Copyright
©The Author(s) 2021.
World J Biol Chem. Jan 27, 2021; 12(1): 1-14
Published online Jan 27, 2021. doi: 10.4331/wjbc.v12.i1.1
Published online Jan 27, 2021. doi: 10.4331/wjbc.v12.i1.1
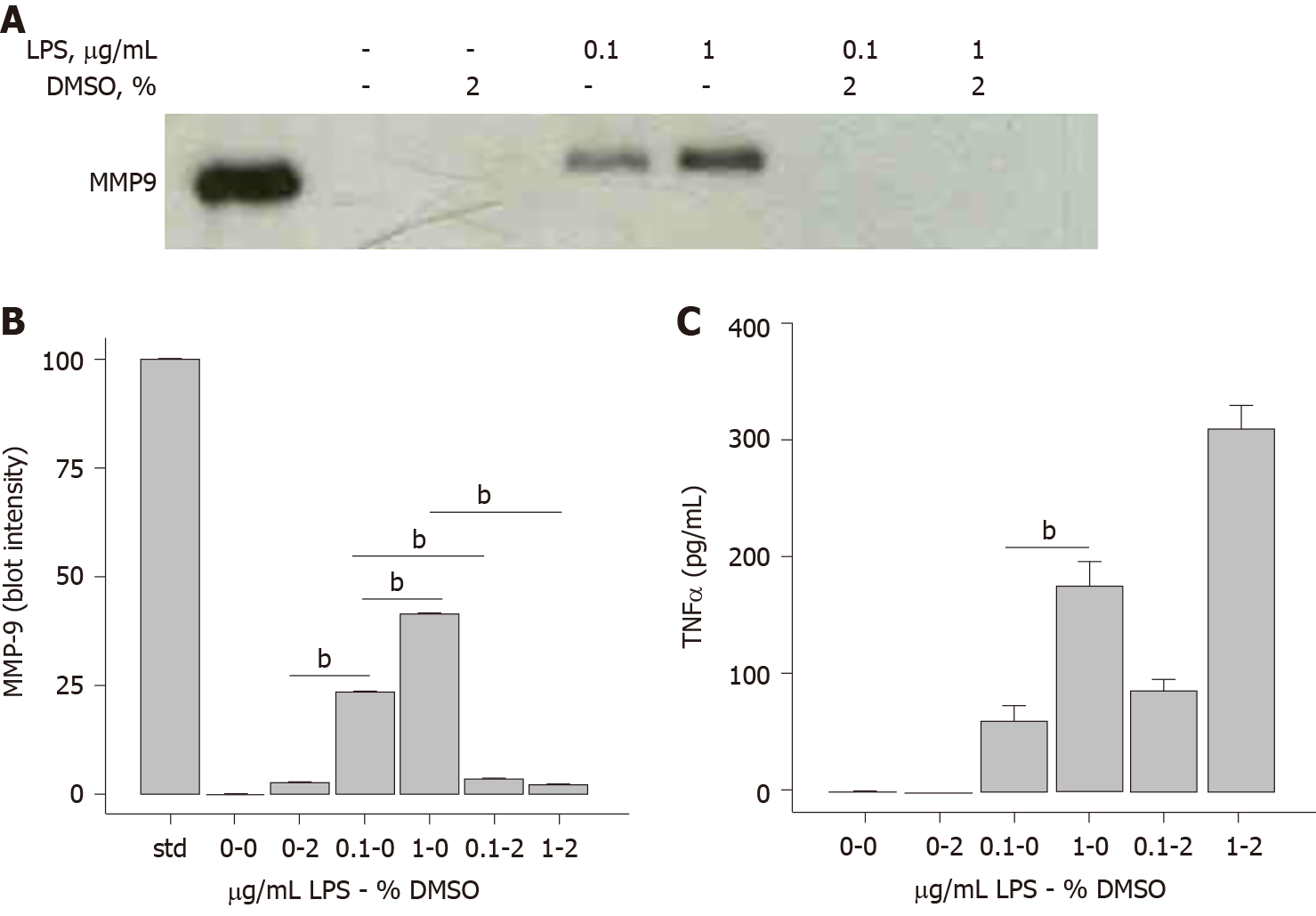
Figure 1 Dimethyl sulfoxide inhibits lipopolysaccharide-induced matrix-metalloproteinase-9, but not tumor necrosis factor α, secretion.
THP-1 monocytes were treated in the absence or presence of dimethyl sulfoxide (2%) and with either sterile water or lipopolysaccharide (0.1 μg/mL or 1 μg/mL) for 72 h. A: Cells were collected, centrifuged at 500 g for 10 min and supernatant assessed for matrix-metalloproteinase-9 (MMP-9), SDS-PAGE/immunoblot of cell supernatants. Lane 1 is an MMP-9 Western blotting standard (10 μL); B: Densitometry of Panel A. Each data bar is the average ± standard error for three independent densitometry measurements; and C: TNFα levels were determined in cell supernatants by enzyme-linked immuno sorbent assay and each data bar is the average ± std error for three independent measurements, Tumor necrosis factor α (TNFα). Statistical differences (aP < 0.05 or bP < 0.01) for 0.1 μg/mL or 1 μg/mL lipopolysaccharide and dimethyl sulfoxide inhibition of MMP-9 or TNFα secretion are shown. DMSO: Dimethyl sulfoxide; LPS: Lipopolysaccharide; MMP-9: Matrix-metalloproteinase-9; TNFα: Tumor necrosis factor α.
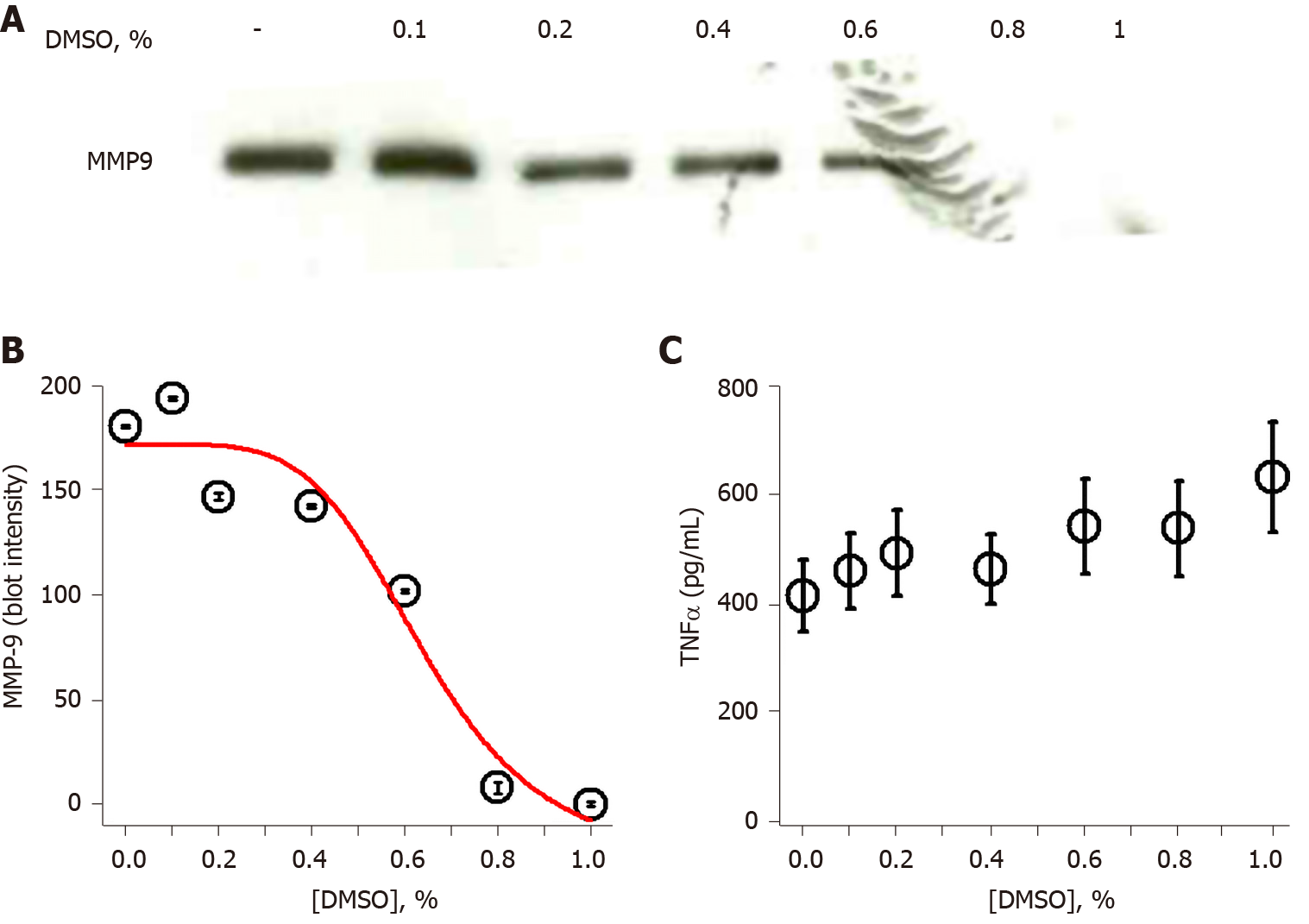
Figure 2 Dimethyl sulfoxide inhibits lipopolysaccharide-induced matrix-metalloproteinase-9 in a dose-dependent fashion.
A: SDS-PAGE/immunoblot of cell supernatants; B: Densitometry of Panel A. Each data bar is the average ± standard error for three independent densitometry measurements. Data were fit to a nonlinear inhibition equation, which produced an IC50 of 0.64%; and C: Tumor necrosis factor α levels were determined in cell supernatants by enzyme-linked immuno sorbent assay and each data bar is the average ± standard error for three independent measurements. THP-1 monocytes were treated with a concentration range of dimethyl sulfoxide (0.1%-1%) followed by lipopolysaccharide (1 μg/mL) for 72 h. Cells were collected, centrifuged at 500 g for 10 min and supernatant assessed for (A) matrix-metalloproteinase-9 and (C) tumor necrosis factor α. DMSO: Dimethyl sulfoxide; MMP-9: Matrix-metalloproteinase-9; TNFα: Tumor necrosis factor α.
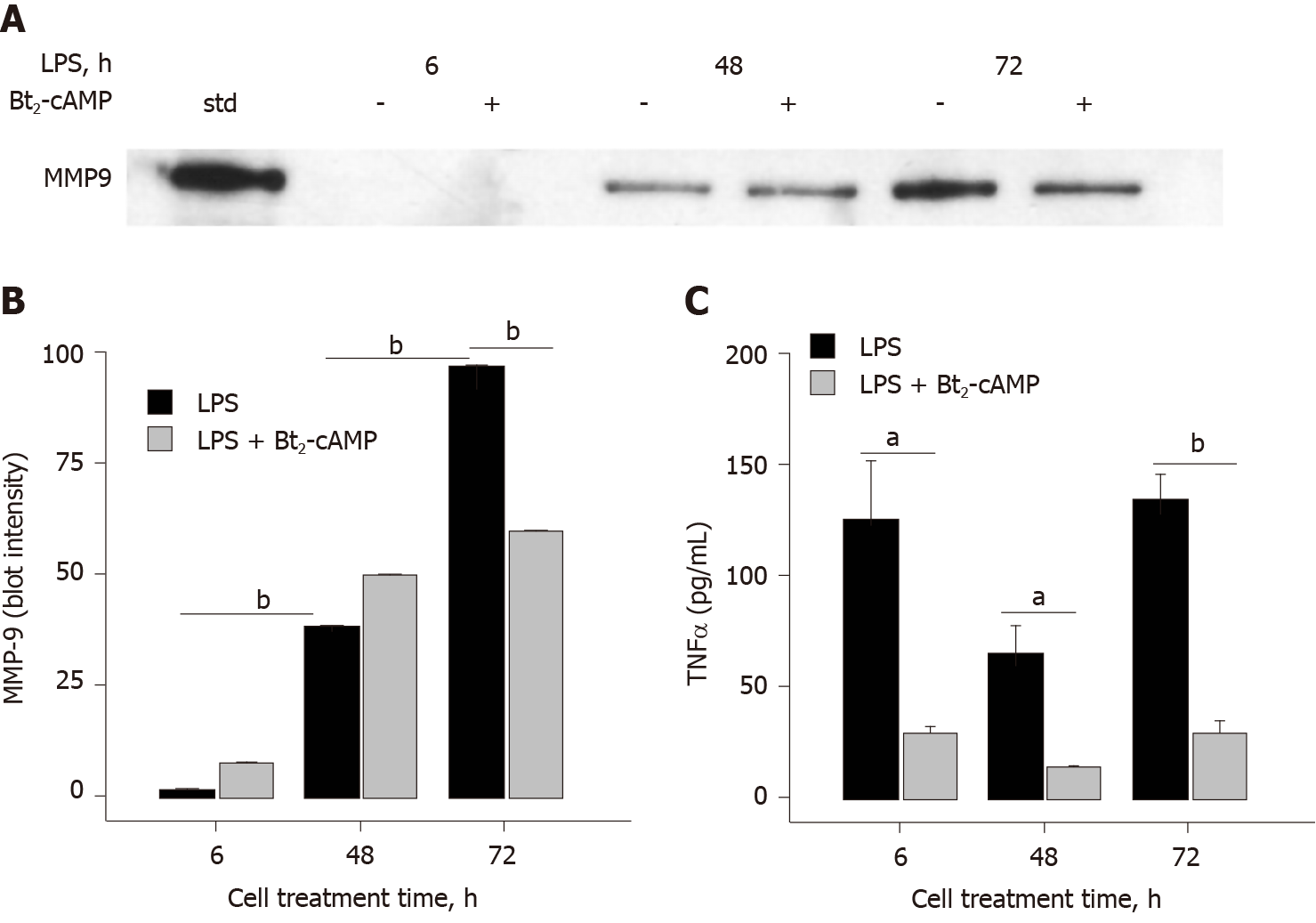
Figure 3 Cyclic adenosine monophosphate inhibits lipopolysaccharide-induced matrix-metalloproteinase-9 and tumor necrosis factor α secretion.
A: SDS-PAGE/immunoblot of cell supernatants. Lane 1 is a matrix-metalloproteinase-9 (MMP-9) standard; B: Densitometry of Panel A. Each data bar is the average ± standard error for three independent densitometry measurements; and C: Tumor necrosis factor α (TNFα) levels were determined in cell supernatants by enzyme-linked immuno sorbent assay and each data bar is the average ± standard error for three independent measurements. THP-1 monocytes were treated with 3',5'-cyclic adenosine monophosphate (300 μmol/L) followed by lipopolysaccharide (0.03 μg/mL) for 6, 48, and 72 h. Cells were collected, centrifuged at 500 g for 10 min and supernatant assessed for (A) MMP-9 and (C) TNFα. Statistical differences (aP < 0.05 or bP < 0.01) between MMP-9 secretion at different lipopolysaccharide treatment times and 3',5'-cyclic adenosine monophosphate inhibition of either MMP-9 or TNFα secretion are shown. LPS: Lipopolysaccharide; MMP-9: Matrix-metalloproteinase-9; TNFα: Tumor necrosis factor α; Bt2-cAMP: 3',5'-cyclic adenosine monophosphate.
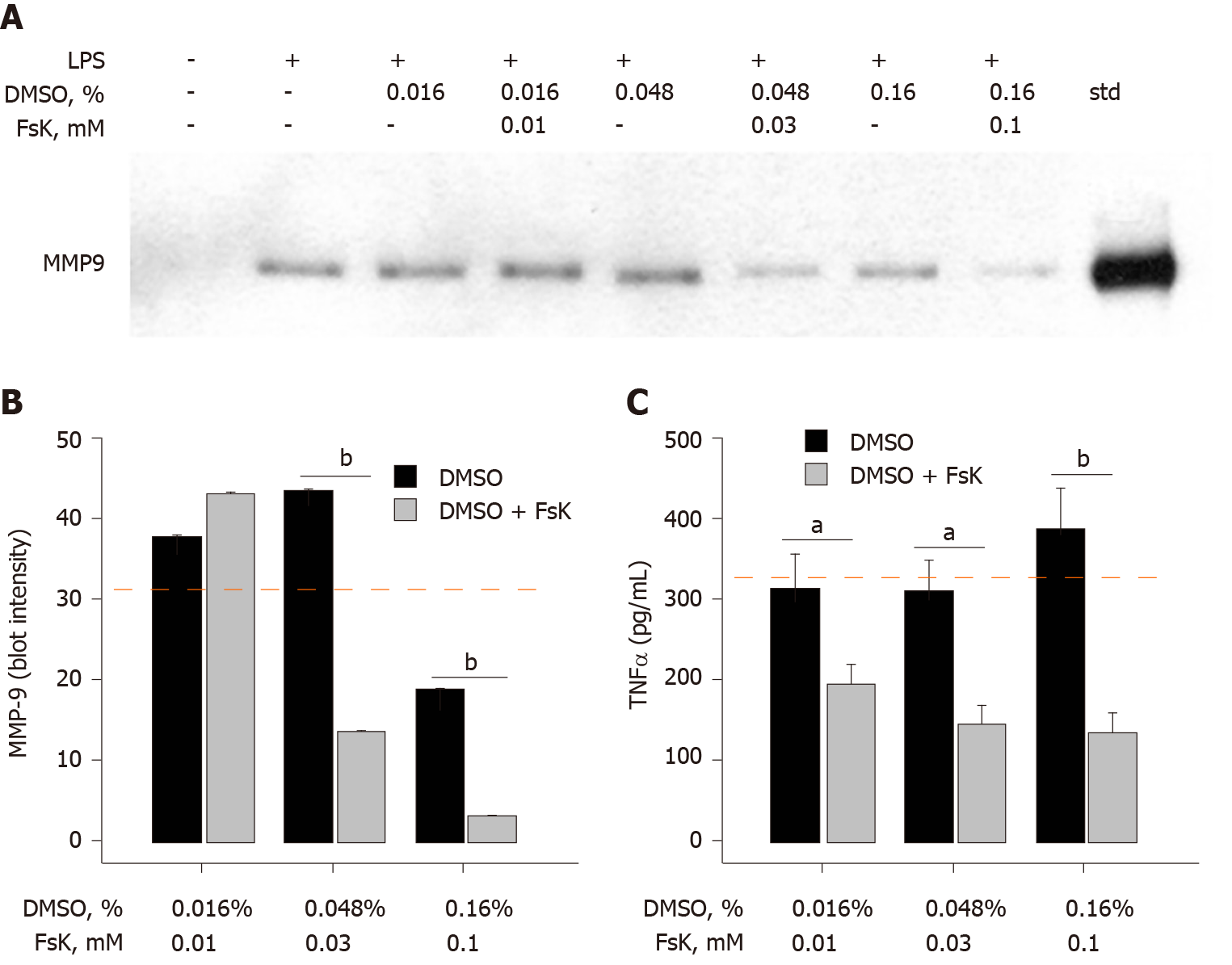
Figure 4 Activation of adenylyl cyclase inhibits lipopolysaccharide-induced matrix-metalloproteinase-9 and tumor necrosis factor α secretion.
A: SDS-PAGE/immunoblot of cell supernatants. Lane 9 is a matrix-metalloproteinase-9 standard; B: Densitometry of Panel A. Each data bar is the average ± standard error for three independent densitometry measurements; and C: Tumor necrosis factor α (TNFα) levels were determined in cell supernatants by enzyme-linked immuno sorbent assay and each data bar is the average ± standard error for three independent measurements. THP-1 monocytes were treated with three forskolin (Fsk) concentrations that contained different percentages of dimethyl sulfoxide (DMSO), followed by lipopolysaccharide (1 μg/mL) for 72 h. Cells were collected, centrifuged at 500 g for 10 min and supernatant assessed for matrix-metalloproteinase-9 and TNFα. The red dashed lines in Panels B and C represent TNFα secretion induced by lipopolysaccharide (1 μg/mL) in the absence of DMSO or Fsk. Statistical differences (aP < 0.05 or bP < 0.01) between DMSO and DMSO + Fsk are shown. LPS: Lipopolysaccharide; DMSO: Dimethyl sulfoxide; MMP-9: Matrix-metalloproteinase-9; TNFα: Tumor necrosis factor α; Fsk: Forskolin.
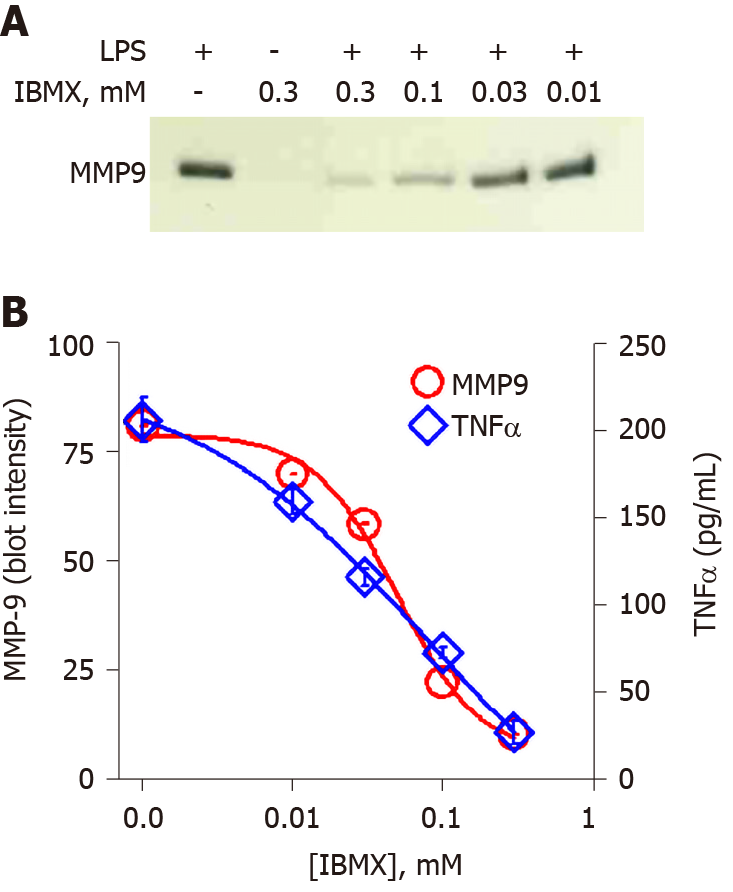
Figure 5 Inhibition of cyclic adenosine monophosphate phosphodiesterase inhibits lipopolysaccharide-induced matrix-metalloproteinase-9 and tumor necrosis factor α in a dose-dependent fashion.
A: SDS-PAGE/WB of cell supernatants; and B: Densitometry of the matrix-metalloproteinase-9 (MMP-9) immunoblot in Panel A (circles). Each data point is the average ± standard error for three independent densitometry measurements. Tumor necrosis factor α (TNFα) levels (diamonds) were determined in cell supernatants by enzyme-linked immuno sorbent assay and each data point is the average ± standard error for three independent measurements. THP-1 monocytes were treated with a concentration range of 3-isobutyl-1-methylxanthine (0.01-0.3 mmol/L) followed by lipopolysaccharide (1 μg/mL) for 48 h. Cells were collected, centrifuged at 500 g for 10 min and supernatant assessed for (A) MMP-9 and (B) TNFα. MMP-9 and TNFα data points were fit to a nonlinear inhibition equation, which produced IC50 values of 0.05 mmol/L and 0.08 mmol/L for MMP-9 and TNFα inhibition respectively. LPS: Lipopolysaccharide; MMP-9: Matrix-metalloproteinase-9; TNFα: Tumor necrosis factor α; IBMX: 3-Isobutyl-1-methylxanthine.
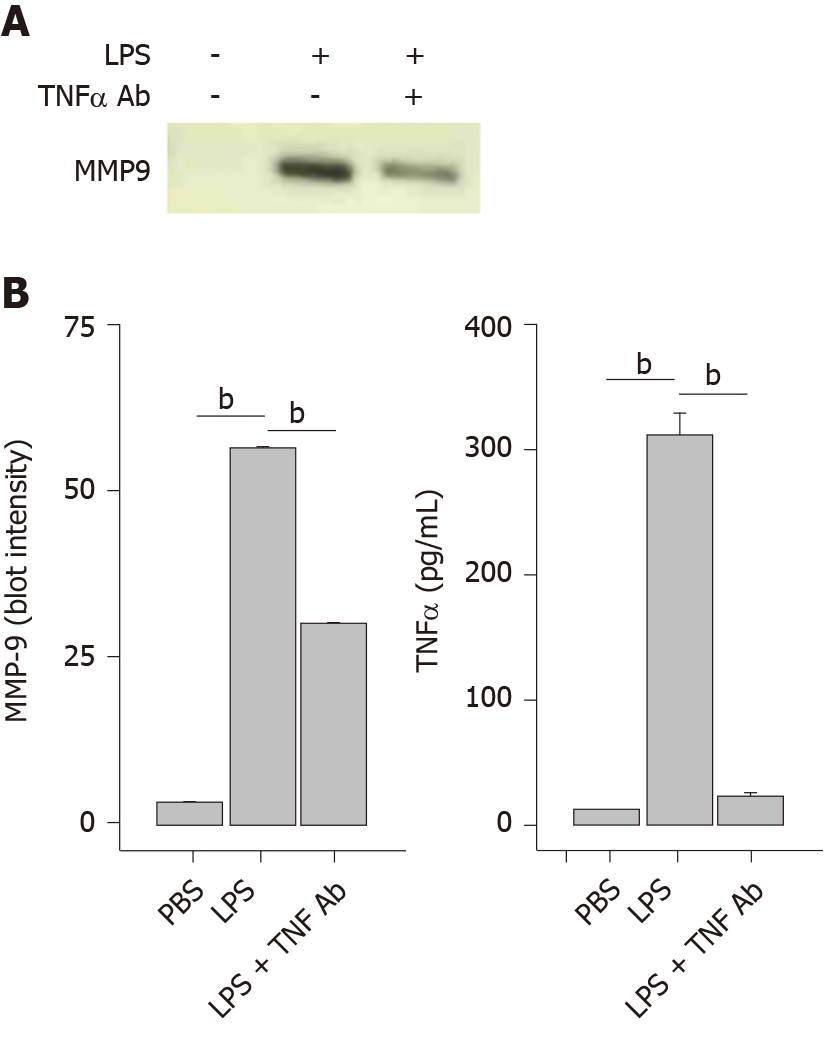
Figure 6 Antif- tumor necrosis factor α antibody blocks lipopolysaccharide-induced matrix-metalloproteinase-9 secretion.
THP-1 monocytes were treated with a tumor necrosis factor α (TNFα) antibody (1 μg/mL) followed by lipopolysaccharide (1 μg/mL) for 48 h. Cells were collected, centrifuged at 500 g for 10 min and supernatant assessed for matrix-metalloproteinase-9 and TNFα. A: SDS-PAGE/WB of cell supernatants; and B: Densitometry of Panel A (left plot). Each data bar is the average ± standard error for three independent densitometry measurements. TNFα levels (right plot) were determined in cell supernatants by enzyme-linked immuno sorbent assay and each data bar is the average ± standard error for three independent measurements. Statistical differences (aP < 0.05 or bP < 0.01) between lipopolysaccharide-stimulated matrix-metalloproteinase-9 or TNFα secretion compared to PBS buffer control and in the absence or presence of an anti-TNFα antibody are shown. LPS: Lipopolysaccharide; MMP-9: Matrix-metalloproteinase-9; TNFα: Tumor necrosis factor α.
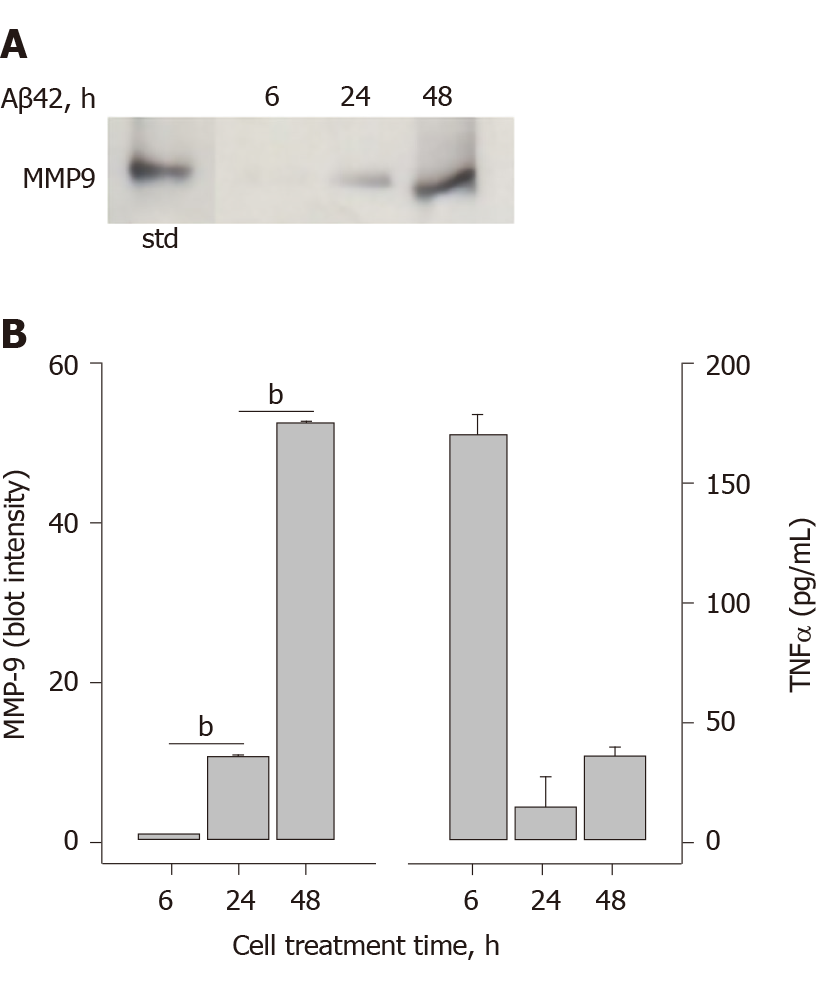
Figure 7 Amyloid-β peptide-induced matrix-metalloproteinase-9 secretion occurs much later than tumor necrosis factor α secretion.
THP-1 monocytes were treated with amyloid-β peptide (17 μmol/L) for 6, 24 and 48 h. Cells were collected, centrifuged at 500 g for 10 min and supernatant assessed for matrix-metalloproteinase-9 (MMP-9) and tumor necrosis factor α (TNFα). A: SDS-PAGE/WB of cell supernatants. Lane 1 is an MMP-9 standard; and B: Densitometry of Panel A (left plot). Each data bar is the average ± standard error for three independent densitometry measurements. Enzyme-linked immuno sorbent assay determination of TNFα levels in cell supernatants (right plot). Each data bar is the average ± standard error for three independent measurements. Statistical differences (aP < 0.05 or bP < 0.01) between MMP-9 or TNFα secretion at different amyloid-β peptide treatment times are shown with an asterisk. Aβ42: Amyloid-β peptide; MMP-9: Matrix-metalloproteinase-9; TNFα: Tumor necrosis factor α.
- Citation: Denner DR, Udan-Johns ML, Nichols MR. Inhibition of matrix metalloproteinase-9 secretion by dimethyl sulfoxide and cyclic adenosine monophosphate in human monocytes. World J Biol Chem 2021; 12(1): 1-14
- URL: https://www.wjgnet.com/1949-8454/full/v12/i1/1.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v12.i1.1