Published online Aug 27, 2014. doi: 10.4240/wjgs.v6.i8.156
Revised: May 11, 2014
Accepted: July 15, 2014
Published online: August 27, 2014
Processing time: 182 Days and 8.9 Hours
Blind loop syndrome after side-to-side ileocolonic anastomosis is a well-recognized entity even though its incidence and complication rates are not clearly defined. The inevitable dilation of the ileal cul-de-sac leads to stasis and bacterial overgrowth which eventually leads to mucosal ulceration and even full-thickness perforation. Blind loop syndrome may be an underestimated complication in the setting of digestive surgery. It should always be taken into account in cases of acute abdomen in patients who previously underwent right hemicolectomy. We herein report 3 patients who were diagnosed with perforative blind loop syndrome a few years after standard right hemicolectomy followed by a side-to-side ileocolonic anastomosis.
Core tip: The authors suggest that we are likely to see more and more cases of blind loop syndrome in the future because more side-to-side ileocolonic anastomoses will be performed in the setting of colonic laparoscopic surgery. A blind loop perforation should immediately be investigated in a patient who presents with acute abdomen years after a right hemicolectomy. Ideally, more end-to-end anastomoses should be performed, whenever suitable, in an effort to prevent the development of a blind loop.
- Citation: Dalla Valle R, Zinicola R, Iaria M. Blind loop perforation after side-to-side ileocolonic anastomosis. World J Gastrointest Surg 2014; 6(8): 156-159
- URL: https://www.wjgnet.com/1948-9366/full/v6/i8/156.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v6.i8.156
In the past, digestive continuity after right hemicolectomy was often restored through either end-to-end or end-to-side ileocolonic anastomosis. Due to the extensive implementation of laparoscopy in colonic surgery, side-to-side ileocolonic anastomosis has gained popularity because of the widespread use of linear staplers in this setting. Even in open surgery, mechanical side-to-side anastomoses are easy, quick and cost-effective because these can be performed using a single linear stapling device, while an end-to-side recontruction would require both linear and circular staplers to complete the anastomosis with additional costs.
Concurrently, intracorporeal anastomoses during laparoscopic right hemicolectomies can be exclusively carried out with linear staplers (endoGIA).
Yet, in 1906, Cannon and Murphy judged side-to-side anastomoses to be far from physiological, and advocated end-to-end reconstruction instead[1]. The same recommendation was reinforced by Pearse[2], Estes et al[3] and Holme[4] in further studies. A key reason why side-to-side anastomoses have been questioned lies in their substantial risk of progressive dilation at the level of the cul-de-sac, which might lead to enlarged pockets prone to stasis and bacterial overgrowth. Such alterations seem to predispose to the so-called “blind loop syndrome”[5,6]. The continuous enlargement of the blind loop may eventually cause mucosal ulcerations, intestinal bleeding and/or full-thickness viscus perforation.
There are limited data in the literature about the actual incidence of blind loop syndrome and its related morbidities because, generally, studies on intestinal anastomoses are exclusively focused on short-term postoperative complications (3 mo), whereas blind loop syndrome tends to develop years after surgery. We retrospectively reviewed 3 cases of blind loop perforation which occurred during long-term follow-up after standard right hemicolectomy followed by mechanical side-to-side ileocolonic anastomosis.
A 76-year-old woman underwent a right hemicolectomy in 2007 for a Duke’s stage C adenocarcinoma of the ascending colon followed by a mechanical isoperistaltic side-to-side ileocolonic anastomosis. In September 2009, she was admitted for acute and diffuse abdominal pain, high-grade fever and leukocytosis. An abdominal X-ray series displayed free intra-peritoneal subphrenic air bilaterally. Since a computed tomography (CT) scan was not immediately available, an exploratory laparoscopy was performed followed by laparotomy. A generalized purulent peritonitis due to a pinpoint perforation of the ileocolonic blind loop was identified. The same blind loop appeared extremely enlarged, measuring about 7 cm, and was resected using a 75 mm GIA stapler (United States Surgical, Norwalk, CT, United States). The patient had an uneventful postoperative course and was discharged home 6 d after surgery. At the 12-mo outpatient clinic follow-up, the patient was asymptomatic with normal bowel function.
An 80-year-old woman had a right hemicolectomy in 2008 for a Duke’s stage B adenocarcinoma of the ileocecal junction, with a mechanical isoperistaltic side-to-side ileocolonic anastomosis. In January 2011, she was admitted with worsening generalized abdominal pain, fever and leukocytosis. Before seeking medical attention, the patient experienced loose stools and mild non-localized abdominal pain for almost 1 wk. Plain abdominal X-rays revealed free subphrenic air. A CT scan detected free air in the abdominal cavity and a slightly dilated small bowel loop. A subsequent laparoscopy identified generalized purulent peritonitis due to a tiny perforation of a 6 cm blind loop. The perforated pouch was resected with a 45 mm endoGIA stapler. She had a straightforward postoperative course and was discharged after 5 d. At a scheduled 6-mo follow-up visit, she was symptom-free except for mild sporadic diarrhea.
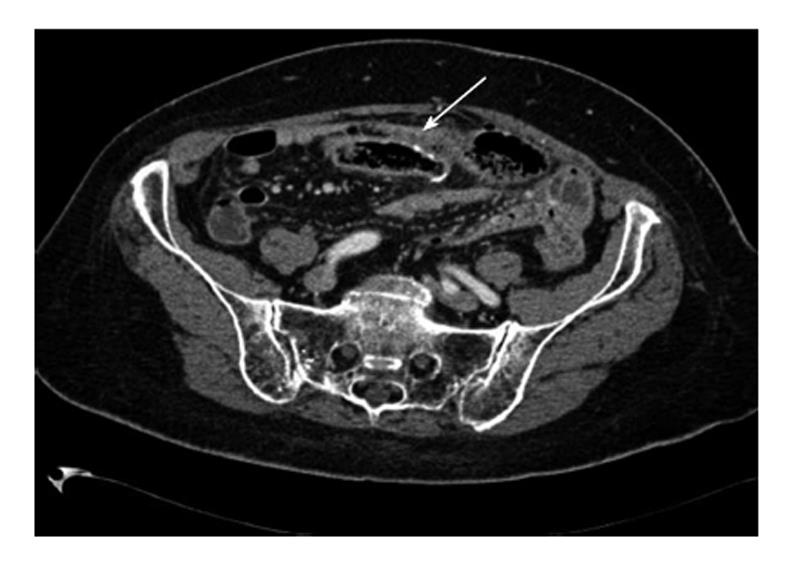
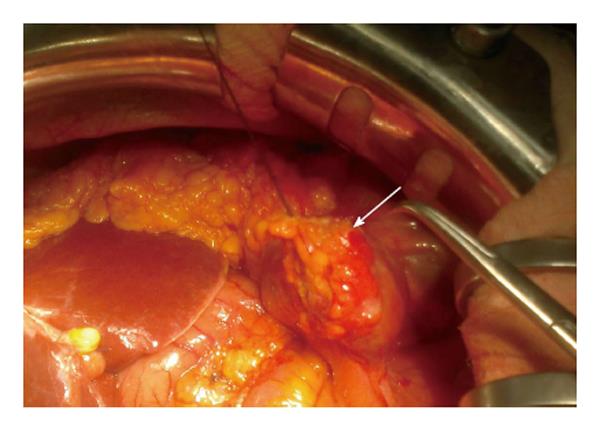
A 58-year-old woman had a right hemicolectomy in 2009 for a Duke’s stage B adenocarcinoma of the hepatic flexure, with a hand-sewn antiperistaltic side-to-side ileocolonic anastomosis. In November 2011, she presented with severe epigastric pain, fever and leukocytosis. Plain abdominal X-rays failed to show free air. A contrast-enhanced CT scan revealed intra-peritoneal free air localized in the lower abdomen with air bubbles near the anastomosis. The blind loop showed thick walls associated with mucosal hyperemia along with focal areas of perivisceral soft tissue fat necrosis (Figure 1). Imaging was compatible with blind loop perforation. The patient underwent laparotomy, which demonstrated generalized purulent peritonitis due to microperforation (Figure 2) of the enlarged blind ileal pouch (about 10 cm long). The blind loop was resected with a 75 mm GIA stapler. She recovered well after surgery and was discharged home in 10 d. At the 6-mo follow-up visit, she had no symptoms and regular bowel movements.
Nowadays, mechanical side-to-side anastomosis after right hemicolectomy is commonly performed either in open or laparoscopic surgery due to its simplicity and speed. In the short-term there is no clear advantage of a specific anastomotic configuration over others. On the other hand, side-to-side anastomoses have been criticized for being theoretically anti-physiologic and for the long-term risk of a blind loop[1].
Blind loops result in abnormal peristalsis causing filling rather than emptying of the pouch. Such dismotility-related stasis predisposes to bacterial overgrowth eventually followed by mucosal and/or transmural inflammatory changes of the intestinal wall. The consequent clinical scenario is referred as blind loop syndrome and may present with a wide spectrum of morbidities such as diarrhea, vitamin B12 deficiency, iron-deficiency anemia, ulcerations, bleeding and enteroliths[5]. At times, a pinpoint perforation of the blind loop may occur[5,7,8]. Partial disruption of the muscle layer in the side-to-side anastomosis causes dysmotility, diverting intestinal content more easily unto the blind loop. The cul-de-sac dilation may occur even after an end-to-side recontruction, though it appears more likely to happen after a side-to-side anastomosis[6]. Conversely, blind loops do not develop after end-to-end anastomoses. Stellamor et al[9] analyzed 66 ileocolonic resections, and identified 9 blind loops (average transverse diameter between 5 and 11 cm) out of 31 side-to-side anastomoses, whereas no blind loops were observed after 12 end-to-end anastomoses.
Only a few cases have been accurately described in literature. Estes et al[3] reported about a 10 cm blind loop which had increased to 46 cm after only a few months. Pollock described a blind loop which started as 2 cm in length and stretched to 15 cm after 1 year[10].
We believe this critical aspect of side-to-side anastomoses will be more intensively taken into consideration in light of their expanding use in the laparoscopic era. The likelihood of enlargement and tearing appear proportional to the span of the blind loop, the bigger the pouch the higher the risk of further lengthening and stretching. It is advisable to leave the bowel stump as short as possible, even if that does not necessarily prevent the loop from enlarging in the long-term. In addition, antiperistaltic anastomoses do not seem to abolish the risk of developing a cul-de-sac, as shown in one of our cases. Some authors reported incidental findings of long blind loops discovered during either autopsy studies or surgical interventions in asymptomatic individuals[6]. Thus, the sole development of a blind loop does not cause clinical manifestations per se.
We found in the literature only 11 cases of blind loop perforation, some presenting with acute, generalized peritonitis due to pinpoint perforation[5,6,8]. Lack of data precludes an accurate appraisal on those factors which might cause symptomatic complications in those with a blind loop.
Usually, blind loop syndrome occurs many years after bowel surgery. In a French review, 45 out of 69 patients with a blind loop developed abdominal symptoms more than 5 years after surgery[5]. In a retrospective study based on abdominal CT examinations of 30 patients with radiological features compatible with a blind loop (eventually resected in 4 cases), the mean and median time between surgery and imaging were 49.4 and 32.2 mo, respectively[11]. In our own series, all patients presented with perforation at least 2 years after a side-to-side ileocolonic anastomosis. A CT scan seems helpful and a focally dilated loop of the small bowel adjacent to surgical clips is a recurrent finding[11]. Even so, blind loops may be mistaken for diverticula, abscesses or obstructed bowel segments. Blind loop syndrome is primarily managed through a redo anastomosis in an end-to-end fashion. We chose to resect the perforated pouch sparing a new anastomosis, deeming this limited procedure safer in the emergency setting. Our patients are free of symptoms albeit after a short clinical follow-up (6-12 mo). Blind loop syndrome along with its complications is probably underestimated and further research is needed to define the real extent of the problem. In addition, a colorectal surgeon should be aware of this potential issue before choosing the ileocolonic anastomosis reconstructive technique.
Three patients presented with perforation of a blind loop years after right hemicolectomy followed by side-to-side ileocolonic anastomosis.
They displayed peritoneal signs along with high-grade fever, one had localized epigastric pain, and the others suffered diffuse abdominal pain.
Complicated acute diverticulitis, peptic ulcer perforation.
All had leukocytosis.
Plain abdominal X-rays usually show peritoneal free-air. Contrast-enhanced computed tomography identified an enlarged blind loop with thick walls, mucosal hyperemia and perivisceral fat necrosis along with intra-abdominal free-air and fluid.
Ileal stump enlargement with full-thickness pinpoint perforation.
Laparoscopic or laparotomic resection of the perforated blind loop with linear stapler.
Only a few series and isolated case reports are available in the literature about the long-term complications of ileocolonic anastomoses associated with a specific anastomosis configuration. The true incidence of blind loop syndrome is probably underestimated but blind loop enlargement after side-to-side digestive anastomoses is a well-known phenomenon. Besides, only 11 cases of blind loop perforation are described in literature thus far.
Blind loop syndrome after right hemicolectomy develops when bacterial overgrowth occurs in the previously interrupted bowel stump, whose dysmotility tends to divert the intestinal contents away from the physiologic route. Clinically it may manifest itself with symptoms of vitamin malabsorption, malnutrition, weight loss, digestive bleeding and even viscus perforation.
Side-to-side anastomoses are strongly related to the development of blind loop syndrome in the long-term. In open right hemicolectomy, end-to-end or end-to-side anastomoses can be used, while in the case of a full laparoscopic procedure requiring a side-to-side reconstruction, surgeons should resect the ileal stump as short as possible.
In general the side-to-side anastomosis is a bad technique to restore intestinal continuity but not unusual in times of laparoscopic surgery of the right colon. It leads to a progressive distention of the cul-de-sac, which produces definite pockets of stasis and infection.
P- Reviewer: Actis GC, Goetze TO, Rabago L S- Editor: Ji FF L- Editor: Cant MR E- Editor: Liu SQ
| 1. | Cannon WB, Murphy FT. IV. The Movements of the Stomach and Intestines in Some Surgical Conditions. Ann Surg. 1906;43:512-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Pearse AE. Experimental Chronic Intestinal Obstruction from Blind Loops. Surg Gynecol Obstet. 1934;59:726. |
| 3. | Estes WL, Holm CE. The Fate of the Obstructed Loop in Intestinal Obstruction following an Anastomosis around the Obstruction without Resection. Ann Surg. 1932;96:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Holm CE. The Fate of the Sidetracted Loop of Ileum Following Lateral Anastomosis for Complete Benign Obstruction. Surg Gynecol Obstet. 1933;56:746. |
| 5. | Frank P, Batzenschlager A, Philippe E. [Blind-pouch syndrome after side-to-side intestinal anastomosis]. Chirurgie. 1990;116:586-596. [PubMed] |
| 6. | Schlegel DM, Maglinte DD. The blind pouch syndrome. Surg Gynecol Obstet. 1982;155:541-544. [PubMed] |
| 7. | Hensler MK, Roosen JU. [A case of perforation of a blind loop secondary to ileocolic side-to-side anastomosis]. Ugeskr Laeger. 1991;153:2835-2836. [PubMed] |
| 8. | Tsugu Y, Shimada N, Kawakami H, Kadomatsu T, Morita H. [Blind loop syndrome after intestinal anastomosis (case of perforated blind loop)]. Shujutsu. 1966;20:650-656. [PubMed] |
| 9. | Stellamor K, Hochberger O. [To the cognizance of the “blind pouch syndrome” following intestinal anastomoses (author’s transl)]. Rontgenblatter. 1974;27:82-90. [PubMed] |
| 10. | Pollock LH. Blind-pouch formation following lateral anastomosis. AMA Arch Surg. 1958;76:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Sandrasegaran K, Maglinte DD, Rajesh A, Tann M, Kopecky KK. CT findings for postsurgical blind pouch of small bowel. AJR Am J Roentgenol. 2006;186:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |