INTRODUCTION
Although research in oncology and surgery has achieved some major milestones, hepatocellular carcinoma (HCC) still represents the fifth most common malignant tumor and the third most common cause of mortality related to cancer in the world[1]. In comparison with other malignant cancers, there is a wide variety of treatments in the armamentarium of surgeons, oncologists and radiologists, such as surgical resection, liver transplantation, chemoembolization, microwave and radiofrequency ablation, or even chemotherapy with sorafenib. However, before deciding on which method to choose from, clinicians ought to first define the clinical stage of the patient’s HCC, which also defines the prognosis.
Especially for HCC, the three important factors determining the patient’s survival are the tumor’s characteristics (size, invasion of the vessels, number of nodules), the patient’s physiologic reserve (for instance, Eastern Cooperative Oncology Group performance status) and the ability of the liver to function properly (Child-Pugh score)[2-4]. In addition, the issue still remains that there is lack of a common language in terms of HCC staging. Histopathology should also be taken into consideration when it comes to staging a type of cancer, and thus a variety of HCC staging systems, such as the Japanese Integrated Staging score, have adopted the American Joint Committee on Cancer TNM staging system[5]. One significant limitation of this system is the fact that it cannot incorporate the unresectable HCCs, because when relying primarily on the pathological characteristics of the tumor, it is a prerequisite that a surgical specimen is needed. Moreover, it does not include two of the three major survival factors mentioned above: physiologic reserve and liver function.
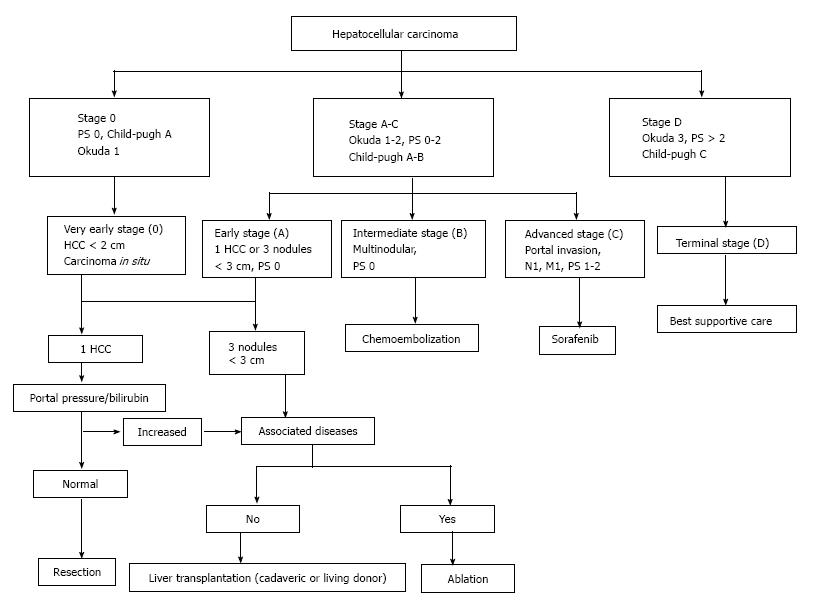
The staging system that seems to be the most inclusive, as well as the most widely verified, is the Barcelona Clinic Liver Cancer (commonly known as BCLC) staging system[6]. Based on this system, HCC patients are classified into subgroups based on their malignancy’s characteristics, the function of the liver and their health in general, and each subgroup is allocated to a different treatment modality according to the treatment algorithm (Figure 1)[7]. On the other hand, a study ranking the different staging systems as to their prognostic value and patient survival, reported the superiority of the Cancer of the Liver Italian Program (commonly known as CLIP) classification and the Chinese University Prognostic Index (commonly known as CUPI)[8]. Although these staging systems differ to a great extent, mostly due to the geographical variation and etiologies of the different HCCs, the EASL-EORTC guidelines suggest that the BCLC classification should be followed when it comes to the management of HCC[9].
Figure 1 Barcelona Clinic Liver Cancer staging system and treatment algorithm.
M: Metastases; N: Nodules; PS: Performance status; HCC: Hepatocellular carcinoma.
In this review, our aim is to thoroughly present the current knowledge around laparoscopic hepatectomy, with a special interest on the indications, general principles and technique, as well as its envisioned future.
Indications for surgical resection for HCC
The fact that HCC arises mostly in a cirrhotic liver, means that any type of treatment of the tumor has to account for factors related to hepatic quality and function. Regarding the liver-related factors, both quantity and quality of the future liver remnant (FLR) should be taken into consideration before performing an excision. One way to achieve hepatic hypertrophy, to ensure adequate liver mass posthepatectomy, is portal vein embolization (PVE), which improves the FLR of the side not embolized[10]. Another important factor is the preoperative liver function status, which can be evaluated by the Child-Pugh classification system (class A patients are suitable for hepatectomy, while class B or C patients are more prone to major complications after surgery due to liver dysfunction)[11]. Nevertheless, a significant contraindication to hepatectomy is high grade portal hypertension, which could be assessed either invasively by measuring hepatic venous pressure gradient (HVPG)[12,13] or noninvasively by measuring the platelet count[14].
The mostly studied tumor-related issues that determine the indications for liver surgical resection are tumor size, number of tumors and vascular invasion. Size alone is not a determining factor for patient survival after surgery, as it has been shown that excision of tumors larger than 10 cm may exhibit equal survival to those smaller than 10 cm, provided that the FLR is sufficient and there is insignificant vascular invasion[15]. Additionally, the management of multinodular HCC is still under discussion, with tumors arising in the cirrhotic liver due to the “field effect” showing improved survival posthepatectomy, in contrast to intrahepatic metastases, which usually present as a sizeable lesion encircled by satellite minor tumor masses[16,17]. Last but not least, it is generally accepted that significant invasion of major vessels remains an important contraindication to surgical resection owing to worse prognosis and early disease recurrence[18].
Laparoscopic liver resection in general
To begin with, there are some challenges in the wider application of laparoscopic surgery. The first one is the loss of tactile sense, such as the margins and staging, but this could be helped by the use of laparoscopic ultrasound and hand-assisted techniques. Another obstacle is that of limited access and instrumentation, which could be solved by hand-assisted maneuvers and improved retractors. The question of bleeding control, while always a significant threat with the liver, can be addressed with devices such as the harmonic scalpel, the vascular stapler and the LigaSure device. In addition, other issues to be addressed include time and money, port side metastases and gas embolism.
Although many studies that compare laparoscopic liver resection (LLR) to open liver resection (OLR) have been carried out to date[19], only one of them was a randomized controlled trial[20]. Despite that, there has been an effort to progress over time from benign to malignant lesions, from smaller to bigger and from normal liver over to the cirrhotic one, by carefully selecting suitable candidates.
Currently, peripheral tumors (segments II, III, IVb, V and VI) are easier to resect laparoscopically[21]. Regarding larger and deeper located tumors, or those located superiorly or posteriorly, which are more difficult to excise, despite the fact that LLR can be implemented[22-24], it is advisable that hand-assisted or a hybrid technique (laparoscopic-assisted open) are performed[25]. On the whole, LLR is currently indicated, especially for solitary HCCs, 5 cm or less, located in the periphery of the liver, especially in segments II through VI, that allow a wedge excision or a segmentectomy[19,26].
Current status of laparoscopic liver resection for HCC
According to Nguyen and Geller from 1992, when the first LLR was performed till 2009, about 2804 LLRs have been carried out. Half of them involved malignant lesions, while 45% were benign and about 1.7% live donor hepatectomies, with the remaining being undetermined[26]. Regarding the technique used 75% were completely laparoscopic, 17% were hand-assisted and about 2% were hybrid, while as it pertains to the resected specimen 45% of them were wedge or segment resections, 20% were anatomic left lateral sectionectomies and 9% were right and 7% were left hepatectomies[26].
Significantly, only a small percentage of the laparoscopic procedures were converted to open (4.1%) and to hand-assisted (0.7%).
Safety
LLR is generally thought of as a safe and feasible operation[27,28]. A previously published world review[26] reported a clearly low rate of mortality (0.3%), without any deaths occurring during the procedure. The most common causes of death were liver dysfunction, multiple organ failure, delirium tremens and hemorrhage. Morbidity, on the other hand, was 10.5%, with postoperative bile leak being the most common complication (1.5%), followed by transient liver failure and liver abscess, as well as bleeding, surgical site infection and collection of fluid inside the abdominal cavity. These low rates could possibly be attributed to several factors, though.
It is clear that careful patient selection and high surgical expertise play important roles. Apart from that, the utilization of the hand-assisted method may decrease bleeding more quickly through direct pressure, while laparoscopic sutures could be more safely executed, thus rendering more difficult cases feasible[29]. Moreover, although keeping a low pressure pneumoperitoneum reduces the incidence of air embolism, if it is increased it can be efficacious in reducing venous leakage[29].
The positive effects of pneumoperitoneum do not stop there, as it is helpful in achieving optimal visualization and as a result bloodless parenchymal transection, which decreases the risk of major hemorrhage and the requirements of blood transfusion, therefore also avoiding the unspecified immunosuppression which increases morbidity and cancer recurrence[30]. In addition, a recent meta-analysis reported lower loss of blood and decreased need for transfusion, rapid recovery and significantly decreased postoperative pain[31]. Finally, data suggest that complications are going to decrease more as the surgeon becomes more experienced.
Operative time
In general, operating time, just as blood loss, is quite challenging to calculate due to the high heterogeneity among the wide range of procedures being performed. Despite this, the world review reported that the operating time may vary from 99 min to 331 min[26], while Soubrane et al[30] estimated a median operating time of 3 h. Similarly, Cannon et al[29] found that for their first 100 patients, the operative time was also 3 h, but as surgeons gained more experience, the time went down to around 2 h for their most recent 100 patients. On the contrary, a meta-analysis of 26 studies showed a significantly increased procedure time as to the open approach[32].
As a matter of fact, OLR involves a larger incision, which needs extra time to be closed; hence, when surgeons become even more expert in this field of hepatobiliary surgery, LLR is not going to be that much more time-consuming. Another meta-analysis found out no difference between LLR and OLR regarding the operative time[28], suggesting that only a minor variance exists. Obviously, the critical factor regarding operative time is the learning curve, something which will also change again in the future as these procedures become more established and they move from the level of the attending to the level of the fellow, and potentially even to the senior resident.
Length of hospital stay
As expected, laparoscopic procedures show a remarkable decrease not only in blood loss and postoperative pain, but also in the length of hospital stay. Specifically, the estimated time for hospital stay is around 2.9 d[29], which is obviously lower than that of the OLR; interestingly, Simillis et al[28] reported a decrease of about 2.6 d in patients treated with LLR compared to those undergoing OLR. The world review[26] exhibited a range between 1.2 d to 15.3 d for LLR, which again was proven to be lower than that of OLR. This variance, though, may be due to nuances among the healthcare providers and cultural habits, as well as due to the fact that some studies included liver cyst excisions, while others did not. This kind of cultural bias tends to play a key role in determining the length of the hospital stay as it ranges only between 1.9 d to 4 d in the United States, while in Europe it is about 3.5 d to 10 d and in Asia 4 d to 20 d for LLR; even so, a constant decrease of about 50% was observed in LLR when compared to OLR[33].
Efficiency
At first, there was great concern regarding LLR and the risk of positive margins, potential tumor seeding and port-site metastasis, which impeded its wide implementation. The results reported by Nguyen et al[26] state categorically that there is no reason for not adopting LLR, as resection with tumor-free margins can be accomplished, and neither significant tumor seeding nor port-site cancer recurrence have ever been reported. The only exception is a patient whose renal cell carcinoma ruptured before the operation, which clearly had nothing to do with the LLR[34]. Moreover, both approaches are equal in terms of oncological survival outcomes[26].
Many studies including patients with HCC or colorectal metastases reported promising survival rates; and, specifically the 5-year survival for colorectal metastases to the liver ranged between 50%-64%, while R0 excision percentages were about the same as those of OLR[21,35]. As to HCC, a study showed that 1-, 3- and 5-year survival rates were 95.4%, 67.5% and 56.2%, respectively, after LLR vs 100%, 73.8% and 53.8% after OLR[36]. Soubrane et al[30] also published a LLR study, in which they achieved R0 marginal resection in 92% of their patients, while 1-, 3- and 5-year overall survival was 90.3%, 70.1% and 65.9%, respectively, and 1-, 3- and 5-year progression-free survival was 85.2%, 55.9% and 40.4%, respectively. In this study, they also proved that LLR fulfills the criteria established by the EASL-EORTC guidelines; hence, it should be used widely for the resection of HCC.
Conversion
Laparoscopic liver resection can be converted to laparotomy if the anatomy is not clear or so as not to endanger patient safety. Although some studies report a high rate of conversion of 13%-17%[30,37], generally rates tend to be as low as about 4%-7%[26,38-40]. Excessive bleeding is the most common cause of conversion, while adhesions, gas embolism, poor visualization and anatomic disorientation or nearby large vessels are some other common causes[26,37,39,40]. Resection of postero-superior segments was found to be an independent factor for conversion, as indicated by a multivariate analysis; major hepatectomy was another significant factor for conversion vs minor hepatectomy[39]. It would be wrong not to mention the relationship between conversion and learning curve. The considerable learning curve indicates that less experienced surgeons may not be able to deal with the numerous difficulties a LLR involves; hence, it has been observed that only after performing about 60 LLRs will the risk of converting LLR to OLR decrease[41].
It is obvious that when a laparoscopic procedure is converted to open, every advantage of the laparoscopic technique is immediately lost. This does not mean, though, that the surgeon should exceed his/her level of competency in order to avoid a conversion, because if it is delayed in some challenging cases, length of hospital stay may increase and complications may be more numerous and devastating[42]. As a result, the hepatobiliary surgeon must first become competent enough in performing LLR, so as to know when to convert or not.
The main reason for conversion, as mentioned previously, has been bleeding. In order to laparoscopically deal with major hemorrhage, the surgeon can intermittently use the Pringle maneuver, compress with gauzes, use clips or staplers or even the hand-assisted approach[43,44]. It is generally advisable that in case of acute bleeding, laparoscopic sutures should be placed after snatching the vessel, which can lead to less blood loss during conversion, and then saline solution should be used in the abdominal cavity when the converting incision is made[39]. The hand-assisted technique is an “in-between” technique used when there is an urgent need to stop bleeding and the decision to convert or not has not yet been made. The other important cause of conversion, gas embolism, can be managed by shifting the operating table into the Trendelenburg position, which increases central venous pressure in case of a damaged vessel[45]. Finally, when resecting a lesion in a postero-superior segment, which represents a higher risk of conversion, robotic-assisted resection is suggested to decrease the risk of conversion[39]; however, a systematic review reported a 6.6% rate of conversion for the robotic procedure[46] and, thus, more research is necessary.
Comparison with the open technique
When comparing techniques, it is important to ensure patient similarity between the different groups. Aiming to prove the advantages of a laparoscopic approach, Ito et al[47] matched 65 patients that received LLR to 65 OLR patients from their archive and then compared them. The results, especially for the short-term, were significantly in favor of the laparoscopic approach, showing a decrease in bleeding, need for transfusion, frequency of the Pringle maneuver, postoperative complications, time to recuperation, length of stay in the hospital and cases of surgical site herniation. As far as the oncologic outcomes are concerned, free-marginal resection and lack of surgical site recurrence were accomplished in both groups, while cancer recurrence rates were also similar. Also, the first study comparing the two techniques for a major liver excision showed that they are equal regarding operative time and postoperative complications, but blood loss, length of hospital stay and general morbidity were significantly reduced in the case of LLR[48].
A meta-analysis comparing the two methods, particularly comparing small resections for solitary tumors in the left lateral lobe or right peripheral subcapsular area, reported that LLR is superior to OLR in short-term outcomes (i.e., loss of blood and postsurgical morbidity), while long-term outcomes (i.e., severity of complications) were similar between the two approaches[49]. Besides, a comparative study reviewing 12 primary studies observed similar mortality rates between the laparoscopic (0.3%) and the open (0.4%) techniques, while liver failure was the most common cause of death in both groups[50].
Other major advantages of the laparoscopic method have to do with improved patient satisfaction and comfort. It is well known that a laparoscopic technique causes less surgical stress than an open one, and this can lead to decreased postsurgical pain, cosmetic advantages (almost no scar) and shorter length of stay in the hospital[51]. Also, time to oral intake and need for opioid analgesics may be reduced[52], the patient may recover faster and get back to his previous activities[53].
A meta-analysis published in 2017 also compared LLR to OLR in terms of short- and long-term outcomes[31]. To elaborate this, the open method showed increased rates of blood loss, requirements for blood transfusion and length of hospital stay, while the only insignificant difference was observed regarding the operating time. Free-marginal resection and width of marginal resection were found to be increased in LLR generally. This study also highlighted the decrease in postsurgical morbidity and in 30-d mortality, in favor of the laparoscopic operation.
Concerning long-term outcomes, although 1-year overall survival was significantly increased in LLR, there was no noticeable difference between the two groups in the 3- and 5-year overall survival. Disease-free survivals after 1, 3 and 5 years, as well as cancer recurrence rates, were also similar for the two methods. Unfortunately, except for one randomized controlled trial from China[20], all the studies included in the meta-analysis are non-randomized comparative studies, which are also characterized as “methodologically adequate”. Although since meta-analysis may over-estimate the effect of sizes in comparison to a meta-analysis of randomized controlled trials[54], the big picture emerges despite the lack of high-quality evidence-based research in LLR. Even though there is a large heterogeneity among the studies regarding surgical expertise, patient selection and tumor-related parameters, this helpful meta-analysis emphasizes the superiority of LLR over OLR for small HCCs.
Cost
Although at first glance one would expect the LLR to be more expensive, given the use of the laparoscopic instruments, this is not necessarily the case. When addressing the issue of cost analysis, the clinical aspect should be taken into consideration and “cost-effectiveness” should be the key concept. Specifically, although using an endoscopic stapler for liver resection is significantly more expensive than the “finger fracture” technique used in an open procedure, the operating room time saved could potentially make up for the difference. Even though, a study reported that the costs of trocars and staplers did not differ between the two groups[55], another from the United Kingdom showed that the devices and disposables utilized in the LLR group were more costly indeed than those in the OLR group[56].
A Canadian study reported no difference in the operative time between the laparoscopic and the open group, which was around 140 min, but an overall theatre time of more than 200 min was documented and the nonsurgical time was occasionally higher than the operative one[55]. Besides, it has been proposed that the theatre usage time is a better indicator of the cost-effectiveness of a procedure than the operative time. This nonsurgical time, though, was similar for the two techniques and was not a result of placing an epidural catheter in the OLR group. However, the aforementioned United Kingdom study[56] showed that although the placement of an epidural anesthesia is beneficial to patients receiving the open operation, it does increase the cost of the procedure compared to the laparoscopic one. As a result, if we add the shorter time of anesthesia and the reduced need for a high-dependency unit admission to the faster recovery time, ambulation time and reduced surgical ward stay observed in the laparoscopic group, it can be seen how the cost of LLR could be lower than that of OLR[56,57]. Additionally, the patient can return to his previous activities quicker, with reduced morbidity, and go to work sooner[58].
In contrast, this financial benefit is not observed in more complex and difficult cases. Specifically, Cannon et al[59] reported that although laparoscopy in general is less expensive than the OLR, when performing a right hepatectomy, which is clearly characterized by higher complexity, the cost-effectiveness of LLR is lost. Nevertheless, segmentectomy and bisegmentectomy clearly emphasize the cost-effectiveness of the laparoscopic approach, as the total hospital cost was lower by around £2.571 (~$3.800) compared to the open approach[56]. Similarly, Koffron et al[38] compared carefully selected and matched patients that received partial and right hemihepatectomy, excluding the outliers, and reported that the overall hospital cost for the laparoscopic group was 98% and 66%, respectively, of that of the open group. Also, they found that the operating room cost for those resections done laparoscopically was 51% and 47% of the overall hospital cost compared to 39% and 36%, respectively, in the case of an open operation.
Vanounou et al[60] used the deviation-based cost modeling to clinically and economically compare the two approaches and showed that the weighted-average median cost of LLR was reduced by about $2.939 in comparison with OLR ($15.104 vs $18.043, respectively). They also expanded this comparison to include malignant disease and they proved again that LLR is more cost-effective than OLR, by about $1.527. On the whole, it is clearly understood that the shorter duration of hospital stay accompanied by the lower morbidity rates, offset the higher intraoperative costs reported in the laparoscopic technique, thus ensuring cost-effectiveness.
SPECIAL SITUATIONS
Patient with cirrhosis
Cirrhosis is seen commonly in patients with HCC, and a different approach may be in order in these patients. The most common postoperative complication observed in cirrhotic patients is ascites, seen even in minor surgeries[61,62]. This could be prevented by the utilization of LLR, which also improves the postsurgical status of those patients in general. The reasons for that are: (1) The less traumatic insult to the abdominal wall and the round ligament, which prevents collateral circulation; (2) the protection of visceral organs from exposure to the atmosphere, which decreases the loss of electrolytes and the need for extra fluid administration; and (3) the restricted loss of blood during the operation[50]. In addition, LLR does not require the total emptying of ascites in the cirrhotic patient, therefore reducing the risk of postsurgical ascites and fluid and electrolyte disturbances[48,63]. Another frequent health issue that patients with cirrhosis usually face is bleeding from intra-abdominal varices. Some experts suggest that such a bleeding incident could be prevented thanks to the pneumoperitoneum produced during a LLR, owing to the tamponade effect[64]. Moreover, as we know, liver transplantation is the only therapeutic modality for cirrhosis. In conjunction to this, a study proved that when resecting a hepatic lesion from a potential future liver transplant candidate, LLR should be adopted over OLR, because it can facilitate liver transplantation due to a lesser degree of postoperative adhesions[65].
On the other hand, a LLR in cirrhotic liver has its own challenges. It is necessary that patient selection criteria are established, so that the early learning curve does not cause more harm than good. In other words, some surgeons suggest that the lesions which are going to be excised should be in the left or anterior right segment of the liver, in order to achieve optimal accessibility, while the lesion’s size should not exceed the 5 cm diameter[64]. This concept is included in the international consensus conference on LLR, and the laparoscopic approach is advocated for surgeons with appropriate expertise and in the beginning for peripherally located solitary lesions that do not exceed 5 cm in diameter[66].
Laparoscopic liver resection, immune system and stress response
Surgery initiates a complex systemic response involving multiple cytokines, immune cells, messenger molecules and metabolic pathways. All of these start with the abdominal trauma induced by the scalpel, but what if we could minimize this incision-induced stress reaction? This is where minimally invasive surgery and laparoscopy come into play.
The utilization of LLR leads to a smaller abdominal incision and decreased damage to the tissues. The initiated stress response is assessed by several measures, such as tumor necrosis factor alpha (TNF-α) and interleukins (IL-1β, -2, -6, -8, -10, -12), C-reactive proteins (CRPs), hormones deriving from the adrenals, lymphocytes in the periphery and by the implementation of delayed-type hypersensitivity skin tests[67,68]. The early stress response to the surgical wound is thought to be mediated by IL-6 produced by monocytes, macrophages and endothelial cells, while the severity of tissue damage can also be evaluated by high serum levels of IL-6[69]. In fact, a study suggested that approaches lowering IL-6 levels, such as laparoscopy, may be more beneficial in the future[70].
LLR, compared to OLR, has shown a decrease in postoperative complications, pain, hospital stay, bleeding and need for blood transfusion, time to oral intake, postoperative need for opioid analgesics and more rapid recovery. All these factors clearly highlight the reduced surgical stress response observed in the laparoscopic group and its superiority over the open method.
Diagnostic laparoscopy in HCC patients prior to resection
Apart from clinical and laboratory examinations, imaging plays a key role in the preoperative work-up and evaluation of HCC. Transabdominal ultrasound, three-phase computerized tomography and magnetic resonance imaging are some of the imaging examinations included in the preoperative work-up. However, as HCC is usually associated with cirrhosis and hepatitis, those may underestimate the level of cirrhosis and the regenerative nodules or peritoneal spread of the tumor, which can be more clearly identified only under direct vision[71]. Indeed, Klegar et al[71] utilized diagnostic laparoscopy in HCC patients undergoing resection, and it changed the decision made to a significant extent in 9 out of 20 cases (45%). The main reasons for this change were advanced level nodular cirrhosis, incorrect evaluation of intrahepatic metastases, difficulty in recognizing a HCC, peritoneal carcinomatosis and intolerability to general anesthesia. Consequently, diagnostic laparoscopy may be kept in mind for the preoperative imaging assessment of HCC.
Ablation
In the beginning of our review we stated that candidates for surgical resection need to fulfill some specific criteria. In the case of the patients that are excluded, a non-surgical approach, such as transarterial chemoembolization, percutaneous ethanol injection, percutaneous radiofrequency and microwave ablation, can be used. Unfortunately, some HCC patients are not suitable even for percutaneous ablation due to liver dysfunction or tumor characteristics necessitating a more controlled approach, and as a result the implementation of laparoscopic ablation could be helpful.
Laparoscopic radiofrequency ablation is a safe procedure used as an alternative to the percutaneous method in subcapsular tumors or in those in contact with adjacent organs. A European study confirmed the safety and efficacy of this procedure, as the reported initial complete response percentage was 94%, while the sustained one was 70% after the follow-up period[72]. Additionally, overall survival rates at 1, 3 and 5 years were 92.6%, 64.5% and 43%, respectively. Buell et al[73] compared laparoscopic radiofrequency ablation to LLR and noticed similar unwanted events and mortality rates (11% vs 16%, respectively and 1.5% vs 1.6%, respectively). Although the rates of overall recurrent disease were equal between the two techniques (24% vs 23%, respectively), local recurrence was more frequently observed in the radiofrequency group (6.3% vs 1.5%, respectively).
An Italian study evaluated the use of laparoscopic microwave ablation in 42 patients and had promising results[74]. Specifically, there was 0% mortality, but the morbidity rate was 24%, while survival and recurrence rates after 2 years were 79% and 55%, respectively. After matching 28 of these patients with 28 others receiving laparoscopic radiofrequency ablation, the 2-year recurrence percentages reported were 55% and 77%, respectively.
Microwave thermosphere ablation is a new method utilizing a single antenna so as to ablate spherical areas. Zaidi et al[75] evaluated microwave thermosphere ablation laparoscopically in 45 patients and reported a morbidity and mortality rate of 11.3% and 0%, respectively. Significantly, the 99.3% complete tumor ablation percentage and the 0.7% local recurrence rate indicate how promising this new technological advance can be in the future.
Learning curve
The combination of technology and technical challenges make the learning curve a critical part of LLR. He et al[76] noticed that the increase in volume of LLRs performed in 2009-2012 vs 2000-2008 may be partially attributed to the Louisville 2009 Consensus[66]. They also observed a decrease in length of hospital stay over time, but no difference regarding morbidity and mortality. Issues that need to be addressed are the qualifications necessary to perform the procedure and the path required to learning it. As expected, the vast majority of LLRs have been performed in liver cancer and liver transplantation centers by experienced surgeons with great knowledge and skills in both laparoscopic and hepatobiliary surgeries. Therefore, Tsinberg et al[77] proposed the formation of a dynamic duo, a laparoscopic surgeon and a hepatobiliary surgeon, who could work together and learn from each other. They also suggest that a surgeon with little experience should start from laparoscopically resecting peripherally located lesions (i.e., segments II, III, IVb, V and VI), as well as benefiting from the usage of the hand-assisted technique.
A study assessing the outcomes of LLR in three different groups in three different eras showed that the last group included more complex and demanding cases, as well as more cirrhotic patients, thus indicating the increased comfort and expertise of surgeons performing LLR during a period of time[29]. Even though cases gradually became more and more complex, operating time was reduced for about 3/4 of an hour from the first till the last group. Blood loss, 30-d mortality and length of hospital stay were similar among the three groups. Vigano et al[41] also evaluated LLRs performed in three different periods of time and concluded that the volume of LLRs increased, rate of conversion, operating time and loss of blood decreased, but most significantly, after adjusting for case-mix, cumulative sum analysis showed that LLRs required a learning curve of 60 patients. On the other hand, a study assessing the LLR learning curve of a single surgeon again in three periods, reported that 50 cases were required, so that a significant reduction in blood loss was observed, while no less than 160 cases were needed so as to perform a wide range of different LLR with safety[78].
There are issues regarding the nature of the learning curve. Even though it is thought of as an “idealized” curve, gradually progressing until reaching a plateau, Villani et al[79] could not but notice several improvements and regressions regarding complications, operative time and blood loss, associated partially to the constantly increasing complexity of the procedures attempted. As a consequence, they proposed the model of the “true” learning curve for LLR, which is characterized by a pattern of “ups and downs” until surgeons become experienced, when their performance reaches peak and the beneficial outcomes are constantly seen.
Koffron et al[38] commented on the need for randomized controlled trials, saying that patients would hesitate to enroll in these studies due to the fear of having OLR. On the contrary, the authors suggested that LLR may become the technique of choice, just as laparoscopic cholecystectomy, and propose a way to deviously avoid the learning curve of LLR. Thus, an inexperienced surgeon should start with using the hybrid method, initially for wedge excision of peripherally located lesions, and as time goes by and he/she becomes more comfortable with it, it is advisable to turn to the hand-assisted approaches. When the surgeon reaches a high level of expertise regarding the laparoscopic skills, it is time to gradually move on to the pure laparoscopic method, again initially for peripheral lesions.
FUTURE PROSPECTS
Nowadays, the swift advances in technology have led to several novel instruments and machines in the everyday surgical routine. Robotic surgery is just one of them. In general, help provided by the robot facilitated a new era for minimally invasive surgery including minor incisions, reduced estimated blood loss, postsurgical pain and length of hospital stay, while concurrently expediting the learning curve for transitioning from the open to minimally invasive approach[80,81]. Inevitably, the da Vinci robot (da Vinci Surgical System; Intuitive Surgical, Inc, Sunnyvale, CA, United States) entered the world of hepatobiliary surgery with increasing popularity. LLR is widely adopted, but mostly for left lateral segmentectomy and less for left and right hepatectomies. Thus, the robotic liver resection through its 3D imaging and advanced-mobility instruments may accommodate such resections[82] and promises to play a key role in the evolution of LLR. However, a study comparing robotic to laparoscopic left lateral sectionectomy reported in the robotic group more admissions to the intensive care unit and more minor complications, as well as increased length of hospital stay and indirect costs[83].
To our knowledge, up to this time, Giulianotti et al[84] have published the largest series for robotic major hepatectomy, consisting of 27 patients (20 right hepatectomies, 5 left hepatectomies and 2 right trisegmentectomies), 74% of which had malignant liver disease. Their median operating time was 313 min, the rate of conversion to open was 4%, while morbidity and mortality rates were 30% and 0%, respectively. Spampinato et al[85] published another large study of 25 patients, 68% of which had malignant disease, with a median operative time of 430 min, 4% conversion rate, but reduced transfusion rate, blood loss and morbidity in contrast to Giulianotti et al[84]. Both studies had a similar length of hospital stay, of 8 d.
Moreover, the largest study, to the best of our knowledge, regarding robotic minor hepatectomy was from Kingham et al[86] in 2016 from the Memorial Sloan Kettering Cancer Center, which included 65 patients (78% with malignant disease). Median operative time was 163 min, conversion rate was 6.3%, morbidity rate was 11% and mortality rate was 2%. Giulianotti et al[84] included 43 cases of robotic minor hepatectomy and reported a median operative time of 198 min, conversion rate of 7%, while morbidity and mortality rates were 16% and 0%, respectively. Data suggest that most published series of robotic major or minor hepatectomy achieved a near 100% R0 resection[87].
The interesting approach of robotic-assisted laparoscopic anatomic hepatectomy has been reported in a study from China[88]. Although this technique was characterized by increased operating time and hospital costs when compared to laparoscopic or open hepatectomy, it was superior in terms of blood loss, transfusion rate and morbidity, hence proving its safety and feasibility over the other two methods. This significant technique is promising because it can overcome the increased surgical trauma, postoperative pain, loss of blood and diminished recovery of the open approach, but simultaneously expand the indications of LLR, therefore representing an efficient combination. The robot’s advantages are the elimination of tremor produced by the surgeon, the accurate resemblance of human wrist movements, the scaling of hand motions into micro-motions, as well as the 3D visualization, which further enhances hand-eye coordination[89,90].
Notably, this robotic-assisted laparoscopic technique can be very helpful when performing hilar dissection, transection of hepatic parenchymal tissue and control of liver outflow, and when dealing with posteriorly located hepatic lesions. Also, robotic surgery can more easily manage bleeding during parenchyma transection, the most common cause of laparoscopic to open conversion[88]. However, there are also disadvantages. For instance, lack of tactile feedback is prominent due to absence of haptic sensors, but the 3D imaging may offset this problem. Additionally, the robotic cart and arms take a great deal of space in the operating room, which may impede additional non-robotic surgical movements or even make the work of the anesthesiologist inconvenient[91]. Robotic surgery is completely different from traditional surgery and many adjustments need to be made, including robotic port placement, development of more advanced surgical instruments and training of table-side surgeons, while hospital costs should always be taken into consideration.
There are other applications of minimally invasive surgery in hepatic surgery. Specifically, the shortage of liver donor grafts is widely known as a major issue in liver transplantation and, thus, many patients resort to live donor liver transplantation, which is a unique procedure given the significant health risk to the living donor; we have to remind ourselves that this is a healthy individual undergoing a high-risk surgery for no benefit to the donor. Consequently, a study compared open to laparoscopic live donor left lateral sectionectomy and reported that the laparoscopic group exhibited a diminished length of hospital stay and time to oral intake, while operative time, estimated blood loss and costs were similar between the two groups with zero mortality observed in both[92]. The same surgical team published in 2017 a study of three pure laparoscopic living donor right hepatectomies, which are very rarely performed, and reported zero complications, reduced surgical trauma morbidity and more rapid recuperation[93]. In 2017, a Japanese study published was the first one to compare laparoscopic to laparoscopy-assisted donor hepatectomy[94]. It showed that although the pure laparoscopic approach may take longer than the laparoscopy-assisted one, it is associated with decreased loss of blood, better cosmetic outcomes and similar complication rates and acceptable liver allograft results.
On the whole, LLR is a challenging procedure requiring a lot of experience, which is not easy to accomplish. Nevertheless, even experienced surgeons may face difficulties intraoperatively. As a result, improved liver and surgical site visualization is needed so as to achieve optimal outcomes. Thus, a surgical simulation 3D system has been developed in order to facilitate surgeons in recognizing vascular structures and the location of the tumor[95]. The aim of this system is to facilitate surgical training, as well as to ultimately provide navigation guidance in real time intra-operatively. Moreover, we have witnessed the evolution of an open liver imaging system to a laparoscopic one, mainly through clinician feedback, which accommodates a high quality intra-operative 3D image, especially useful in LLRs[96]. The future seems quite promising for laparoscopic liver surgery, both in terms of surgical technique, as well as in terms of navigation guidance in the operating room.