Published online Apr 27, 2013. doi: 10.4240/wjgs.v5.i4.115
Revised: February 21, 2013
Accepted: March 8, 2013
Published online: April 27, 2013
Processing time: 246 Days and 0.3 Hours
AIM: To evaluate the clinical usefulness of Daikenchuto (DKT) in hepatecomized patients.
METHODS: Twenty patients were enrolled with informed consent. Two patients were excluded because of cancelled operations. The remaining 18 patients were randomly chosen for treatment with DKT alone or combination therapy of DKT and lactulose (n = 9, each group). Data were prospectively collected. Primary end points were Visual Analogue Scale (VAS) score for abdominal bloating, total Gastrointestinal Symptoms Rating Scale (GSRS) score for abdominal symptoms, and GSRS score for abdominal bloating.
RESULTS: The VAS score for abdominal bloating and total GSRS score for abdominal symptoms recovered to levels that were not significantly different to preoperative levels by 10 d postoperation. Combination therapy of DKT and lactulose was associated with a significantly poorer outcome in terms of VAS and GSRS scores for abdominal bloating, total GSRS score, and total daily calorie intake, when compared with DKT alone therapy.
CONCLUSION: DKT is a potentially effective drug for postoperative management of hepatectomized patients, not only to ameliorate abdominal bloating, but also to promote nutritional support by increasing postoperative dietary intake.
- Citation: Hanazaki K, Ichikawa K, Munekage M, Kitagawa H, Dabanaka K, Namikawa T. Effect of Daikenchuto (TJ-100) on abdominal bloating in hepatectomized patients. World J Gastrointest Surg 2013; 5(4): 115-122
- URL: https://www.wjgnet.com/1948-9366/full/v5/i4/115.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v5.i4.115
Despite developments in surgical skills and perioperative management, uncomfortable abdominal symptoms such as bloating and pain are unavoidable after abdominal surgery. A combination of stool softeners, laxatives, antispasmodics, antidepressants, and diet changes are often used in postoperative management; however, the full effects of the above drugs and diet changes remain unclear, and stimulant laxatives may themselves induce abdominal bloating and pain[1].
Daikenchuto (TJ-100, DKT) is a frequently prescribed traditional Japanese herbal (or Kampo) medicine in Japan, comprising extract granules of Japanese pepper, processed ginger, ginseng radix, and maltose powder derived from rice. DKT extract powder (Tsumura and Co., Tokyo, Japan) is manufactured as an aqueous extract containing 2.2% Japanese pepper, 5.6% processed ginger, 3.3% ginseng radix, and 88.9% maltose syrup powder. The standard dosage of DKT is 15 g/d[2].
The effects of DKT in the gastrointestinal (GI) tract are mediated mainly by cholinergic and serotonergic nerves. DKT enhances GI motility both in vitro and in vivo[3]. Clinically, DKT plays pivotal roles in the management of gastroenterological disorder after surgery by improving bowel blood flow and bowel movement[4].
Hepatectomy is an effective method for treating various liver diseases, such as hepatocellular carcinoma (HCC), metastatic liver cancer (MLC), and cholangiocarcinoma (CCC). Recent advances in surgical techniques and perioperative care have made hepatectomy a safer therapeutic option than previously observed, with less morbidity and mortality. However, postoperative liver failure remains an unsolved problem, especially in patients who have undergone major liver resection and have limited hepatic functional reserve. Bacterial translocation (BT) is a key factor in liver failure after hepatic resection, and leads to high rates of morbidity and mortality[5]. To prevent BT, we have routinely used drugs such as lactulose to promote release of flatus and defecation after hepatic resection. DKT is often used for the same purpose as lactulose in many Japanese institutes[6].
DKT has been used to treat patients following GI surgery, and has been shown to prevent postoperative ileus in these patients. In Japan, many surgeons have successfully used DKT to promote release of flatus and defecation after GI surgery[3-6]. However, there have been few scientific studies of DKT in hepatectomized patients, and the effects of DKT in such patients remain unclear. Therefore, this study sought to evaluate the clinical usefulness of DKT in hepatectomized patients.
Abdominal bloating and pain can be accurately and quantitatively evaluated by using the Visual Analogue Scale (VAS) score, and various abdominal symptoms can be evaluated by using the Gastrointestinal Symptoms Rating Scale (GSRS) score[1]. Here, we used VAS and GSRS scores to evaluate abdominal symptoms in hepatectomized patients. To the best of our knowledge, this is the first study to evaluate the effects of DKT using VAS and GSRS scores in hepatectomized patients.
From November 2009 to June 2011, 20 patients with liver malignancy were enrolled in this study. Informed consent was obtained from each patient according to the guidelines for clinical studies established by Kochi Medical School. Two patients were eliminated from the study because their operation was cancelled. Data for the remaining 18 patients were prospectively collected.
Three primary end points were used to assess the severity of abdominal symptoms. First, patients subjectively evaluated their abdominal bloating by using VAS[7], which is a 10 cm horizontal line scoring system ranging between 0 (no abdominal bloating) and 10 (extremely strong or frequent). VAS evaluations were performed the day before the operation, prior to administration of DKT, and 2, 4, 6, 8 and 10 d after the operation. Second, the patients subjectively evaluated their abdominal symptoms by using the GSRS, Japanese edition[8], which comprises 15 items that are each rated according to severity on a scale of 1 (absence of the symptom) to 7 (maximal intensity of the symptom); thus, higher GSRS scores indicate more severe symptoms. The GSRS questionnaire was administered twice to each patient on the day before the operation, prior to administration of DKT, and 10 d after the operation. We used the total GSRS score for all 15 GI symptoms to evaluate abdominal symptoms. Third, in a sub-analysis, we used the GSRS score for abdominal bloating to evaluate abdominal bloating specifically.
Secondary end points were levels of serum ammonia, C-reactive protein (CRP), and interleukin-6 (IL-6), the length of time from the end of general anesthesia until the first release of flatus or defecation, and the presence or absence of postoperative adhesive ileus.
Each of the 18 patients was treated orally with a 15 g/d dosage of DKT from 3 d before the operation to 10 d after the operation. Nine of these patients were selected at random, and cotreated with at least 16 g of lactulose 3 times a day (≥ 48 g/d) over the same period. Thus, the 18 patients were divided equally into 2 groups: DKT alone therapy group (D group; n = 9) and combination therapy of DKT and lactulose group (D + L group; n = 9).
Results are expressed as mean ± SD unless stated otherwise. The treatment responses in the D and D + L groups were evaluated based on the changes in the VAS and GSRS scores between before and after treatment by using the Wilcoxon signed rank test. Background factors with a continuous distribution, such as patient age and body mass index (BMI), were compared between X and Y by using the Wilcoxon rank sum test. The distributions of various categorical factors, such as gender, diagnosis, and Child-Pugh score[9,10] were compared between X and Y by using the Fisher’s exact test. The incidences of morbidity were compared between the D and D + L groups by using the χ2 test. P values less than 0.05 were considered statistically significant.
The patient characteristics are listed in Table 1. The 18 patients comprised 15 men and 3 women, with 12 cases of HCC, 5 cases of MLC, and 1 case of CCC. There was no mortality and no side effects after treatment with DKT. Six patients (33%) had morbidity in the form of minor bile leakage (5 cases) and slight cholangitis (1 case), but all affected patients recovered with conservative therapy.
| Variable | |
| Age (yr) | 64.9 ± 6.7 |
| Male/female | 15/3 |
| Height (cm) | 161.4 ± 10.5 |
| Weight (kg) | 60.6 ± 9.9 |
| BMI (%) | 23.3 ± 3.7 |
| Diagnosis | |
| HCC | 12 (67) |
| MLT | 5 (28) |
| CCC | 1 (5) |
| Child-Pugh score | |
| A | 18 (100) |
| B | 0 (0) |
| C | 0 (0) |
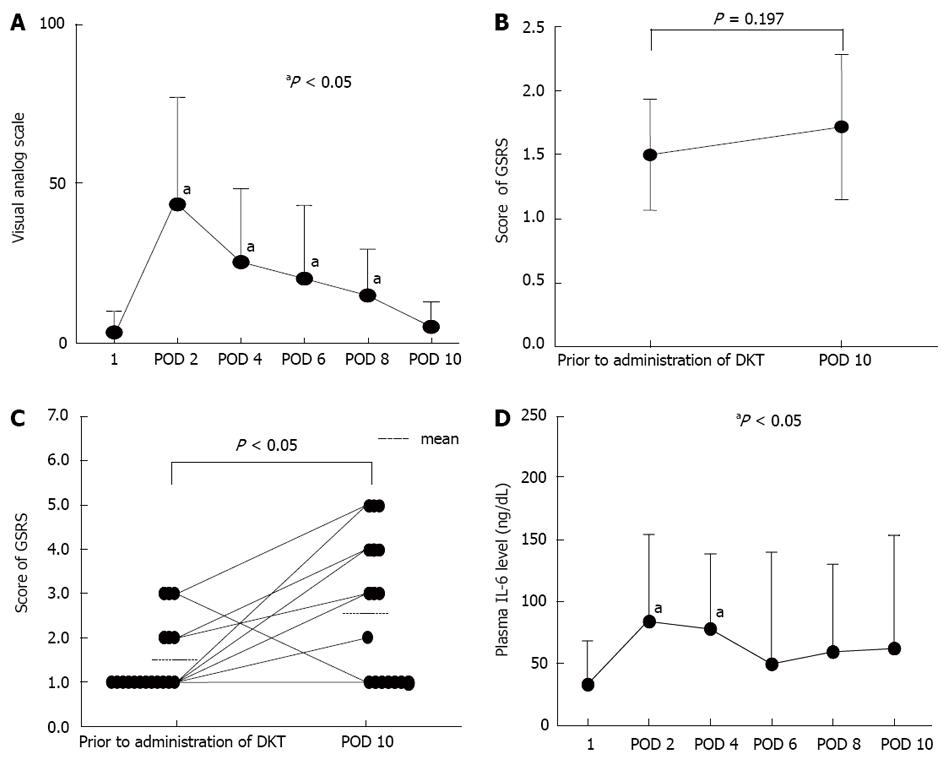
The VAS score for abdominal bloating peaked at postoperative day 2, and then decreased gradually to a level similar to the preoperative level by postoperative day 10 (Figure 1A). The scores measured on postoperative days 2 to 8 were significantly higher than the preoperative score measured prior to administration of DKT.
Although total GSRS score for abdominal symptoms was slightly elevated at postoperative day 10 (1.72) compared with the level before surgery and prior to administration of DKT (1.51), the difference was not statistically significant (Figure 1B). In contrast, the GSRS score sub-analysis showed a significantly elevated GSRS score for abdominal bloating at postoperative day 10 compared with the level prior to surgery (Figure 1C). The use of lactulose in combination with DKT was a significant risk factor for a reduced (i.e., worse) GSRS score after hepatic resection, compared with the preoperative score (Table 2). Other factors such as intraoperative blood loss, operative method, and tumor marker levels were not significant risk factors (Table 2).
| Variable | P value |
| Use of lactulose (yes/no) | 0.001 |
| Preserved liver function (ChE) | 0.115 |
| Marker of liver fibrosis (hyarulonic acid) | 0.553 |
| Intraoperative blood loss (mL) | 0.080 |
| Type of hepatic resection1 | 0.093 |
| BMI | 0.192 |
| Operation time (min) | 0.466 |
| Anesthesia time (min) | 0.556 |
Although plasma IL-6 levels increased significantly at postoperative days 1 and 2 after hepatic resection compared with the level before the operation and prior to DKT administration, they recovered to a level that was not significantly different from the preoperative level by postoperative day 4 (Figure 1D).
The characteristics of patients treated with DKT (15 g/d) alone (D group; n = 9) and patients subjected to combination therapy of DKT (15 g/d) and lactulose (48 g/d) (D + L group; n = 9) are listed in Table 3. Between the two groups, there were no significant differences in preoperative data such as Child-Pugh score[9,10], CLIP score[11], and liver damage score[12] or intraoperative data such as type of hepatic resection, blood loss, and operation time.
| Variable | DKT group | DKT + lactulose group | P value |
| Age (yr) | 65.9 ± 6.1 | 64.0 ± 7.6 | 0.567 |
| Male/female | 7/2 | 8/1 | 1.000 |
| Height (cm) | 164.6 ± 7.3 | 158.2 ± 12.6 | 0.206 |
| Weight (kg) | 65.1 ± 7.4 | 56.1 ± 10.5 | 0.052 |
| BMI (%) | 24.0 ± 1.7 | 22.6 ± 5.0 | 0.455 |
| Child-Pugh score | Grade A: 9 | Grade A: 9 | 0.585 |
| CLIP score | Score 0: 8 and Score 1: 1 | Score 0: 7 and Score 1: 2 | 1.000 |
| Liver damage score | Grade A: 9 | Grade A: 7 and Grade B: 2 | 0.166 |
| Type of hepatic resection | 0.188 | ||
| Right lobectomy: 1 | Left lobectomy: 1 | ||
| Segmentectomy: 1 | Segmentectomy: 3 | ||
| Wedge resection: 7 | Wedge resection: 5 | ||
| Intraoperative blood loss (mL) | 428.9 ± 294.0 | 852.2 ± 928.4 | 0.211 |
| Operation time (min) | 307.8 ± 107.5 | 347.2 ± 125.0 | 0.483 |
| Anesthesia time (min) | 392.8 ± 118.0 | 420.6 ± 128.4 | 0.639 |
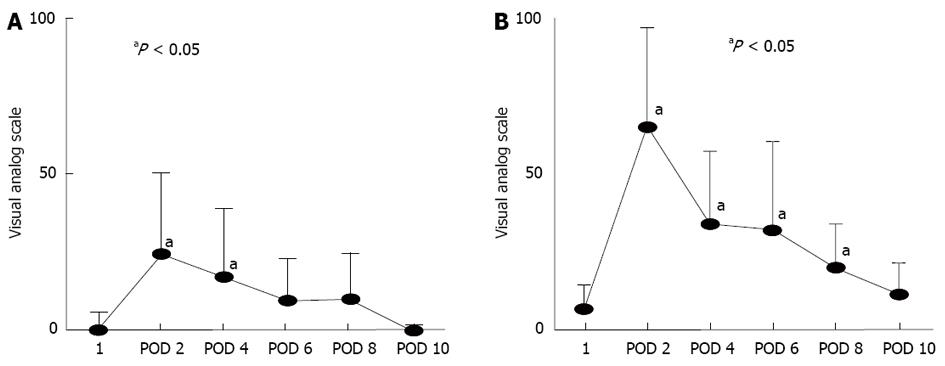
By postoperative day 6, the VAS scores for abdominal bloating in D group had recovered to levels that were not significantly different from the preoperative levels prior to administration of DKT (Figure 2A). In contrast, the VAS scores of the D + L group did not return to preoperative levels until postoperative day 10 (Figure 2B). At postoperative days 2 and 10, the VAS scores for abdominal bloating in D group were significantly lower than those in the D + L group (Figure 2).
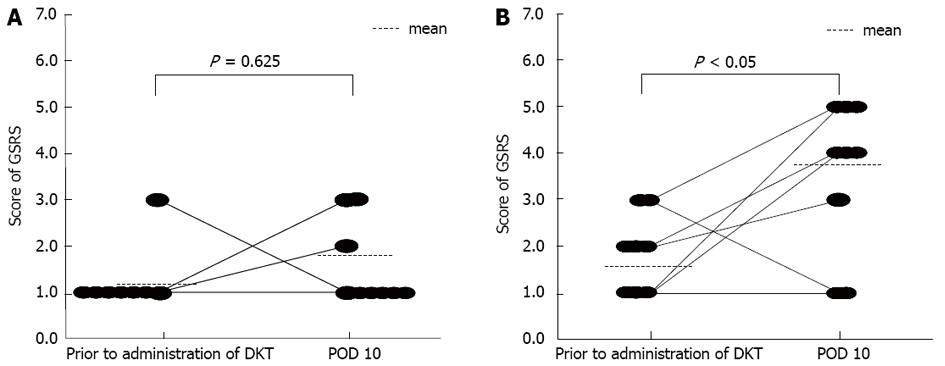
The total GSRS score at postoperative day 10 was significantly lower in D group than in D + L group (P < 0.05), whereas there was no significant difference in this score between the groups before the operation. Also, in the sub-analysis, although there was no significant difference between preoperative and postoperative GSRS scores for abdominal bloating in D group (Figure 3A), and the postoperative GSRS score for abdominal bloating was significantly higher than the preoperative score in the D + L group (Figure 3B). Regarding the secondary end points, there were no significant differences in postoperative serum ammonia or CRP values between the two groups. The median times from the end of general anesthesia until the first release of flatus or defecation in D vs D + L groups were 39.7 vs 29.3 h (release of flatus) and 77.7 vs 42.7 h (defecation), respectively. Although the time until the first release of flatus did not differ significantly between the two groups, the time until the first defecation was significantly smaller in the D + L group than that in the D group (P < 0.05). No patients experienced postoperative adhesive ileus.
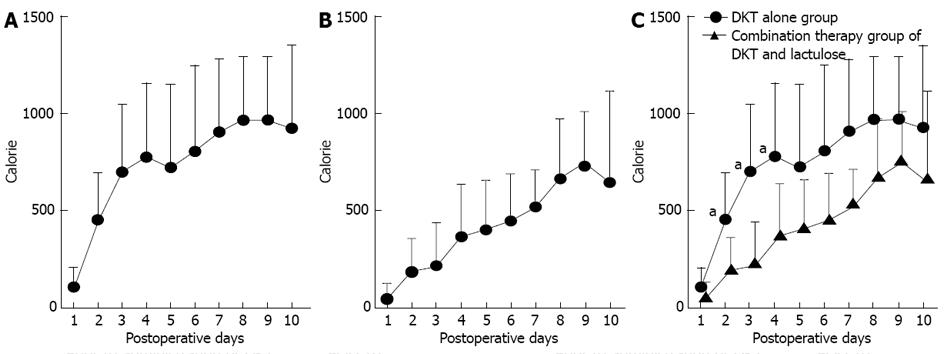
In terms of patient nutrition, the mean total calorie intake over the period from postoperative day 1 to day 10 (7305.0 kcal, Figure 4A) was significantly higher in the D group than in the D + L group (4201.6 kcal, P = 0.027, Figure 4B) and the mean total calorie intake at each of postoperative days 2, 3, and 4 was significantly higher in the D group than in the D + L group (P < 0.05, Figure 4C).
The incidence of morbidity after hepatic resection in the D group (1/9 patients experienced cholangitis) was lower than that in the D + L group (5/9 patients experienced bile leakage); however, this difference was not statistically significant (P = 0.34). Also, between the groups, there was no significant difference in the mean or median period of hospital stay after hepatic resection (P = 0.30, P = 0.29, respectively), although D group patients showed a tendency for shorter postoperative hospital stays compared with the D + L group.
In this prospective study, we found that DKT therapy produced an effective surgical outcome for hepatic resection in that the VAS score for abdominal bloating in patients treated with DKT returned to close to the preoperative level by postoperative day 10, and the preoperative level of the total GSRS score for abdominal symptoms was well preserved after the operation. We also found that combination therapy of DKT and lactulose produced a significantly poorer outcome, not only in terms of total GSRS score for abdominal symptoms, but also in VAS and GSRS scores for abdominal bloating, compared with DKT alone therapy. As a result, DKT alone therapy was superior to the combination therapy as a perioperative nutritional support by enabling higher postoperative dietary intake.
Horiuchi et al[1] suggested that both the VAS and GSRS scores of patients treated with DKT for chronic constipation were superior for dosages of 15 g/d compared with 7.5 g/d. In that study, DKT showed a dose-dependent effect on abdominal bloating and abdominal pain. Therefore, in our study, every patient was treated orally with a dosage of 15 g/d, and we recommend that DKT should be administered to hepatectomized patients with abdominal bloating or pain by using a standard dose of 15 g/d.
Lactulose is an artificially synthesized disaccharide (1, 4-β galactosido-fructose). Following oral administration, lactulose reaches the colon in intact form, where it is split by lactobaccili into lower molecular-weight organic acids such as lactic acid and acetic acid. As a result, gram-negative rods, which are the main cause of ammonia production in the GI tract, reduce in number and lactobaccili increase in number. Diminished ammonia production after lactulose treatment has been explained by alteration of bacterial flora in the gut, changes in bacterial metabolism, and an effect of lactulose on the metabolism of crypt and villus cells of the bowel wall. Lactulose also inhibits the production of tumor necrotizing factor in endotoxin-stimulated monocytes and binds endotoxin[6]. In our study, if lactulose was used in combination with DKT, the first release of flatus and first defecation after hepatic resection occurred earlier than when DKT was used alone; this effect was statistically significant for defecation, but not for release of flatus; however, we do not recommend combination therapy of DKT and lactulose for patients with uncomfortable abdominal symptoms after hepatic resection because of significantly worse VAS and GSRS scores for abdominal bloating compared with those observed for DKT alone therapy.
Kaiho et al[6] reported that levels of postoperative serum ammonia in hepatectomized patients in a DKT-treatment (15 g/d) group were significantly lower than those in the control group (no DKT or lactulose treatment) or the lactulose-treatment (48 g/d) group. These results, albeit from a retrospective study, suggested that DKT might be a more effective and safe agent than lactulose in postoperative management of hepatic resection. The lack of a significant difference in postoperative serum ammonia levels between the D and D + L groups in our study suggests that prevention of increased levels of ammonia and associated hepatic coma after hepatic resection does not require lactulose treatment if DKT is used.
Kono et al[2,3] reported that DKT activates the endogenous adrenomedullin (ADM)/calcitonin gene-related peptide (CGRP) system. ADM and CGRP belong to the calcitonin family and are potent endogenous vasodilators. ADM plays important roles in the regulation of microcirculation, angiogenesis, anti-fibrosis, antibiosis, and down-regulation of proinflammatory cytokines. CGRP is present in most sensory neurons supplying the GI tract and plays important roles in the regulation of microcirculation and immune suppression; moreover, CGRP has anti-inflammatory effects. Therefore, DKT has a substantial anti-inflammatory effect through its activation of ADM and CGRP. We also observed that DKT increased the serum CGRP level and contributed to the early recovery of abdominal symptoms in patients with adhesive ileus (data not shown).
Previously we performed absorption, distribution, metabolism, and excretion studies of DKT[13,14]. In the first study[13], after oral administration of 15 g of DKT in healthy volunteers, 6 ingredients of DKT (i.e., hydroxy-α- and hydroxy-β-sanshool derived from Japanese pepper, 6- and 10-shogaol derived from ginger, and ginsenoside Rb1 and Rg1 derived from ginseng) were detected in intravenous blood. In the second study[14], four of these compounds (i.e., hydroxy-α and hydroxyl-β-sanshool, and 6- and 10-shogaol) were dose-dependently detected in venous blood[14]. Of note, CGRP and ADM are known to be activated by hydroxy-α and hydroxy-β-sanshool and/or 6-shogaol[14].
The increase of inflammatory cytokines after surgery is responsible for a substantial portion of the loss of epithelial barrier function observed. Impaired integrity of the intestine can cause BT, which is a key factor leading to high morbidity and mortality by causing conditions such as sepsis and multiple organ failure[5]. Thus, to improve surgical outcomes, it is important to prevent BT after surgery. Yoshikawa et al[5] reported that DKT prevents BT in rats through the morphological preservation of microvilli and the suppression of various inflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-6, and interferon-γ (IFN-γ). These experimental data support our clinical finding that, in patients treated with DKT, the significant elevation of IL-6 observed immediately after hepatic resection recovered by postoperative 4 day to a level that was not significantly different to the preoperative level (Figure 1D). Recently, we found that DKT played important roles in the suppression of inflammatory cytokines such as IL-6 and INF-γ in patients with adhesive ileus (data not shown). In addition, a recent clinical report demonstrated that postoperative DKT therapy (7.5 g/d for 7 d) significantly suppressed the CRP level correlating to IL-6 in general, and shortened the time until first release of flatus following laparoscopic colorectal resection[15]. Infection control plays pivotal roles in the prevention of liver failure after hepatic resection. Our finding that DKT has anti-inflammatory effects by reducing the elevation of plasma IL-6 after hepatic resection (Figure 1D) suggests that DKT may be a useful drug for infection control and the prevention of BT.
Enhanced recovery after surgery (ERAS) is a novel concept of perioperative management derived from Western countries[16-18]. Briefly, the ERAS protocol, which consists of appropriate nutritional support and good infection control including prevention of hyperglycemia and/or insulin resistance, aims to shorten hospital stays, improve quality of life in patients, and reduce costs to the hospital[16-19]. In our study, treatment with DKT alone was more effective in improving VAS and GSRS scores for abdominal bloating than was treatment with DKT combined with lactulose. Furthermore, the results of our comparison of total calorie intake between patients treated with DKT alone and those treated with combination therapy, suggest that DKT alone therapy is the more effective regimen to promote early recovery associated with dietary nutrition. Also, although it was not a statistically significant difference, patients treated with DKT alone showed a lower morbidity rate and had a tendency towards a shorter hospital stay compared with those subjected to the combination therapy. Furthermore, Ogasawara et al[20] found that DKT increased portal blood flow in the early phase after oral administration (2.5 g dose) without any significant changes in the blood pressure and heart rate in the healthy volunteers, cirrhotic patients and liver transplant patients, suggesting that DKT therapy might improve liver function by increasing portal blood flow in patients after hepatic resection. Therefore, in terms of ERAS, DKT alone therapy after hepatic resection may be useful not only for improvement of postoperative abdominal symptoms, and enhanced dietary nutrition, but also for preservation of hepatic function through increasing portal blood flow. Further examinations with larger series of patients will be required to address these issues regarding ERAS.
Besides the small sample size, a limitation of our study is the absence of a control group that was not subjected to DKT treatment. Placebos of Kampo medicine such as DKT are difficult to prepare[4-7], but even if a placebo could be prepared successfully, we consider that the use of such a placebo might give rise to ethical problems, for the following reasons. Almost all hepatectomized patients suffer from abdominal bloating and pain associated with delayed release of flatus and defecation; therefore, if no drugs such as DKT and/or lactulose were used, the situation might grow more serious, especially in patients with chronic hepatitis and cirrhosis. Ammonia levels and the incidence of postoperative BT might increase, and infection control might become poor. In the worst situation, this may lead to high morbidity and mortality caused by hepatic encephalopathy and acute liver failure. At all events, the setting of control group with no DKT and no lactulose group might be needed for more strict evaluation in this study. However, the superior effectiveness of DKT for post-hepatectomy abdominal discomfort compared with lactulose alone group or no DKT and no lactulose group has been already established by previous clinical study[6].
In conclusion, DKT is a potentially effective drug for hepatectomized patients, not only to ameliorate VAS and GSRS scores for abdominal bloating, but also to promote ERAS by increasing postoperative dietary intake. Additional studies of DKT in prospective randomized clinical trials with a large patient series are warranted to further evaluate the effects of DKT against uncomfortable abdominal symptoms such as bloating and pain after hepatic resection.
Daikenchuto (DKT) has been used to treat patients following gastrointestinal (GI) surgery, and has been shown to prevent postoperative ileus in these patients. In Japan, many surgeons have successfully used DKT to promote release of flatus and defecation after GI surgery. However, there have been few scientific evidences of DKT in hepatectomized patients, and the effects of DKT in such patients remain unclear. This study evaluates the clinical usefulness of DKT in hepatectomized patients.
Abdominal bloating and pain can be accurately and quantitatively evaluated by using the Visual Analogue Scale (VAS) score, and various abdominal symptoms can be evaluated by using the Gastrointestinal Symptoms Rating Scale (GSRS) score. VAS and GSRS scores are used to evaluate abdominal symptoms.
In hepatectomized patients, combination therapy of DKT and lactulose was associated with a significantly poorer outcome in terms of VAS and GSRS scores for abdominal bloating, total GSRS score, and total daily calorie intake, when compared with DKT alone therapy.
To promote enhanced recovery after surgery (ERAS), perioperative DKT treatment may play an important role in the improvement of abdominal symptoms and nutritional status after hepatic resection. Therefore DKT may contribute to reduce morbidity, mortality due to liver failure, and hospital staying after hepatectomy.
ERAS is a novel concept of perioperative management. The ERAS protocol, which consists of appropriate nutritional support and good infection control, aims to shorten hospital stays, improve quality of life in patients, and reduce costs to the hospital. VAS and GSRS are useful scores to objectively evaluate abdominal symptoms in surgical patients.
This is an early study to evaluate the effects of DKT using VAS and GSRS scores in hepatectomized patients. The results suggest that perioperative DKT treatment for surgical patients may have a potential effect to ameliorate abdominal symptoms and contribute to nutrition support and ERAS protocol.
P- Reviewer Ikegami T S- Editor Wen LL L- Editor A E- Editor Lu YJ
| 1. | Horiuchi A, Nakayama Y, Tanaka N. Effect of traditional Japanese medicine, Daikenchuto (TJ-100) in patients with chronic constipation. Gastroenterol Research. 2010;3:151-155. |
| 2. | Kono T, Kanematsu T, Kitajima M. Exodus of Kampo, traditional Japanese medicine, from the complementary and alternative medicines: is it time yet? Surgery. 2009;146:837-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Kono T, Kaneko A, Hira Y, Suzuki T, Chisato N, Ohtake N, Miura N, Watanabe T. Anti-colitis and -adhesion effects of daikenchuto via endogenous adrenomedullin enhancement in Crohn’s disease mouse model. J Crohns Colitis. 2010;4:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Manabe N, Camilleri M, Rao A, Wong BS, Burton D, Busciglio I, Zinsmeister AR, Haruma K. Effect of daikenchuto (TU-100) on gastrointestinal and colonic transit in humans. Am J Physiol Gastrointest Liver Physiol. 2010;298:G970-G975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Yoshikawa K, Kurita N, Higashijima J, Miyatani T, Miyamoto H, Nishioka M, Shimada M. Kampo medicine “Dai-kenchu-to” prevents bacterial translocation in rats. Dig Dis Sci. 2008;53:1824-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Kaiho T, Tanaka T, Tsuchiya S, Yanagisawa S, Takeuchi O, Miura M, Saigusa N, Miyazaki M. Effect of the herbal medicine Dai-kenchu-to for serum ammonia in hepatectomized patients. Hepatogastroenterology. 2005;52:161-165. [PubMed] |
| 7. | Mukaida K, Hattori N, Kondo K, Morita N, Murakami I, Haruta Y, Yokoyama A, Kohno N. A pilot study of the multiherb Kampo medicine bakumondoto for cough in patients with chronic obstructive pulmonary disease. Phytomedicine. 2011;18:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Hongo M, Fukuhara S, Green J. QOL (quality of life) in gastroenterological series; evaluation of QOL by Gastrointestinal Symptom Rating Scale (GSRS) in Japanese. Diagnosis and Therapy. 1999;87:731-736. |
| 9. | Child CG, Turcotte JG. Surgery and portal hypertension. The liver and portal hypertension. Philadelphia: Saunders 1964; 50-64. |
| 10. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5726] [Article Influence: 110.1] [Reference Citation Analysis (2)] |
| 11. | Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 372] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 12. | Kokudo N, Makuuchi M. Evidence-based clinical practice guidelines for hepatocellular carcinoma in Japan: the J-HCC guidelines. J Gastroenterol. 2009;44 Suppl 19:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Iwabu J, Watanabe J, Hirakura K, Ozaki Y, Hanazaki K. Profiling of the compounds absorbed in human plasma and urine after oral administration of a traditional Japanese (kampo) medicine, daikenchuto. Drug Metab Dispos. 2010;38:2040-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Munekage M, Kitagawa H, Ichikawa K, Watanabe J, Aoki K, Kono T, Hanazaki K. Pharmacokinetics of daikenchuto, a traditional Japanese medicine (kampo) after single oral administration to healthy Japanese volunteers. Drug Metab Dispos. 2011;39:1784-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Yoshikawa K, Shimada M, Nishioka M, Kurita N, Iwata T, Morimoto S, Miyatani T, Komatsu M, Kashihara H, Mikami C. The effects of the Kampo medicine (Japanese herbal medicine) “Daikenchuto” on the surgical inflammatory response following laparoscopic colorectal resection. Surg Today. 2012;42:646-651. [PubMed] |
| 16. | Kehlet H, Mogensen T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg. 1999;86:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 350] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 17. | van Dam RM, Hendry PO, Coolsen MM, Bemelmans MH, Lassen K, Revhaug A, Fearon KC, Garden OJ, Dejong CH. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg. 2008;95:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Gustafsson UO, Ljungqvist O. Perioperative nutritional management in digestive tract surgery. Curr Opin Clin Nutr Metab Care. 2011;14:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 19. | Tsukamoto Y, Okabayashi T, Hanazaki K. Progressive artificial endocrine pancreas: The era of novel perioperative blood glucose control for surgery. Surg Today. 2011;41:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Ogasawara T, Morine Y, Ikemoto T, Imura S, Fujii M, Soejima Y, Shimada M. Influence of Dai-kenchu-to (DKT) on human portal blood flow. Hepatogastroenterology. 2008;55:574-577. [PubMed] |