Published online Jul 27, 2015. doi: 10.4240/wjgs.v7.i7.116
Peer-review started: March 3, 2015
First decision: March 20, 2015
Revised: April 6, 2015
Accepted: June 9, 2015
Article in press: June 11, 2015
Published online: July 27, 2015
Processing time: 146 Days and 6.3 Hours
AIM: To predict node-positive disease in colon cancer using computed tomography (CT).
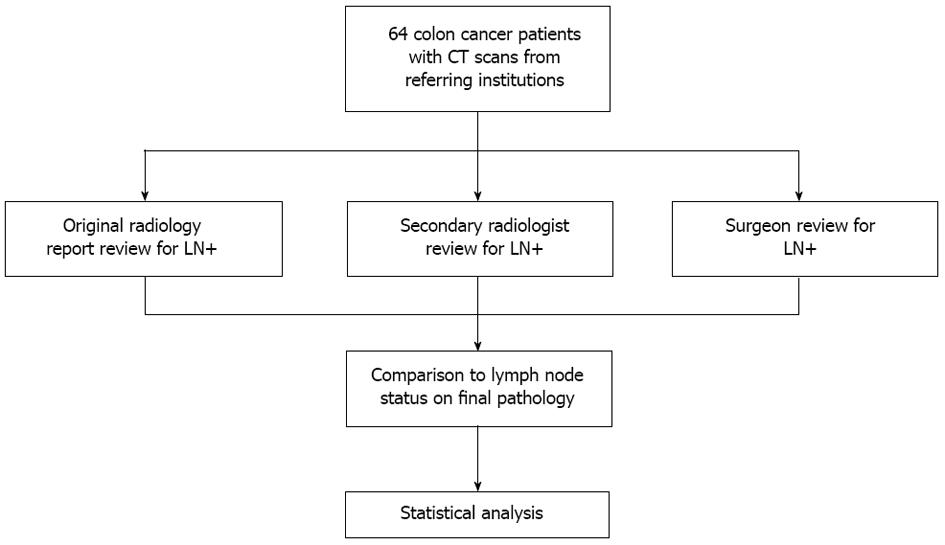
METHODS: American Joint Committee on Cancer stage I-III colon cancer patients who underwent curavtive-intent colectomy between 2007-2010 were identified at a single comprehensive cancer center. All patients had preoperative CT scans with original radiology reports from referring institutions. CT images underwent blinded secondary review by a surgeon and a dedicated abdominal radiologist at our institution to identify pericolonic lymph nodes (LNs). Comparison of outside CT reports to our independent imaging review was performed in order to highlight differences in detection in actual clinical practice. CT reviews were compared with final pathology. Results of the outside radiologist review, secondary radiologist review, and surgeon review were compared with the final pathologic exam to determine sensitivity, specificity, positive and negative predictive values, false positive and negative rates, and accuracy of each review. Exclusion criteria included evidence of metastatic disease on CT, rectal or appendiceal involvement, or absence of accompanying imaging from referring institutions.
RESULTS: From 2007 to 2010, 64 stageI-III colon cancer patients met the eligibility criteria of our study. The mean age of the cohort was 68 years, and 26 (41%) patients were male and 38 (59%) patients were female. On final pathology, 26 of 64 (40.6%) patients had node-positive (LN+) disease and 38 of 64 (59.4%) patients had node-negative (LN-) disease. Outside radiologic review demonstrated sensitivity of 54% (14 of 26 patients) and specificity of 66% (25 of 38 patients) in predicting LN+ disease, whereas secondary radiologist review demonstrated 88% (23 of 26) sensitivity and 58% (22 of 38) specificity. On surgeon review, sensitivity was 69% (18 of 26) with 66% specificity (25 of 38). Secondary radiology review demonstrated the highest accuracy (70%) and the lowest false negative rate (12%), compared to the surgeon review at 67% accuracy and 31% false negative rate and the outside radiology review at 61% accuracy and 46% false negative rate.
CONCLUSION: CT LN staging of colon cancer has moderate accuracy, with administration of NCT based on CT potentially resulting in overtreatment. Active search for LN+ may improve sensitivity at the cost of specificity.
Core tip: Clinical staging to determine eligibility for neoadjuvant trials requires accurate imaging. This study compares lymph node identification on preoperative computed tomography (CT) scans by outside radiologists, a tertiary cancer center radiologist and a surgeon, mirroring referral patterns to tertiary care facilities. While re-review of CT scans by a tertiary center radiologist improved sensitivity of lymph node detection, CT staging of colon cancer demonstrated moderate accuracy overall. Our findings suggest that the administration of neoadjuvant chemotherapy based on preoperative CT staging would potentially result in overtreatment of colon cancer patients.
- Citation: Choi AH, Nelson RA, Schoellhammer HF, Cho W, Ko M, Arrington A, Oxner CR, Fakih M, Wong J, Sentovich SM, Garcia-Aguilar J, Kim J. Accuracy of computed tomography in nodal staging of colon cancer patients. World J Gastrointest Surg 2015; 7(7): 116-122
- URL: https://www.wjgnet.com/1948-9366/full/v7/i7/116.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v7.i7.116
Adjuvant chemotherapy is well-established for treating colon cancer patients with American Joint Committee on Cancer (AJCC) stage III disease[1]. More recently, there has been growing interest in administering neoadjuvant chemotherapy (NCT) prior to planned surgical resection to reduce disease recurrence in high-risk tumors. Preliminary results from the Fluoropyrimidine, Oxaliplatin and Targeted-Receptor preOperative Therapy (FOxTROT) trial for patients with high-risk operable colon cancer, an ongoing phase III randomized controlled trial in the United Kingdom, have demonstrated that NCT for operable, locally-advanced colon cancer can downstage tumors[2]. Patients for the study were selected on the basis of having either T3 tumors with ≥ 5 mm extramural tumor depth or T4 tumors by computed tomographic (CT) imaging. Nodal stage was not specifically used as inclusion criteria for the study and only 52% of patients randomized to the adjuvant chemotherapy group demonstrated nodal involvement on final pathologic exam.
Unlike rectal cancer, where neoadjuvant chemoradiation is frequently utilized based on staging with endorectal ultrasound (ERUS) or magnetic resonance imaging (MRI)[3,4], the administration of NCT for patients with resectable colon cancer is controversial. In order to appropriately select colon cancer patients for NCT, an accurate and reliable imaging modality for detecting involved lymph nodes (LN) is mandatory. Due to low sensitivity, MRI and positron emission tomography (PET) are not favorable imaging studies for preoperative pathologic LN detection[5-8]. In contrast, CT is currently the most commonly used imaging study used to stage colon cancer patients preoperatively, particularly to identify liver, lung, and other sites of distant metastases that may exclude patients from NCT trials[9-11]. Our objective was to determine the utility and accuracy of preoperative CT scan in detecting regional colon cancer LN metastases by comparing outside CT reports to independent imaging review at a referral center in order to highlight differences in detection in actual clinical practice.
After obtaining approval from the Institutional Review Board, we identified and analyzed the medical records of 64 colon cancer patients with AJCC stageI-III disease who underwent curative resection between 2007 and 2010 at City of Hope Comprehensive Cancer Center. Exclusion criteria included evidence of metastatic disease on CT, rectal or appendiceal involvement, or absence of accompanying imaging from referring institutions. Medical records were reviewed for demographic and treatment-related variables.
Prior to treatment at our institution, patients had CT imaging performed at outside community hospitals or imaging centers. Outside CT images and radiology reports were obtained on all patients. Secondary imaging review of the original outside CT scans was conducted by a surgeon and an abdominal imaging radiologist at our institution. They were blinded to the original radiologic report final pathology exam and reviewed the images with the specific goal to identify mesenteric LNs (Figure 1). Once reviewed, each observer’s results were compared with the final pathology.
Patients were staged according to the AJCC 7th edition TNM classification system. Variables examined in our study included age, sex, location of primary tumor, T stage, and N stage. Radiographic LN involvement was defined when the longest LN diameter was > 1.0 cm or was 0.7-1.0 cm in size with round shape, heterogeneity, eccentricity, hilar thinning, calcification, central necrosis, or perinodal infiltration. Based on the radiographic review, each patient was designated either lymph node positive (LN+) or lymph node negative (LN-). The reports from outside radiologists were reviewed and the absence of pathologic LN identification was recorded as LN-.
Results of the outside radiologist review, secondary radiologist review, and surgeon review were compared with the final pathologic exam to determine sensitivity, specificity, positive (PPV) and negative predictive values (NPV), false positive and negative rates, and accuracy of each review. Both binomial 95%CI and asymptotic P-values were calculated to determine the statistical significance of each observer’s results compared to a null hypothesis of 50% (i.e., results expected due to random chance). The association between clinical factors and the accuracy of LN detection was also examined.
From 2007 to 2010, 64 stage I-III colon cancer patients met the eligibility criteria of our study (Table 1). The mean age of the cohort was 68 years, and 26 (41%) patients were male and 38 (59%) patients were female. Tumors were located in the sigmoid colon (n = 18, 28%), the ascending colon (n = 16, 25%), or the cecum (n = 14, 22%). On final pathology, 19 (30%) patients were stage I, 19 (30%) were stage II, and 26 (40.6%) patients were stage III. LN- disease was diagnosed in 38 patients and LN+ disease in 26 patients. In the LN+ cohort, 17 patients had N1 disease and 9 patients had N2 disease. All patients in our study had ≥ 12 LNs removed with a median of 22 LNs.
| Characteristics | n = 64 (%) |
| Age (yr)1 | 67.6 ± 12.8 |
| Sex | |
| Male | 26 (40.6) |
| Female | 38 (59.4) |
| Tumor location | |
| Cecum | 14 (21.9) |
| Ascending colon | 16 (25.0) |
| Transverse colon | 6 (9.4) |
| Splenic flexure | 1 (1.6) |
| Descending colon | 6 (9.4) |
| Sigmoid colon | 18 (28.1) |
| Rectosigmoid | 3 (4.7) |
| Pathologic stage | |
| Stage I | 19 (29.7) |
| Stage II | 19 (29.7) |
| Stage III | 26 (40.6) |
| N stage | |
| N0 | 38 (59.4) |
| N1 | 17 (26.5) |
| N2 | 9 (14.1) |
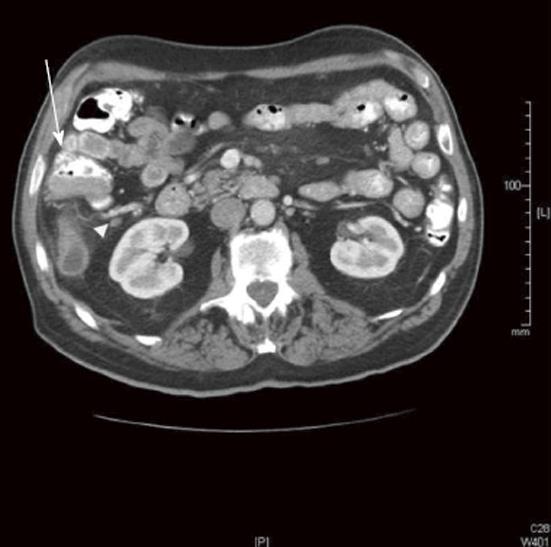
Outside radiology review only identified 14 of 26 LN+ patients and 25 of 38 LN- patients (Table 2). The sensitivity, specificity, and accuracy for the original radiology review for predicting LN disease were calculated, as were PPV, NPV, false positive rate, and the false negative rate (Table 3). The original radiology review had the lowest sensitivity and highest false negative rate compared with the secondary radiologist and surgeon review. Figure 2 shows an example of a LN- CT by the original radiologist; however, this case was LN+ on final pathology, secondary radiology, and secondary surgical reads.
| Final pathology (n = 64) | ||
| LN+ (n = 26) | LN- (n = 38) | |
| Original radiologist | ||
| LN+ | 14 | 13 |
| LN- | 12 | 25 |
| Secondary radiologist | ||
| LN+ | 23 | 16 |
| LN- | 3 | 22 |
| Surgeon | ||
| LN+ | 18 | 13 |
| LN- | 8 | 25 |
| Sensitivity(95%CI, P-value) | Specificity(95%CI, P-value) | PPV(95%CI, P-value) | NPV(95%CI, P-value) | FPR(95%CI, P-value) | FNR(95%CI, P-value) | Accuracy(95%CI, P-value) | |
| Original radiologist | 54% (35%-73%, P = 0.69) | 66% (51%-81%, P = 0.05) | 52% (33%-71%, P = 0.85) | 68% (52%-83%, P = 0.03) | 34% (19%-49%, P = 0.05) | 46% (27%-65%, P = 0.69) | 61% (49%-73%, P = 0.08) |
| Secondary radiologist | 88% (76%-100%, P < 0.01) | 58% (42%-74%, P = 0.33) | 59% (44%-74%, P = 0.26) | 88% (75%-100%, P < 0.01) | 42% (26%-58%, P = 0.33) | 12% (0%-24%, P < 0.01) | 70% (59%-82%, P < 0.01) |
| Surgeon | 69% (51%-87%, P = 0.05) | 66% (51%-81%, P = 0.05) | 58% (41%-75%, P = 0.37) | 76% (61%-90%, P < 0.01) | 34% (19%-49%, P = 0.05) | 31% (13%-49%, P = 0.05) | 67% (56%-79%, P = 0.01) |
The secondary radiologist correctly identified 23 of 26 LN+ cases and 22 of 38 LN- cases (Table 2). Of the three observers, the secondary radiologist demonstrated the highest sensitivity and accuracy for LN+ detection, 88% (95%CI: 76%-100%, P < 0.01) and 70% (95%CI: 59%-82%, P < 0.01), respectively. The accuracy of the secondary radiologist was approximately 10% higher than that of outside radiologist review (Table 3).
Surgeon review correctly predicted 18 of 26 LN+ patients and 25 of 38 LN- patients (Table 2). Of the three observers, sensitivity and accuracy of the surgeon review were better than the original radiology review, but not as high as the secondary radiology review (Table 3). The surgeon review had comparable specificity to original radiology review.
Location of the tumor, sex, body mass index (BMI), and number of LN examined on final pathology were analyzed to determine whether these variables correlated with improved accuracy of LN detection on preoperative CT scan reviewed by the secondary radiologist. LN detection in female patients tended to be more accurate than male patients (76% vs 63%, P = 0.27) and BMI < 25 also tended to improve accuracy of LN detection (84% vs 67%, respectively; P = 0.16). Total number of LNs examined and location of the tumor did not predict LN detection accuracy (P = 0.91 and P = 0.87, respectively).
Given the promising outcomes of preoperative and perioperative therapies in other gastrointestinal malignancies[4,12,13], NCT for node-positive colon cancer remains of great interest. The theoretical benefits of NCT include the reduction of micrometastatic disease and tumor shedding during surgery, and use of tumor response to neoadjuvant chemotherapy to guide further adjuvant therapies if needed after surgery. In addition, patients may be better able to tolerate full-dose chemotherapy regimens in the preoperative rather than postoperative setting. To determine which patients may benefit most from NCT, accurate preoperative imaging to assess nodal disease is essential.
Our study compared CT reviews by the original radiologist and two secondary reviewers (a radiologist and a surgeon) with the final pathology. While the original radiology reviews had low sensitivity, the results from the secondary radiologist and surgeon reviews were comparable to contemporary studies on LN staging by CT. For example, in a meta-analysis of 19 studies that included 907 patients, the overall sensitivity of CT for LN+ detection was 70% and the specificity was 78%[14]. While the majority of prior reports used results obtained only by dedicated abdominal radiologists[10,11,15,16], our study sought to investigate CT reviews performed by three different clinical perspectives in order to compare and contrast the reading results. This approach was designed to mirror actual clinical practice, particularly in tertiary care and referral centers, as patients frequently arrive for initial consultation with outside imaging and reports of variable quality. The sensitivity rates from the original radiology reviews were lower than those from the secondary reviewers, and it is possible that these higher rates of false negatives exist because LN+ detection and staging were not the primary focus of the original review. Compared with the outside radiology review, sensitivity and accuracy for lymph node detection improved with active search for lymphadenopathy on secondary review, while specificity tended to decrease. These findings highlight the importance of independently reviewing outside imaging studies prior to clinical decision making. Of note, in order to avoid multiple insurance charges for preoperative imaging, the majority of patients did not undergo repeat CT scans at our institution. Thus, we were unable to make comparisons in LN detection between outside CT scans and our institutional CT scans.
While CT is the most commonly utilized imaging modality for preoperative staging in colon cancer, the use of PET and MRI for metastatic lymph node detection has been studied by other investigators. PET/CT generally demonstrates lower sensitivity than CT alone[5-7]. Because PET lacks the spatial resolution of CT, even when combined with CT, increased fluorodeoxyglucose (FDG) uptake in lymph nodes can be difficult to interpret, particularly when nodes are in close proximity to a primary tumor with high standardized uptake value (SUV). Similarly, MRI is associated with lower sensitivity, but increased specificity for LN involvement when compared to CT[8], and this may in part due to the fact that MRI criteria for lymph node positivity other than size, such as border criteria or signal criteria, can be subjective and have less reliable inter-observer differences[17].
CT appears to have comparable sensitivity and specificity to ERUS for LN+ detection, although preoperative ERUS staging for rectal cancer depends on the combination of the T and N stage[18]. The ability of ERUS to accurately determine the T stage in rectal cancer is likely better than the ability of CT to determine the T stage for colon cancer. CT can differentiate tumor invasion through the muscularis propria (T1/T2 vs T3/T4) in colon cancer with high accuracy[19], but depending on the operator, ERUS better distinguishes invasion of rectal tumors through the layers of the rectal wall with very high accuracy[20]. For these reasons, CT staging for lymph node involvement in colon cancer has not been utilized to select patients for neoadjuvant chemotherapy administration, in contrast to the use of ERUS in rectal cancer staging.
Other groups have also examined the role of preoperative staging with CT in colon cancer patients. Currently, the FOxTROT trial is randomizing patients on the basis of preoperative T staging by CT to determine whether administration of neoadjuvant oxaliplatin, folinic acid and fluorouracil prior to surgical resection impacts long-term outcomes when compared with the current standard of surgical resection followed by adjuvant chemotherapy[2]. Preliminary results showed 91% of patients who were classified as high risk by CT had T3 tumors or above confirmed by final pathology. Of the 99 patients randomized to the preoperative chemotherapy group, 39.4% (39/99) were LN+ on final pathology[2]. Stratification by T stage on CT scan may result in the administration of neoadjuvant chemotherapy to LN- patients, particularly because CT tends to overstage nodal disease compared with the final pathologic diagnosis. In the preliminary results of the FOxTROT trial, 48% of patients were LN- in the postoperative chemotherapy group, but would have received neoadjuvant chemotherapy according to the trial’s CT T staging criteria.
The current clinical staging of colon cancer by CT has moderate sensitivity and specificity for detecting lymph node involvement. By implementing a study design that mirrors actual clinical practice, our study demonstrated that although sensitivity increases by actively re-reviewing CT imaging from referral centers for metastatic nodal disease, specificity may be negatively impacted. The patient derived benefit of accurate preoperative CT identification of LNs would be the reliable diagnosis of stage III disease prior to surgery with the potential eligibility for neoadjuvant treatment strategies. However, at the current level of CT technology, administration of neoadjuvant chemotherapy based on preoperative CT LN involvement would potentially result in overtreatment of these selected colon cancer patients. Currently, CT scanning is used to determine T stage as entry criteria for clinical trials of neoadjuvant chemotherapy for colon cancer, but the results of these trials are needed before CT becomes the standard imaging modality for detecting presumed LN+ colon cancer and guiding neoadjuvant therapy.
Adjuvant chemotherapy is well-established for treating colon cancer patients with American Joint Committee on Cancer stage III disease. More recently, there has been growing interest in administering neoadjuvant chemotherapy (NCT) prior to planned surgical resection to reduce disease recurrence in high-risk tumors. In order to appropriately select colon cancer patients for NCT, an accurate and reliable imaging modality for detecting involved lymph nodes (LN) is mandatory. The authors’ objective was to determine the utility and accuracy of preoperative computed tomography (CT) scan in detecting regional colon cancer LN metastases by comparing outside CT reports to independent imaging review at a referral center in order to highlight differences in detection in actual clinical practice.
Currently, there is growing interest in preoperatively identifying colon cancer patients who would benefit from neoadjuvant therapy. One such study (Fluoropyrimidine, Oxaliplatin and Targeted-Receptor preOperative Therapy trial) is randomizing patients on the basis of preoperative T staging by CT to determine whether administration of neoadjuvant oxaliplatin, folinic acid and fluorouracil prior to surgical resection impacts long-term outcomes when compared with the current standard of surgical resection followed by adjuvant chemotherapy.
Although previous studies have also demonstrated that CT has modest accuracy for preoperative identification of LNs, this study utilizes comparison of three different clinical perspectives to highlight differences in LN detection in actual clinical practice.
From a practical standpoint, this results highlight the importance of independently reviewing outside imaging studies prior to surgical resection. The authors have demonstrated that sensitivity for LN detection increases with active search on re-review by the authors’ surgeon and dedicated abdominal radiologist compared to the original outside radiology assessments.
Node-positive disease in colon cancer involves the metastatic spread of cells from the primary tumor to the regional mesenteric LNs.
This is a timely presentation of important results in clinical oncology.
P- Reviewer: Actis GC, Nasir O, Sijens PE S- Editor: Gong XM L- Editor: A E- Editor: Yan JL
| 1. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Colon Cancer. Version 3. 2013;. |
| 2. | Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13:1152-1160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 348] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 3. | Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, Müller L, Link H, Moehler M, Kettner E. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 350] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 4. | O’Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, Pitot HC, Shields AF, Landry JC, Ryan DP. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32:1927-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 331] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 5. | Kwak JY, Kim JS, Kim HJ, Ha HK, Yu CS, Kim JC. Diagnostic value of FDG-PET/CT for lymph node metastasis of colorectal cancer. World J Surg. 2012;36:1898-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Llamas-Elvira JM, Rodríguez-Fernández A, Gutiérrez-Sáinz J, Gomez-Rio M, Bellon-Guardia M, Ramos-Font C, Rebollo-Aguirre AC, Cabello-García D, Ferrón-Orihuela A. Fluorine-18 fluorodeoxyglucose PET in the preoperative staging of colorectal cancer. Eur J Nucl Med Mol Imaging. 2007;34:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Tsunoda Y, Ito M, Fujii H, Kuwano H, Saito N. Preoperative diagnosis of lymph node metastases of colorectal cancer by FDG-PET/CT. Jpn J Clin Oncol. 2008;38:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Zerhouni EA, Rutter C, Hamilton SR, Balfe DM, Megibow AJ, Francis IR, Moss AA, Heiken JP, Tempany CM, Aisen AM. CT and MR imaging in the staging of colorectal carcinoma: report of the Radiology Diagnostic Oncology Group II. Radiology. 1996;200:443-451. [PubMed] |
| 9. | Burton S, Brown G, Bees N, Norman A, Biedrzycki O, Arnaout A, Abulafi AM, Swift RI. Accuracy of CT prediction of poor prognostic features in colonic cancer. Br J Radiol. 2008;81:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Huh JW, Jeong YY, Kim HR, Kim YJ. Prognostic value of preoperative radiological staging assessed by computed tomography in patients with nonmetastatic colon cancer. Ann Oncol. 2012;23:1198-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Smith NJ, Bees N, Barbachano Y, Norman AR, Swift RI, Brown G. Preoperative computed tomography staging of nonmetastatic colon cancer predicts outcome: implications for clinical trials. Br J Cancer. 2007;96:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4609] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 13. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4080] [Article Influence: 313.8] [Reference Citation Analysis (0)] |
| 14. | Dighe S, Purkayastha S, Swift I, Tekkis PP, Darzi A, A’Hern R, Brown G. Diagnostic precision of CT in local staging of colon cancers: a meta-analysis. Clin Radiol. 2010;65:708-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Dighe S, Blake H, Koh MD, Swift I, Arnaout A, Temple L, Barbachano Y, Brown G. Accuracy of multidetector computed tomography in identifying poor prognostic factors in colonic cancer. Br J Surg. 2010;97:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Kanamoto T, Matsuki M, Okuda J, Inada Y, Tatsugami F, Tanikake M, Yoshikawa S, Narabayashi I, Kawasaki H, Tanaka K. Preoperative evaluation of local invasion and metastatic lymph nodes of colorectal cancer and mesenteric vascular variations using multidetector-row computed tomography before laparoscopic surgery. J Comput Assist Tomogr. 2007;31:831-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Akasu T, Iinuma G, Takawa M, Yamamoto S, Muramatsu Y, Moriyama N. Accuracy of high-resolution magnetic resonance imaging in preoperative staging of rectal cancer. Ann Surg Oncol. 2009;16:2787-2794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Puli SR, Reddy JB, Bechtold ML, Choudhary A, Antillon MR, Brugge WR. Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers: a meta-analysis and systematic review. Ann Surg Oncol. 2009;16:1255-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Dighe S, Swift I, Magill L, Handley K, Gray R, Quirke P, Morton D, Seymour M, Warren B, Brown G. Accuracy of radiological staging in identifying high-risk colon cancer patients suitable for neoadjuvant chemotherapy: a multicentre experience. Colorectal Dis. 2012;14:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Schaffzin DM, Wong WD. Endorectal ultrasound in the preoperative evaluation of rectal cancer. Clin Colorectal Cancer. 2004;4:124-132. [PubMed] |