Copyright
©The Author(s) 2016.
World J Biol Chem. Feb 26, 2016; 7(1): 14-43
Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.14
Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.14
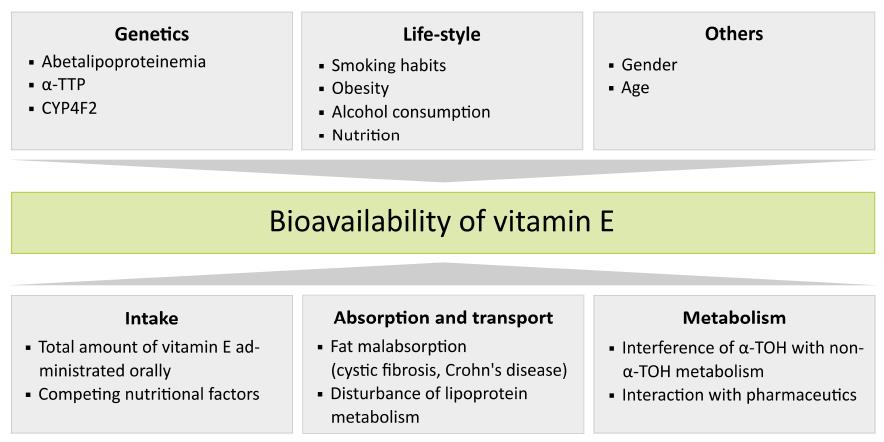
Figure 1 Factors influencing the bioavailability of vitamin E.
Some factors affecting bioavailablity cannot be influenced, such as gender, age or genetic disposition, whereas others depend on individual habits and can be summarized as life-style factors. Variations in the physiological handling of vitamin E can also change its distribution status in the body. While uptake of vitamin E can be actively modulated, for example by the total amount of vitamin E intake and competing nutritional factors, absorption and transport principally depend on the state of health of the individual. In contrast, regulation of vitamin E metabolism is just partly influenced by exogenous factors, such as interfering pharmaceuticals.
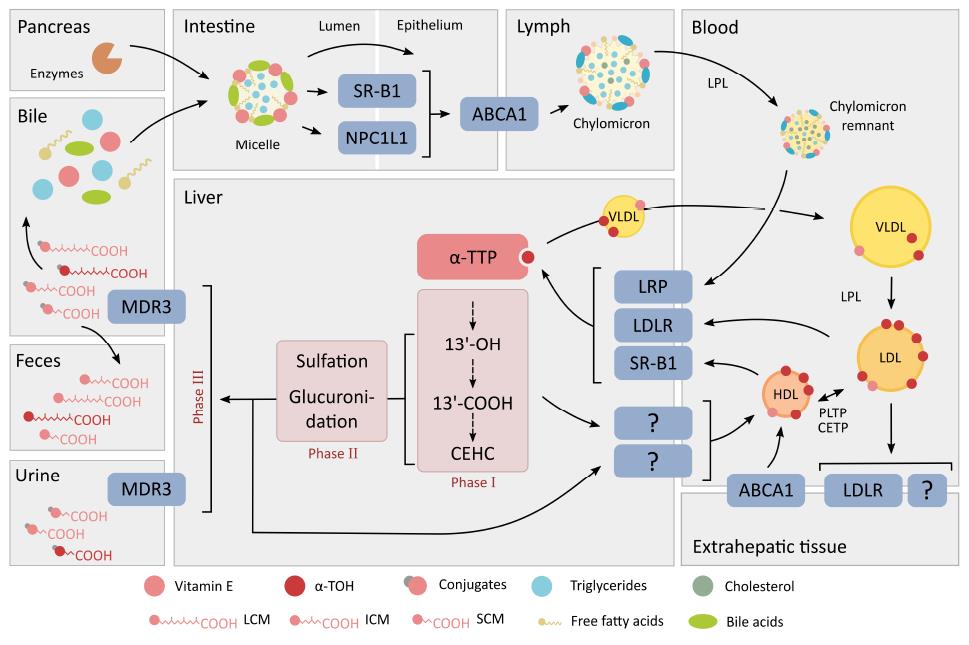
Figure 2 Absorption, transport and metabolism of vitamin E.
The route of vitamin E after oral intake follows in general the pathway of other lipids. Pancreatic and intestinal enzymatic digestion followed by the circulation and distribution to the liver and non-hepatic tissues is the same for all vitamin E forms. Discrimination between the different forms of vitamin E in favor of α-TOH occurs mainly in the liver by α-TTP, which protects α-TOH from excessive degradation and excretion. The figure was modified from[43,46,56,60]. SR-B1: Scavenger receptor class B type 1; LPL: Lipoprotein lipase; NPC1L1: Niemann-Pick C1-like 1; VLDL: Very low density lipoproteins; HDL: High density lipoproteins; α-TOH: α-tocopherols; α-TTP: α-TOH transfer protein; LDL: Low density lipoproteins; LRP: LDL receptor-related proteins; LDLR: LDL receptor; 13’-OH: 13’-hydroxychromanol; 13’-COOH: 13’-carboxychromanol; CEHC: Carboxyethylhydroxychromanols; HDL: High density lipoproteins; PLTP: Phospholipid transfer protein; CETP: Cholesteryl ester transfer protein; LCM: Long-chain metabolites; ICM: Intermediate-chain metabolites; SCM: Short-chain metabolites.
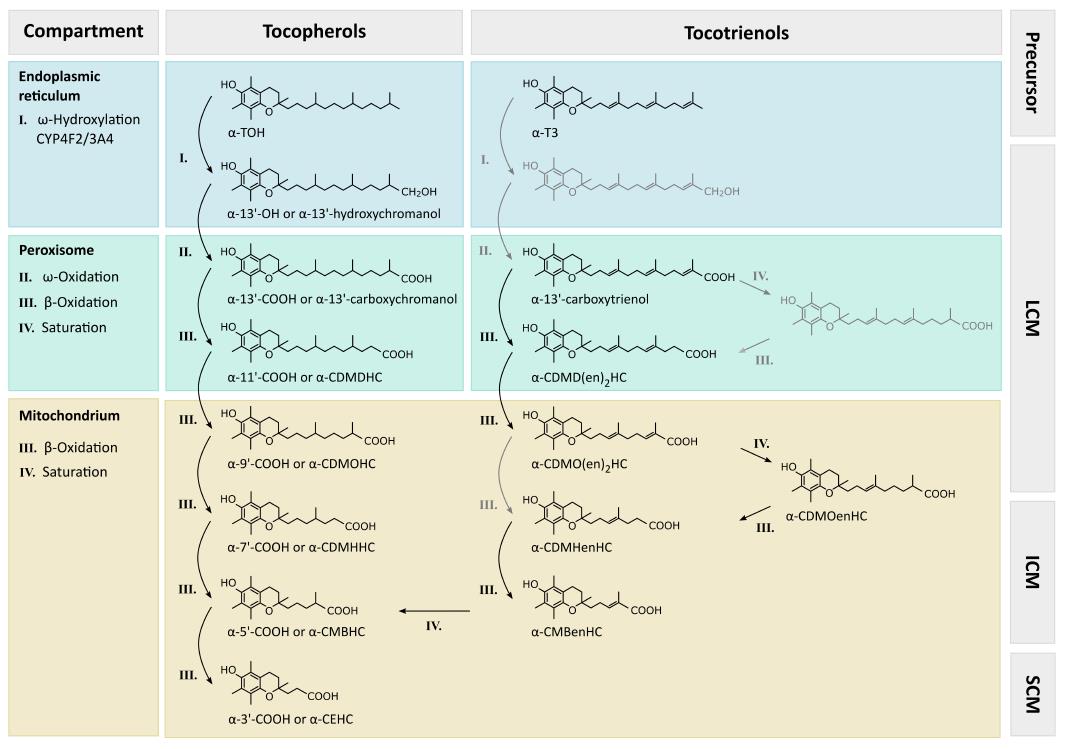
Figure 3 Metabolism of vitamin E.
The metabolism of vitamin E starts with one cycle of ω-hydroxylation followed by five cycles of β-oxidation. The principal catabolic pathway is independent of the saturation of the side-chain[136,137] or the substitution of the chromanol ring system[136], whereas the rate of metabolic degradation is modulated mostly by these two factors[136] (for further details, see the sections on α-TTP in chapter “Intracellular binding proteins” as well as CYP4F2 and kinetics in chapter “Metabolism of vitamin E”). While the intracellular compartmentation of the different reaction steps is known for TOHs[138], it remains unresolved for T3s. The same applies to the structures and arrows marked in grey; they reflect molecules or principle steps which fit into the general concept so far but need further experimental confirmation and molecular characterization. α-TOH: α-tocopherols; α-T3: α-tocotrienols; 13’-OH: 13’-hydroxychromanol; 13’-COOH: 13’-carboxychromanol; LCM: Long-chain metabolites; ICM: Intermediate-chain metabolites; SCM: Short-chain metabolites; CDMD(en)2HC: Carboxydimethyldecadienylhydroxychromanol; CDMOenHC: Carbodimethyloctenylhydroxychromanol; CDMHenHC: Carboxymethylhexenylhydroxychromanol; CMBenHC: Carboxymethylbutadienylhydroxychromanol; CDMOHC: Carboxymethyloctylhydroxychromanol; CDMHHC: Carboxymethylhexylhydroxychromanol; CMBHC: Carboxymethylbutylhydroxychromanol; CEHC: Carboxyethylhydroxychromanols.
- Citation: Schmölz L, Birringer M, Lorkowski S, Wallert M. Complexity of vitamin E metabolism. World J Biol Chem 2016; 7(1): 14-43
- URL: https://www.wjgnet.com/1949-8454/full/v7/i1/14.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v7.i1.14