Published online Aug 27, 2010. doi: 10.4240/wjgs.v2.i8.265
Revised: March 22, 2010
Accepted: March 29, 2010
Published online: August 27, 2010
Non-carcinoid appendiceal malignancies are rare entities, representing less than 0.5% of all gastrointestinal malignancies. Because of their rarity and particular biological behavior, a substantial number of patients affected by these neoplasms do not receive appropriate surgical resection. In this report, we describe a rare case of primary signet-ring cell carcinoma of the appendix with peritoneal seeding which occurred in a 40-year old man admitted at the Emergency Surgery Department with the clinical suspicion of acute appendicitis. After a surgical debulking and right hemicolectomy, the patient had systemic chemotherapy according to FOLFOX protocol. After completion of the latter, the patient underwent cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. This report offers a brief review of the literature and suggests an algorithm for the management of non-carcinoid appendiceal tumors with peritoneal dissemination.
- Citation: Bertuzzo VR, Coccolini F, Pinna AD. Peritoneal seeding from appendiceal carcinoma: A case report and review of the literature. World J Gastrointest Surg 2010; 2(8): 265-269
- URL: https://www.wjgnet.com/1948-9366/full/v2/i8/265.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v2.i8.265
Appendiceal cancers represent less than 0.5% of all gastrointestinal tumors. In one series, the age-adjusted incidence of cancer of the appendix was 0.12 cases per 1 000 000 people per year[1].
While the most common histopathologic type of appendiceal tumor is carcinoid, adenocarcinoma including the histological variants of mucinous adenocarcinoma, signet-ring cell carcinoma (SRCC) and goblet cell carcinoma[2-4] follow in the incidence rate. Benign histology includes leiomyomas, neuromas, lipomas and angiomas.
Primary SRCC of colon and rectum, described for the first time by Laufman and Shapir in 1951[5], is a rare histological finding in colorectal carcinomas (CRC); in western countries it accounts for 0.01% to 2.6% of all CRC[6-9]. SRCC of the appendix (A-SRCC) is an exceedingly rare entity, diagnosed in just 4% of appendiceal malignancies[9]; in fact, only a few cases are reported in the literature[1,9-16] (Table 1).
| No. of cases | Manifestations | Treatment | Survival | |
| McCusker at al[1] | 70 | - | Hemicolectomy or more (72%) less than hemicolectomy (26%) | 5-yr survival 20% |
| McGory at al[9] | 113 | - | - | 5-yr survival 18% |
| Ko at al[10] | 1 | Abdominal distension | Appendectomy, bilateral salpingo-oophorectomy | Alive after 12 mo |
| No authors[11] | 1 | Adnexal mass | Appendectomy and bilateral salpingo-oophorectomy | - |
| Suzuki at al[12] | 1 | Intestinal obstruction | Hartmann’s operation, ileocecal resection, total hysterectomy, bilateral salpingo-oophorectomy | - |
| Niesel at al[13] | 1 | Scrotal metastasis | Ileocecal resection, omentectomy, right testis, right testicular cord and right hemiscrotum resection | - |
| Glehen at al[14] | 36 | - | Cytoreductive surgery plus perioperative intraperitoneal chemotherapy | 3-yr survival -25% |
| Özakyol at al[15] | 1 | Crampy abdominal pain and weight loss | Total hysterectomy, bilateral salpingo-oophorectomy, appendectomy, pelvic and para aortic lymphadenectomy | - |
| Uharcek at al[16] | 1 | Lower abdominal mass | Total hysterectomy, bilateral salpingo-oophorectomy, appendectomy, omentectomy, pelvic and para aortic lymphadenectomy | Died after 18 mo |
A 40-year old man reported to the Emergency Department with sudden onset of acute abdominal pain localized in his right lower quadrant; on examination, the patient was found to be feverish (body temperature 37.6°C/99.68°F),
pulse was 96 beats/min and blood pressure 100/60 mmHg. Abdominal examination showed guarding and tenderness in the lower abdomen with positive rebound tenderness. Blood tests showed neutrophil leucocytosis (WBC 17.830/mm3, neutrophils 78.9%) and an elevation of C-reactive protein (CRP 12.6 mg/dL). The patient’s significant medical history was bilateral inguinal hernioplasty.
Abdominal radiography showed intestinal distension with some air-fluid levels in the central abdomen. The ultrasound scan revealed a swollen hypoechoic appendix with a 10-mm diameter; the lumen was occupied by a thin liquid layer and coprolites. All other abdominal organs were found to be within normal limits.
The surgical intervention took place on the same day as arrival. At laparoscopy, we found a bulky abscess at the appendiceal site involving the cecum and terminal ileum. As this surrounded the appendix making a direct intervention impossible, we decided to do a laparotomic McBurney incision.
The appendix was completely replaced by a purulent and lardaceous material; the extemporaneous histological exam revealed a signet-ring cell carcinoma diffusely infiltrating the vermiform appendix and extending to the appendix mesentery and surrounding adipose tissue. The immunohistochemical research was negative for chromogranin, serotonin, somatostatin and pancreatic polypeptide, which excluded a mixed carcinoid and adenocarcinoma of the appendix[17].
Then, following most current guidelines[9], we undertook a midline incision from the xyphoid bone to the pubic bone. Due to the presence of peritoneal seeding on the ileal mesentery and the parietal peritoneum, we performed a right hemicolectomy enlarged to 35 cm of the ileum and the abdominal wall. With the intention of staging the disease, we also removed some other peritoneal nodules in the Douglas pouch and on the jejunal mesentery.
Definitive histological examination showed cecum neoplastic infiltration of SRCC with neoplastic mucosal ulceration and massive nodal metastases in 9 of 29 analyzed lymph nodes. All the nodules removed from the peritoneal surface were metastatic localizations of SRCC.
The patient recovered uneventfully and was released from hospital 7 d after the operation.
A complete staging with total body-CT revealed no distant metastases but the presence of three peritoneal nodules suggested neoplastic nodules so our patient had systemic chemotherapy with 8 cycles of FOLFOX protocol (5-fluorouracil, folinic acid plus oxaliplatin).
After completion of the latter, a second complete staging was done which demonstrated an increase in the number and dimensions of the peritoneal nodules and numerous enlarged lymph nodes in the retroperitoneum and along the aorto-mesenteric trunk.
Since the patient fulfilled the necessary criteria (Table 2)[18-20], he was again admitted to our Institution and, after a prophylactic ureteral stent placement as previously reported[21], he underwent surgical intervention. Peritoneal cavity exploration revealed peritoneal nodules along the transverse and descending colon, greater and lesser omentum, Glisson’s capsule, gallbladder, hepatic hilum, spleen, bilateral diaphragmatic peritoneum, pelvic and paracaval peritoneum. The calculated peritoneal cancer index was 22[22]. We performed total colectomy, splenectomy, cholecystectomy and complete peritonectomy followed by a 90-min long hyperthermic intraperitoneal chemotherapy (HIPEC) with cisplatin and mytomicin C. The intervention was completed by an ileorectal anastomosis with protective ileostomy. On the first post-operative day, the patient underwent an explorative laparotomy due to an acute hemorrhage. Diaphragmatic bleeding was found and brought under control by means of local hemostatic agents. The remaining post-operative course was regular and the patient was discharged 22 d after surgery.
| First international symposium on regional cancer therapies rules | |
| 1 | Eastern cooperative oncology group performance status two or less[9] |
| 2 | No evidence of extra-abdominal disease |
| 3 | Up to three small, resectable parenchymal hepatic metastases |
| 4 | No evidence of biliary obstruction |
| 5 | No evidence of ureteral obstruction |
| 6 | No evidence of intestinal obstruction at more than one site |
| 7 | Small bowel involvement: no evidence of gross disease in the mesentery with several segmental sites of partial obstruction |
| 8 | Small volume disease in the gastro-hepatic ligament |
Five months later, the patient is well and has a good quality of life. He completed systemic chemotherapy and follow-up imaging revealed no peritoneal recurrence or metastatic lesions.
Appendiceal malignancies represent less than 0.5% of all gastrointestinal tumors with an age-adjusted incidence of 0.12 cases per 1 000 000 people per year[1]. The international classification of diseases for oncology divides tumors of the appendix into malignant carcinoid, goblet cell carcinoma, colonic type adenocarcinoma, mucinous adenocarcinoma and signet-ring cell carcinoma[4].
Because adenocarcinoma of the appendix is so rare, its clinical presentation and natural history are still not well described. The clinical presentation of the majority of patients reported in the literature is acute appendicitis or an abdominal mass but preoperative diagnosis is rarely evident and most patients are not identified until the disease is advanced[1,9-16]. Moreover, up to 70% of cases are not diagnosed intraoperatively[2,23].
The anatomic peculiarities of the appendix allow for several considerations in regard to appendiceal neoplasms to be made. The narrow appendiceal diameter predisposes to occlusion of the lumen by the neoplasm early in its course. This creates the potential for superimposed appendicitis and a marked tendency to rupture and, in fact, appendiceal adenocarcinoma represents the gastrointestinal malignancy most commonly linked to perforation[23]. Furthermore, the appendix often has deficiencies of both longitudinal and circular muscle fibres which predisposes to perforation but also leads to the potential for early peritoneal dissemination.
Primary SRCC of the appendix is an exceedingly rare entity with an incidence rate of 0.15 for 1 000 000 people[9]; just 4% of all appendiceal malignancies are SRCC[9]. In fact, there are only few cases reported in the literature[1,9-16] (Table 1).
Due to the intrinsic characteristics of SRCC and to the particular anatomy of the appendix, in patients with primary A-SRCC the possibility that the tumor extends beyond the colon and/or metastases at the time of diagnosis is higher than in other histological types. In particular,
McCusker et al[1] reported that 76% of people with A-SRCC have distant localization compared to 37% with colonic type adenocarcinoma. According to the same report, lymph node involvement was present in 64% of people with A-SRCC but only in 31% with colonic type adenocarcinoma.
Similarly to SRCC of stomach and colon, SRCC of the appendix causes survival chances to be lower compared to other histological cases. Using multivariate analysis, the presence of signet-ring cells has to be considered as an independent prognostic indicator of poor survival[14]. In fact, the overall 5-year survival for A-SRCC is 18%, ranging from 42%, 46%, 76% and 83% for adenocarcinoma, mucinous carcinoma, goblet-cell carcinoma and carcinoid respectively[9].
The stage-adjusted survival for A-SRCC ranges from 55% in localized disease to 7% in cases of a distant involvement[9]. Moreover, the risk of dying is higher for patients with tumor spread beyond colon and/or metastases in cases of SRCC compared to colonic type adenocarcinoma (HR = 1.82, 95% CI: 1.09-3.04) and with goblet cell carcinoma (HR = 1.66, 95% CI: 1.13-2.44)[1].
According to most current guidelines, a right hemicolectomy should be performed for all non-carcinoid invasive tumors of the appendix: this is also recommended in cases of a secondary procedure[9,23-25]. The 5 year survival rate of patients who underwent a right hemicolectomy was 68% vs 20% for those who only underwent appendectomy[23].
However, the optimal therapeutic approach for appendiceal tumors with peritoneal seeding is the object of discussion in the current scientific literature[9,10,14,23-29]. Although complete cytoreductive surgery and HIPEC have recently become the treatment of choice for peritoneal disseminated disease[24-27] and were shown to ensure a 3-year overall survival of 86% for HIPEC patients compared to 29% for unresectable patients[28], it remains unclear which is the best surgical approach when a non-carcinoid appendiceal neoplasm associated with peritoneal carcinomatosis is incidentally found during surgery. While Murphy et al[24] have recently suggested that, for an optimal outcome, any neoplasm greater than 2 cm and any other neoplasm involving the base of the appendix or appendix mesentery should undergo an immediate right hemicolectomy, González-Moreno et al[27] demonstrated that a right hemicolectomy does not ensure any survival advantage in patients with mucinous appendiceal tumors with peritoneal seeding without peritonectomy and perioperative hyperthermic intraperitoneal chemotherapy. An explanation may be that adhesions induced by prior operations can entrap tumor cells and allow them to progress[27,30]. In addition, Ortega-Perez et al[31] studied four patients with a paracaval tissue invasion by an appendiceal mucinous tumor previously removed by right hemicolectomy and argued that open tissue planes at the site of a right hemicolectomy result in deep invasion of the tumor.
For these reasons, some authors have suggested that a right hemicolectomy should be performed only if it is necessary to clear the primary tumor, when lymph node involvement is histologically demonstrated and/or when a non-mucinous histological type is identified[27].
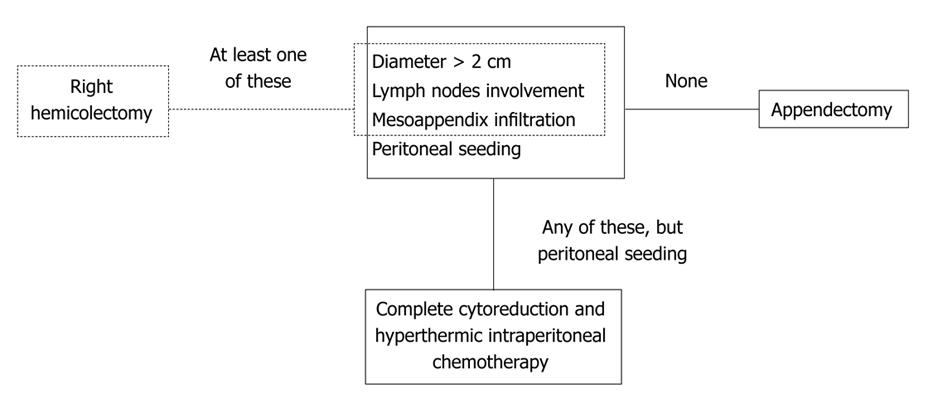
In conclusion, non-carcinoid appendiceal malignancy is a rare entity. Because of its incidental finding in clinical practice and as a general postoperative diagnosis, the correct approach is often not known. Reviewing the literature, we suggest that the therapeutic algorithm for these histological types should be (Figure 1): (1) tumor size less than 2 cm, without appendix mesentery infiltration, lymph node involvement and peritoneal seeding - appendectomy; (2) tumor size more than 2 cm, appendix mesentery infiltration and/or histologically demonstrated lymph node involvement without peritoneal seeding - right hemicolectomy; and (3) any tumor size, lymph node involvement, appendix mesentery infiltration with peritoneal seeding - complete cytoreduction and HIPEC as treatment of choice; if not feasible, appendectomy and refer the patient to an appropriate specialist center.
Peer reviewers: Theodoros E Pavlidis, MD, PhD, Professor, Department of Surgery, University of Thessaloniki, Hippocration Hospital, A Samothraki 23, Thessaloniki 54248, Greece; Giulio A Santoro, Professor, Pelvic Floor Unit and Colorectal Unit, Department of Surgery, Regional Hospital, Treviso 31000, Italy
S- Editor Wang JL L- Editor Roemmele A E- Editor Yamg C
| 1. | McCusker ME, Coté TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer. 2002;94:3307-3312. |
| 2. | Connor SJ, Hanna GB, Frizelle FA. Appendiceal tumors: retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum. 1998;41:75-80. |
| 3. | Jass JR, Sobin LH. Histological typing of intestinal tumors. 2nd ed World Health Organization. New York: Springer-Verlag 1989; . |
| 4. | Percy C, Fritz A, Jack A. Shanmugarathan S, Sobin L, Parkin DM, Whelan S. International Classification of Diseases for Oncology (ICD-O). Third edition. Geneva: WHO 2000; Available from: http://www.who.int/classifications/icd/en. |
| 5. | Laufman H, Saphir O. Primary linitis plastica type of carcinoma of the colon. AMA Arch Surg. 1951;62:79-91. |
| 6. | Anthony T, George R, Rodriguez-Bigas M, Petrelli NJ. Primary signet-ring cell carcinoma of the colon and rectum. Ann Surg Oncol. 1996;3:344-348. |
| 7. | Nissan A, Guillem JG, Paty PB, Wong WD, Cohen AM. Signet-ring cell carcinoma of the colon and rectum: a matched control study. Dis Colon Rectum. 1999;42:1176-1180. |
| 8. | Messerini L, Palomba A, Zampi G. Primary signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 1995;38:1189-1192. |
| 9. | McGory ML, Maggard MA, Kang H, O'Connell JB, Ko CY. Malignancies of the appendix: beyond case series reports. Dis Colon Rectum. 2005;48:2264-2271. |
| 10. | Ko YH, Jung CK, Oh SN, Kim TH, Won HS, Kang JH, Kim HJ, Kang WK, Oh ST, Hong YS. Primary signet ring cell carcinoma of the appendix: a rare case report and our 18-year experience. World J Gastroenterol. 2008;14:5763-5768. |
| 11. | WEEKLY clinicopathological exercises; signet-ring cell carcinoma of appendix, with metastases to both ovaries. N Engl J Med. 1950;242:797-800. |
| 12. | Suzuki J, Kazama S, Kitayama J, Uozaki H, Miyata T, Nagawa H. Signet ring cell carcinoma of the appendix manifesting as colonic obstruction and ovarian tumors: report of a case. Surg Today. 2009;39:235-240. |
| 13. | Niesel T, Böhm J, Paul R, Breul J, Hartung R. Rare metastases of signet ring cell carcinomas to the scrotum: report of two cases. Urology. 1996;47:769-771. |
| 14. | Glehen O, Mohamed F, Sugarbaker PH. Incomplete cytoreduction in 174 patients with peritoneal carcinomatosis from appendiceal malignancy. Ann Surg. 2004;240:278-285. |
| 15. | Ozakyol AH, Sariçam T, Kabukçuoğlu S, Cağa T, Erenoğlu E. Primary appendiceal adenocarcinoma. Am J Clin Oncol. 1999;22:458-459. |
| 16. | Uharcek P, Mlyncek M, Durcanský D. Appendiceal adenocarcinoma presenting with bilateral Krukenberg tumors. J Obstet Gynaecol Res. 2007;33:211-214. |
| 17. | Lin BT, Gown AM. Mixed carcinoid and adenocarcinoma of the appendix: report of 4 cases with immunohistochemical studies and a review of the literature. Appl Immunohistochem Mol Morphol. 2004;12:271-276. |
| 18. | Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R, Baratti D, Bartlett D, Barone R, Barrios P. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol. 2007;14:128-133. |
| 19. | Glockzin G, Schlitt HJ, Piso P. Peritoneal carcinomatosis: patients selection, perioperative complications and quality of life related to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2009;7:5. |
| 20. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. |
| 21. | Coccolini F, Ansaloni L, Concetti S, Schiavina R, Martorana G. The importance of ureteral stenting in major debulking surgery. Int J Gynecol Cancer. 2010;20:479. |
| 22. | Esquivel J, Farinetti A, Sugarbaker PH. [Elective surgery in recurrent colon cancer with peritoneal seeding: when to and when not to proceed]. G Chir. 1999;20:81-86. |
| 23. | Nitecki SS, Wolff BG, Schlinkert R, Sarr MG. The natural history of surgically treated primary adenocarcinoma of the appendix. Ann Surg. 1994;219:51-57. |
| 24. | Murphy EM, Farquharson SM, Moran BJ. Management of an unexpected appendiceal neoplasm. Br J Surg. 2006;93:783-792. |
| 25. | Marrie A. Chirurgia dellâappendice ileo-cecale. EMC Roma-Parigi. Tecniche Chirurgiche-Addominale. 1991;40-500:1-15. |
| 26. | Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727-731. |
| 27. | González-Moreno S, Sugarbaker PH. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg. 2004;91:304-311. |
| 28. | Marcotte E, Sideris L, Drolet P, Mitchell A, Frenette S, Leblanc G, Leclerc YE, Dubé P. Hyperthermic intraperitoneal chemotherapy with oxaliplatin for peritoneal carcinomatosis arising from appendix: preliminary results of a survival analysis. Ann Surg Oncol. 2008;15:2701-2708. |
| 29. | Walters KC, Paton BL, Schmelzer TS, Gersin KS, Iannitti DA, Kercher KW, Heniford BT. Treatment of appendiceal adenocarcinoma in the United States: penetration and outcomes of current guidelines. Am Surg. 2008;74:1066-1068. |