Copyright
©The Author(s) 2016.
World J Biol Chem. Feb 26, 2016; 7(1): 1-13
Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.1
Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.1
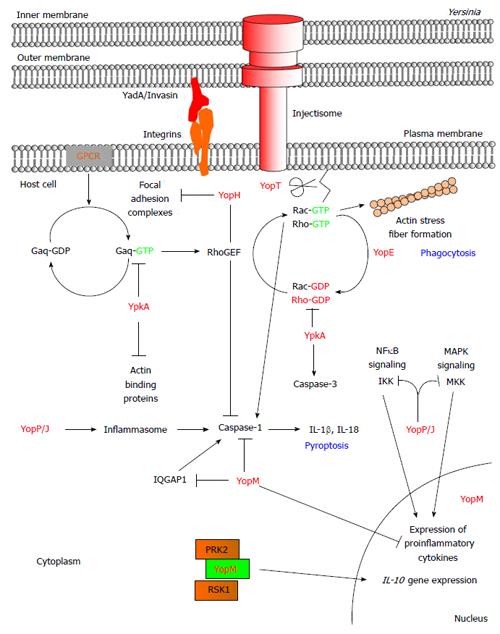
Figure 1 Modulation of host signaling pathways by the Yersinia effector proteins.
Pathogenic Yersinia utilizes the T3SS to subvert the host innate immune response. During an infection, the Yersinia bacterium adheres onto the target host cell by using adhesion proteins such as YadA and invasin to bind onto host β1 integrin. This enables the injectisome of the T3SS to form a pore at the host plasma membrane and the subsequent translocation of the Yersinia effector proteins directly into the cytoplasm. The Yersinia effector proteins localize to distinct subcellular locations where they mimic host proteins/functions to disrupt host signaling pathways. The Ser/Thr kinase YpkA binds actin monomers resulting in YpkA autophosphorylation and kinase activation. Upon GPCR activation of the host G protein Gαq, YpkA binds to and phosphorylates Gαq to inhibit Gαq-mediated activation of the Rho GTPases and subsequent actin stress fiber formation. YpkA also uses actin as bait for actin binding proteins (i.e., VASP, cofilin, and WASP) whereby YpkA then phosphorylates these proteins. In addition, YpkA binds onto the Rho GTPases, RhoA and Rac1, to inhibit the exchange of GDP for GTP. YopE is a GAP protein that facilitates the intrinsic GTPase activity of the Rho proteins resulting in their inactivation. The cysteine protease, YopT, disrupts the actin cytoskeleton by cleaving post-translationally modified Rho GTPases. Cleavage of the Rho proteins results in the mislocalization and inactivation of the Rho proteins. YopH, a PTPase, dephosphorylates focal adhesion components such as FAK, p130Cas, paxillin, Fyb, SKAP-HOM, PRAM-1, and SLP-76 to disrupt focal adhesion complexes, β1 integrin signaling, and activation of the Rho protein, Rac1. Together, the enzymatic activity of YpkA, YopE, YopT, and YopH affects organization of the actin cytoskeleton to inhibit phagocytosis. YopP/J is an acetyltransferase with deubiquitinase activity and a putative cysteine protease function. YopP/J targets signaling components of the MAPK and NFκB signaling pathways following activation of Toll-like receptor 4 to inhibit production of proinflammatory cytokines. In addition to inhibiting the MAPK and NFκB signaling pathways, YopP/J activates the inflammasome complex resulting in the maturation of caspase-1, the production of IL-1β and IL-18, and the cell death process termed pyroptosis. YopE and YopT were implicated in inhibiting inflammasome and caspase-1 activation by targeting Rac1. YopM is a protein with leucine-rich repeats that localizes to the cytoplasm and the nucleus of the cell. The translocation of YopM results in the inhibition of proinflammatory cytokine production. Cytoplasmically-localized YopM inhibits caspase-1 activity by binding to the upstream component, IQGAP1, to pro-caspase-1, or to mature caspase-1. YopM also binds onto the PRK and RSK isoforms and inhibits phosphatase-mediated deposphorylation of these two host kinases. The underlying function of targeting these two kinases by YopM is still unclear, but is linked to the production of the anti-inflammatory cytokine, IL-10. T3SS: Type III secretion system; GPCR: G protein-coupled receptor; IL: Interleukin; FAK: Focal adhesion kinase; MAPK: Mitogen-activated protein kinase; NFκB: Nuclear factor kappa b; IQGAP1: IQ motif containing GTPase activating protein 1; PRK: Protein kinase C-related kinase; RSK: Ribosomal S6 protein kinase; GAP: GTPase activating protein; VASP: Vasodilator-stimulated phosphoprotein; WASP: Wiskott-Aldrich syndrome protein; p130Cas: Crk associated tyrosine kinase substrate; Fyb: Fyn-binding protein; SKAP-HOM: Src kinase-associated phosphoprotein 55 homologue; PRAM-1: PML-retinoic acid receptor alpha regulated adaptor molecule 1; SLP-76: SH2 domain containing leukocyte protein of 76 kDa.
- Citation: Pha K, Navarro L. Yersinia type III effectors perturb host innate immune responses. World J Biol Chem 2016; 7(1): 1-13
- URL: https://www.wjgnet.com/1949-8454/full/v7/i1/1.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v7.i1.1