies on bacteriology and pathogenesis of S. equi
S. equi is a horse pathogen causing strangles. Although S. equi and GAS have different host specificity, they have similar genetic make-up. We have taken a comparative approach in our studies on bacteriology and pathogenesis of both organisms. The two-component regulatory system VicRK is important to virulence and growth of both S. equi and GAS[27,28]. The secreted esterases produced by the two organisms have similar substrate specificity[26]. S. equi also has the Shp/HtsABC machinery for heme uptake[29]. However, the homologue of the GAS Mac protein does not inhibit opsonophagocytosis of S. equi by horse neutrophils[30]. These studies highlight the similarity and difference between the two pathogens.
CONCLUSION
Heme is a major source of essential iron for many bacterial pathogens, and the machineries for heme acquisition are potential targets for prevention and treatment of bacterial infections. Dr. Lei’s laboratory has made significant contributions to the understanding of heme acquisition in Gram-positive pathogens regarding the machinery, pathway, reaction, kinetic and biochemical mechanisms, and structural basis of heme transfer along the pathway. His studies have the potential to provide clinically relevant antibiotic strategies to inhibit the heme acquisition process for treating bacterial infections. Dr. Lei’s studies on pathogenesis of GAS identified the secreted esterase as a CovRS-regulated virulence factor that is a protective antigen and is critical for GAS spreading in the skin and systemic dissemination. Further studies on the esterase will reveal novel mechanism of GAS pathogenesis.