Copyright
©The Author(s) 2018.
World J Biol Chem. Oct 18, 2018; 9(1): 1-15
Published online Oct 18, 2018. doi: 10.4331/wjbc.v9.i1.1
Published online Oct 18, 2018. doi: 10.4331/wjbc.v9.i1.1
Figure 1 Process for the synthesis and oxidative folding of secretory proteins and membrane proteins within endoplasmic reticulum.
During synthesis in the endoplasmic reticulum, both secretory proteins and membrane proteins need to be oxidatively folded before being translocated to final destination via the Golgi body. While endoplasmic reticulum oxidoreductin 1 utilizes molecular oxygen to oxidize reactive sulfhydryl groups on protein disulfide isomerase family proteins, peroxiredoxin 4 and glutathione peroxidase 7/8 oxidize them by means of hydrogen peroxide. ROS: Reactive oxygen species; ER: Endoplasmic reticulum; ERO1: Endoplasmic reticulum oxidoreductin 1; PDI: Protein disulfide isomerase; PRDX4: Peroxiredoxin 4; GPX: Glutathione peroxidase.
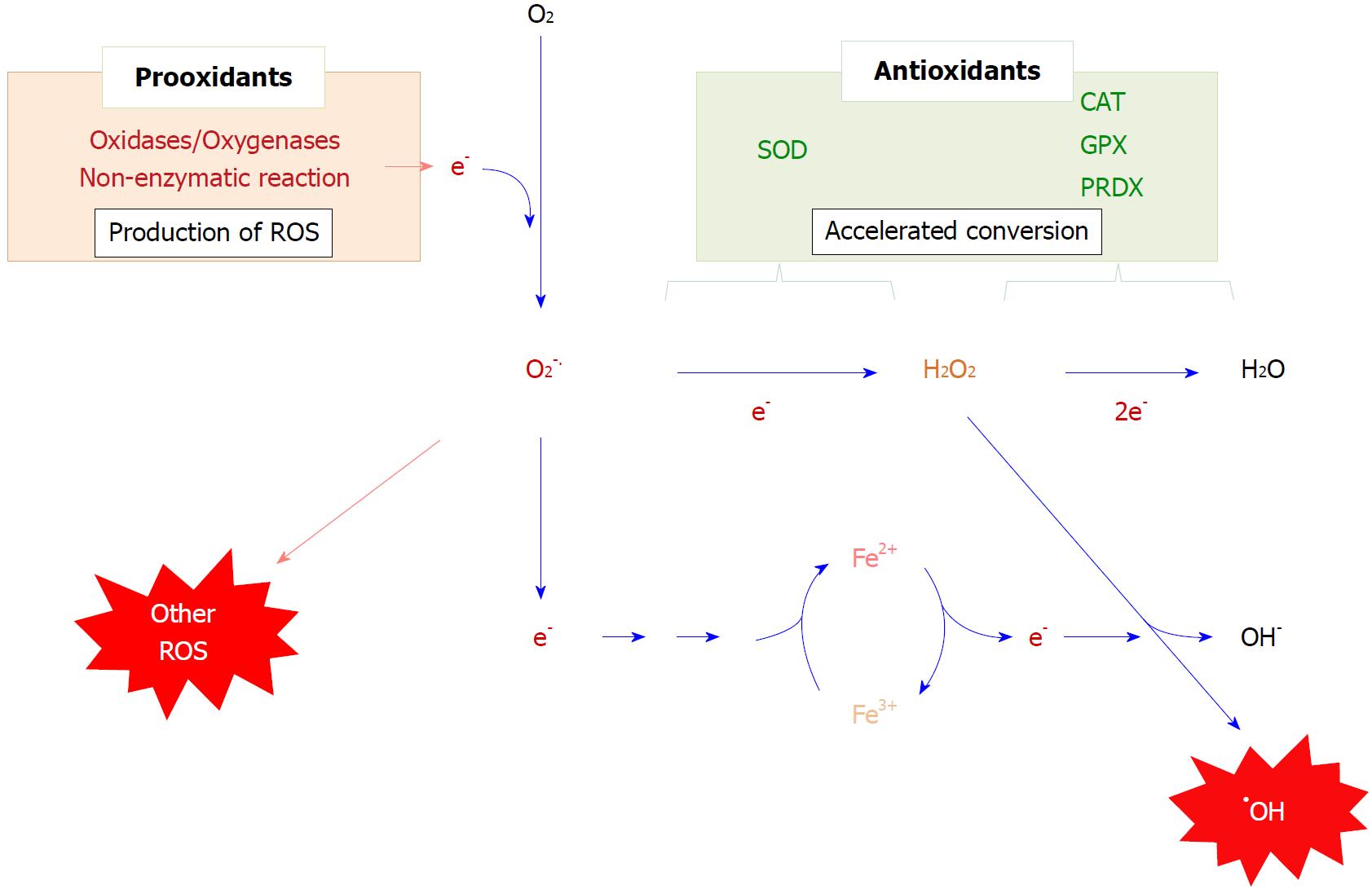
Figure 2 Production, conversion, and suppression of reactive oxygen species.
The production of reactive oxygen species is largely initiated by a one-electron donation to an oxygen molecule, resulting in the production of superoxide (O2.-). Superoxide undergoes spontaneous dismutation to hydrogen peroxide, a process that is markedly accelerated by superoxide dismutase. The resulting hydrogen peroxide can be rapidly eliminated by peroxidases, such as catalase, glutathione peroxidase and peroxiredoxin In the meantime, a one-electron reduction of a transition metal, notably ferric to ferrous ion, results in the conversion of hydrogen peroxide to hydroxyl radicals (•OH). ROS: Reactive oxygen species; SOD: Superoxide dismutase; CAT: Catalase; GPX: Glutathione peroxidase; PRDX: Peroxiredoxin.
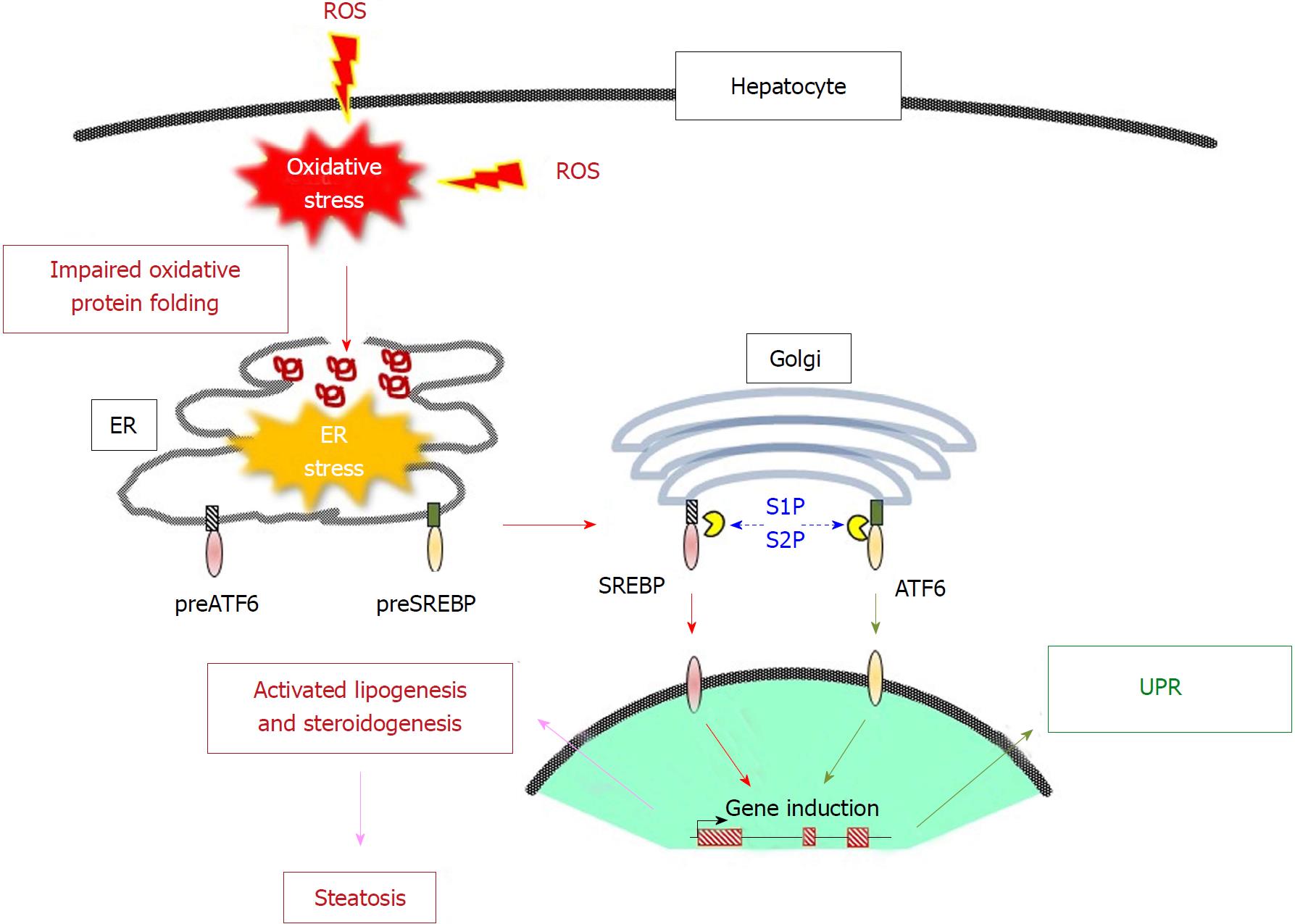
Figure 3 Coordinate action of oxidative stress and endoplasmic reticulum stress in liver steatosis.
Excessively produced reactive oxygen species (ROS) cause the misfolding of proteins in the endoplasmic reticulum (ER), which leads to ER stress. The precursor forms of activating transcription factor 6 (ATF6) and sterol regulatory element-binding proteins (SREBPs) in the ER membrane are translocated to the Golgi body by an independent mechanism but are proteolytically activated there by site-1 protease (S1P) and S2P. The transcriptionally active ATF6 and SREBP then move to the nucleus. While ATF6 exerts a protective function by activating the genes involved in the unfolded protein response, SREBPs activates genes that are involved in lipogenesis and steroidogenesis, which may lead to the development of nonalcoholic fatty liver disease. ROS: Reactive oxygen species; ER: Endoplasmic reticulum; ATF6: Activating transcription factor 6; SREBP: Sterol regulatory element-binding protein; S1P: Site-1 protease; UPR: Unfolded protein response.
- Citation: Fujii J, Homma T, Kobayashi S, Seo HG. Mutual interaction between oxidative stress and endoplasmic reticulum stress in the pathogenesis of diseases specifically focusing on non-alcoholic fatty liver disease. World J Biol Chem 2018; 9(1): 1-15
- URL: https://www.wjgnet.com/1949-8454/full/v9/i1/1.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v9.i1.1