Published online Mar 27, 2025. doi: 10.4240/wjgs.v17.i3.94286
Revised: September 17, 2024
Accepted: December 25, 2024
Published online: March 27, 2025
Processing time: 346 Days and 15.1 Hours
Gastric signet ring cell carcinoma (GSRC) is a distinctive type of gastric cancer. It is a mucus-secreting adenocarcinoma that may progress to distant metastasis at an early stage. Because of poor differentiation, aggressive invasion, rapid progre
To explore the clinical efficacy of fluorouracil (5-FU) combined with paclitaxel and oxaliplatin for the treatment of advanced GSRC.
A total of 85 patients with advanced GSRC were selected between January 2020 and June 2021 and randomly divided into a control group (n = 42, receiving standard chemotherapy) and a treatment group (n = 43, receiving monotherapy with oxaliplatin, 5-FU, and paclitaxel). Patients in the treatment group received a 135 mg/m2 infusion of paclitaxel for 3 hours, a 400 mg/m2 infusion of calcium folate (or 200 mg/m2 of levocalcium folate) for 2 hours, and an 85 mg/m2 infusion of oxaliplatin for 2 hours. This was followed by a continuous intravenous infusion of 2200-2400 mg/m2 5-FU for 46 hours using a portable pump.
The treatment group showed a median survival time of 11.7 months and an objective response rate (ORR) of 32.5%, significantly higher than the control group (P < 0.05). Serum carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and albumin levels were correlated with treatment effectiveness in advanced GSRC (P < 0.01), but total serum protein was not correlated (P > 0.05). Safety and survival were assessed in all patients. Short-term efficacy was evaluated in 66 patients, with a disease control rate of 89.4% and an ORR of 48.5%. Median progression-free survival was 7.0 months (95% confidence interval [CI]: 6.85-7.15), and median overall survival was 10.6 months (95%CI: 9.86-11.3). Primary grade III/IV adverse events included neutropenia (22.1%) and peripheral neurotoxicity (10.3%).
This treatment regimen is more effective for patients with advanced GSRC. Serum levels of CEA, CA19-9, and albumin predicted chemotherapy efficacy, while total protein concentration correlated minimally and insignificantly.
Core Tip: This study explored the clinical efficacy of fluorouracil combined with paclitaxel and oxaliplatin for the treatment of patients with advanced gastric signet ring cell carcinoma, and analyzed the role of serum carcinoembryonic antigen, carbohydrate antigen 19-9, albumin, and total protein levels in evaluating chemotherapy efficacy.
- Citation: Liu M, Feng B, He N, Yan R, Qin J. Efficacy of fluorouracil combined with paclitaxel and oxaliplatin for the treatment of advanced gastric signet ring cell carcinoma. World J Gastrointest Surg 2025; 17(3): 94286
- URL: https://www.wjgnet.com/1948-9366/full/v17/i3/94286.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i3.94286
Gastric signet ring cell carcinoma (GSRC) is a highly malignant undifferentiated gastric cancer (GC) originating from the epithelial cells of the gastric mucosa. It is characterized by the production of a large amount of mucus by cancer cells, causing the nucleus to be pushed to one side by a large amount of cytoplasmic mucus, so that under the microscope, the cancer cells appear like a ring “seal ring shape,” hence the name[1]. Although the overall incidence of GC has decreased in recent decades, the proportion of GRCC is increasing, reaching 35% to 45%[2]. The histological manifestations of GRCC are early spread, mainly in the mucosal layer or submucosal layer of the stomach. However, once the tumor penetrates the submucosa, its invasion is significantly enhanced, rapidly spreading to the muscle layer of the stomach, the serous layer, and nearby lymph nodes[3]. The progression of population aging has led to a significant increase in the incidence of GC. Clinical symptoms are often nonspecific, and it is typically diagnosed at an advanced stage. GSRC is a distinct subtype of mucinous adenocarcinoma that can present in the early stage of GC causing distant metastasis. GSRC is poorly differentiated, highly invasive, progresses rapidly, and exhibits other high-risk characteristics, making early surgical intervention critical. According to the guidelines of the World Health Organization, GSRC is histologically diagnosed by the presence of “signet ring” cells in > 50% of the tumor cells. The incidence of GSRC increased 10-fold between 1970 and 2000. Current studies suggest that the lymph node metastasis rate in early GSRC is low, and that the prognosis is generally favorable. However, compared with non-SRCC, advanced GSRC shows lower sensitivity to chemotherapy and has a poorer prognosis. There is a lack of appropriate standardized treatment options for advanced GSRC, and the sensitivity of patients to chemotherapy with fluorouracil (5-FU) in combination with paclitaxel and oxaliplatin remains unclear.
Multiple meta-analyses have shown that patients with locally advanced or metastatic GC can benefit from chemotherapy. However, patients and their families often discontinue treatment because due to concerns about adverse effects. This highlights the importance of timely evaluation of a patient’s condition, identifying factors that are likely to affect prognosis, and determining which patients will benefit most from chemotherapy, all of which have significant clinical implications. Multiple studies have shown that blood clearance of biomarkers such as carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), albumin, and total protein have high diagnostic and prognostic value in cancer. However, further research is needed to confirm whether these parameters can be reliably used to monitor and evaluate the effect of chemotherapy[4]. A meta-analysis of randomized clinical trials in patients with advanced-stage oral and GC showed that palliative chemotherapy can not only reduce symptoms but also significantly improves the quality of life compared to optimal supportive care. According to the European Society for Medical Oncology guidelines, 5-FU plus cisplatin is recommended as the reference regimen for advanced GC. In Phase 3 trials, the docetaxel + cisplatin + 5-FU regimen was shown to be superior to the 5-FU + cisplatin regimen, but is not widely used because of its poor tolerance[5]. Paclitaxel has been shown to have comparable efficacy to docetaxel, with fewer adverse effects. As a third-generation platinum drug, oxaliplatin offers better safety and greater efficacy than cisplatin. The combination of 5-FU, paclitaxel, and oxaliplatin (POF regimen) is reportedly as effective as first-line treatment for advanced GC with response rates ranging from 52.1% to 57.1%, median progression-free survival (PFS) of 7.1-9.2 months, and median overall survival (OS) of 11.6-11.7 months[6]. These findings suggest that paclitaxel-based treatment regimens are effective in treating advanced GSRC[7,8]. However, the efficacy and safety of treatments for advanced GSRC have not yet been systematically studied or evaluated.
The primary objective of this study was to comprehensively investigate the complex correlations among serum levels of CEA, CA19-9, albumin, and total protein, and their impact on chemotherapy outcomes in patients with advanced GSRC. Additionally, this study assessed the predictive potential of alterations in these biomarker levels to predict the efficacy of chemotherapy in this patient cohort. A key objective was to evaluate the clinical efficacy and safety profile of the POF regimen as a first-line treatment strategy for advanced GSRC.
Patients admitted at The First Affiliated Hospital of Xi’an Medical College (Xi’an, China) between January 2020 and June 2021, for the treatment of advanced GSRC, were included in the study. All patients met the following inclusion criteria: Confirmed diagnosis of advanced GSRC; ≥ 65 years of age; had not received chemotherapy or radiation therapy in the past month; Karnofsky Performance Status score ≤ 70; had normal routine hematuria, liver, and kidney function test outcomes; normal electrocardiogram; no serious comorbidities of important organs or contraindications to chemotherapy; and expected survival of 3 months or longer. Patients with infection, bleeding, or tissue trauma were excluded. The study protocol was approved by the Hospital Ethics Committee (Grant No. XYYFYLL-KTSB-2024-10), and all patients provided written informed consent prior to chemotherapy. Our research was carried out in accordance with the Convention in accordance with the Declaration of Helsinki.
The random number table method was used to divide the patients into two groups. We used the envelope method for grouping. Specifically, the envelope method means that the randomization scheme is stored in an opaque envelope; the envelopes are opened successively according to the order of enrollment, and the grouping of patients is determined according to the allocation scheme in the envelope. For example, in this study, the results can be divided into 85 envelopes and randomized: The paper grouping control group was put into the envelope marked with number 001, the paper grouping treatment group was put into the envelope marked with number 002, and the paper grouping control group was put into the envelope marked with number 003. The envelope was distributed to the research implementation personnel after the envelope packaging was completed. When the first patient appeared, the inclusion and exclusion criteria were used to determine whether the patient was included in the study. If so, envelope No. 001 was opened. At this time, the study object number of this patient was 001, and the group was the control group. There were 43 patients in the treatment group, including 26 males and 17 females aged 65-78 years, with a median age of 69 years. There were 11 patients with mucous adenocarcinoma with indolescular carcinoma, 23 patients with mucosal adenocarcinoma with indolescular carcinoma, 6 patients with moderate and low-differentiation adenocarcinoma with cytocellular carcinoma, 18 patients with initial treatment, and 27 patients who were retreated. A total of 18 patients underwent surgery and 28 had postoperative recurrence or metastasis, including 12 patients with liver metastasis, 8 patients with metastasis to the supraclavicular lymph nodes, and 27 patients with intraperitoneal lymph node metastasis. There were 42 patients in the control group, including 24 males and 18 females, with an age range of 65-79 years and a median age of 72 years. There were 10 patients with mucous adenocarcinoma with indolescular carcinoma, 18 patients with mucosal adenocarcinoma with indolescular carcinoma, 7 patients with moderate and low-differentiated adenocarcinoma with cytocellular carcinoma, 16 patients with initial treatment, and 16 patients with retreated disease. In total, 24 patients were not treated; 23 had postoperative recurrence or metastasis, 13 had liver metastasis, 10 had supraclavicular lymph node metastasis, and 18 had intraperitoneal lymph node metastasis. A comparison of the general data of the two patient groups revealed that the difference was not statistically significant (P > 0.05).
Patients were examined for objective efficacy evaluation of chemotherapy following every three cycles of treatment. The baseline assessments included clinical history, comprehensive physical examination, Eastern Cooperative Oncology Group (ECOG) score, blood count, liver and kidney function, tumor marker levels, medical comorbidities, chest computed tomography (CT), abdominal and pelvic CT, and magnetic resonance imaging (MRI). Following every three cycles of treatment, patients were examined for objective efficacy evaluation of chemotherapy, for which they were divided into complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD) groups according to RECIST 1.1 evaluation. PFS was defined as the time from the initiation of chemotherapy to disease progression, death, or loss to follow-up for any reason. OS was defined as the time from the initiation of chemotherapy to death or loss to follow-up for any reason[9]. Adverse reactions were evaluated according to Common Terminology Criteria for Adverse Events v3.0 and classified as 0-IV.
Control group: The POF regimen consisted intravenous infusions of 135 mg/m2 paclitaxel for 3 hours, 400 mg/m2 calcium folinic acid (levofalinate calcium 200 mg/m2) for 2 hours, and synchronized oxaliplatin 85 mg/m2 infusion for 2 hours, followed by continuous intravenous infusion of 5-FU (200-2400 mg/m2) for 46 hours (using a portable pump) for 14 days. Efficacy was evaluated after every three cycles of treatment. Pretreatment with glucocorticoids, antihistamines, and H2 receptor antagonists was performed prior to paclitaxel infusion. Prophylactic administration of antiemetics was performed before the infusion of chemotherapeutic drugs[10]. During the treatment, measures were taken to keep the patients warm and to avoid exposure to cold stimuli. Granulocyte colony-stimulating factors were administered as needed, particularly in cases where leukocyte counts dropped below acceptable levels or neutropenia developed. In instances of treatment-related grade III/IV toxicity, the dosage for the subsequent chemotherapy cycle was reduced by 25%. Treatment was continued until one of the following occurred: Disease progression, development of intolerable toxicity, or the patient’s decision to discontinue treatment.
In the treatment group, 5-FU was administered in combination with paclitaxel and oxaliplatin as follows: Oxaliplatin and 5-FU (Jiangsu Hengrui Pharmaceutical Co., Ltd.; 20 mg; Lot number: 16110496) were combined with a taxol injection (Ai Heng, 100 mg, batch number not provided; Jiangsu Hengrui Pharmaceutical Co., Ltd., Jiangsu, China). The dosing regimen included 130 mg/m2, intravenously, on day 1 of each cycle, with each cycle spanning 21 days. Patients in both groups received minimum two cycles of chemotherapy, or 2-4 cycles if the disease did not progress[11].
Prior to chemotherapy, all patients were routinely administered antiemetics, such as tosane sparron, to prevent nausea and vomiting. Granulocyte colony-stimulating factor was administered if peripheral white blood cell count fell below the lower limit of normal post-chemotherapy.
One week before treatment and two cycles post-chemotherapy, the patient was examined via analysis of chest and abdomen CT, bone emission computed tomography, skull MRI, as well as serum CEA, CA19-9, albumin, and total protein levels. CT and other imaging assessments were performed every treatment two cycles. Enhanced CT scans were repeated to evaluate the immediate efficacy of the therapy. Assessments continued until disease progression was observed or the development of intolerable adverse drug reactions (ADRs) necessitated discontinuation of treatment.
The median survival time (MST) was calculated as the survival time based on a cumulative survival rate of 50% from the beginning of chemotherapy[12].
Treatment efficacy was assessed over a 4-week period based on RECIST 1.1 guidelines according to the following evaluation criteria: (1) CR: Complete disappearance of all lesions; (2) PR: Maintenance of at least one PR with an estimated tumor size shrinkage by more than 50% sustained for at least 4 weeks; (3) SD: After at least two treatment cycles (after 6 weeks), no significant change in the lesion size with an estimated tumor shrinkage of less than 50% or enlargement less than 25%; and (4) PD: Emergence of new lesions or tumor size increase exceeding 25%. The ORR included CR and PR, while the disease control rate (DCR) comprised CR, PR, and SD (CR + PR + SD).
The diagnostic reagents used for CEA, CA19-9, albumin, and total protein were purchased from Roche Diagnostic Reagent Company (Basel, Switzerland). Quality control was ensured as follows: CEA and CA19-9 measured using the electrochemiluminescence method, and albumin and total protein measured using the turbidity method. Blood parameters, liver and kidney function, electrolytes, serum markers (CEA, CA19-9, albumin, and total protein) were routinely reviewed before and after chemotherapy.
ADRs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events. Reactions were categorized into five grades, ranging from Grade 0 (no ADR) to Grade 5 (severe ADRs)[13].
The data were processed using Statistical Package for the Social Sciences software (version 26.0). OS and PFS were analyzed using the Kaplan-Meier method. Significance was analyzed using the log-rank test. P < 0.05 was considered statistically significant. The counting data were compared, and survival analysis was performed via Kaplan-Meier analysis, which included multilinear regression analysis of detection indicators and treatment efficacy.
The MST was 11.7 months in the treatment group and 10.0 months in the control group, and the difference was statistically significant (P < 0.05). The 1-year survival rate was 27.0% in the treatment group and 15.0% in the control group. After two chemotherapy cycles, the ORR was 32.5% in the treatment group and 19.0% in the control group, and the difference was statistically significant (P < 0.05). The clinical efficacies of these regimens are shown in Table 1.
| Treatment prescription | Sample | PR | SD | PD | ORR (%) | DCR (%) |
| Control group | 42 | 8 | 20 | 14 | 19 | 66.7 |
| Treatment group | 43 | 14 | 20 | 9 | 32.5 | 79.1 |
Following chemotherapy, the serum CEA and CA19-9 Levels in the PR group were significantly lower than those before chemotherapy, and the serum albumin level was significantly higher (P < 0.05). The blood clearance of patients with PD increased before chemotherapy, and the serum albumin level decreased from that before chemotherapy (P < 0.05). There were no significant changes in serum CEA, CA19-9, albumin, or total protein levels in patients with SD compared with those before treatment (P > 0.05). Multiple linear regression analysis revealed that changes in serum CEA, CA19-9, and albumin concentrations were related to the efficacy of the chemotherapy for preventing cell carcinoma in advanced GSRC (P < 0.01); however, the total serum protein concentration was not significantly correlated with the efficacy of chemotherapy (P > 0.05; Table 2).
| Detect items | Patients with PR (n = 22) | Patients with SD (n = 40) | Patients with PD (n = 23) | P value | |||
| Before chemotherapy | After chemotherapy | Before chemotherapy | After chemotherapy | Before chemotherapy | After chemotherapy | ||
| CEA (ng/mL) | 28.5 ± 27.8 | 22.6 ± 21.6 | 24.8 ± 25.7 | 23.4 ± 22.5 | 32.8 ± 31.8 | 44.6 ± 34.8 | < 0.01 |
| CA19-9 (ng/mL) | 145 ± 117 | 116 ± 91 | 159 ± 127 | 164 ± 136 | 166 ± 115 | 186 ± 145 | < 0.01 |
| Serum albumin (g/L) | 267 ± 155 | 278 ± 193 | 409 ± 267 | 405 ± 260 | 470 ± 258 | 413 ± 256 | < 0.01 |
| Serum total protein (g/L) | 569 ± 385 | 509 ± 314 | 548 ± 256 | 526 ± 215 | 587 ± 258 | 596 ± 274 | > 0.05 |
The most commonly observed ADRs included nausea, vomiting, leukopenia, and peripheral sensory reactions. These were managed with symptomatic treatment, and no chemotherapy-related deaths occurred during the study. The incidence of ADRs in the control group and treatment group was 16.7% and 18.6%, respectively, and this difference was not statistically significant (P > 0.05; Table 3).
| Treatment options | Nausea and vomiting | White blood cell decrease | Diarrhea | Peripheral sensation adverse reactions | Bone marrow suppression |
| Control group, n = 42 | 4 (9.5) | 3 (7.14) | 1 (2.38) | 2 (4.76) | 1 (2.38) |
| Treatment group, n = 43 | 5 (11.62) | 2 (4.65) | 2 (4.65) | 3 (6.97) | 1 (2.32) |
Clinical characteristics of the 85 patients, who received the POF regimen from January 2020 to June 2021, are presented in Table 4. The safety and survival rates were evaluated in 83 patients.
| Characteristic | Value |
| Sex | |
| Women | 50 (58.8) |
| Men | 35 (41.2) |
| Age (year) | |
| Median | 69 |
| Range | 65-78 |
| ECOG | |
| 0 | 48 (56.5) |
| 1 | 28 (32.9) |
| 2 | 9 (10.6) |
| Primary tumor site | |
| Esophagogastric junction | 28 (32.9) |
| Gastric body/fundus | 23 (27.1) |
| Gastric antrum | 19 (22.4) |
| Leather stomach | 15 (17.6) |
| Organs involved | |
| Lymph nodes | 51 (60.0) |
| Liver | 31 (36.4) |
| Lung | 9 (10.6) |
| Peritoneum | 30 (35.3) |
| Other | 10 (11.8) |
| Number of organs involved | |
| 1 | 23 (27.1) |
| 2 | 33 (38.8) |
| > 2 | 29 (34.1) |
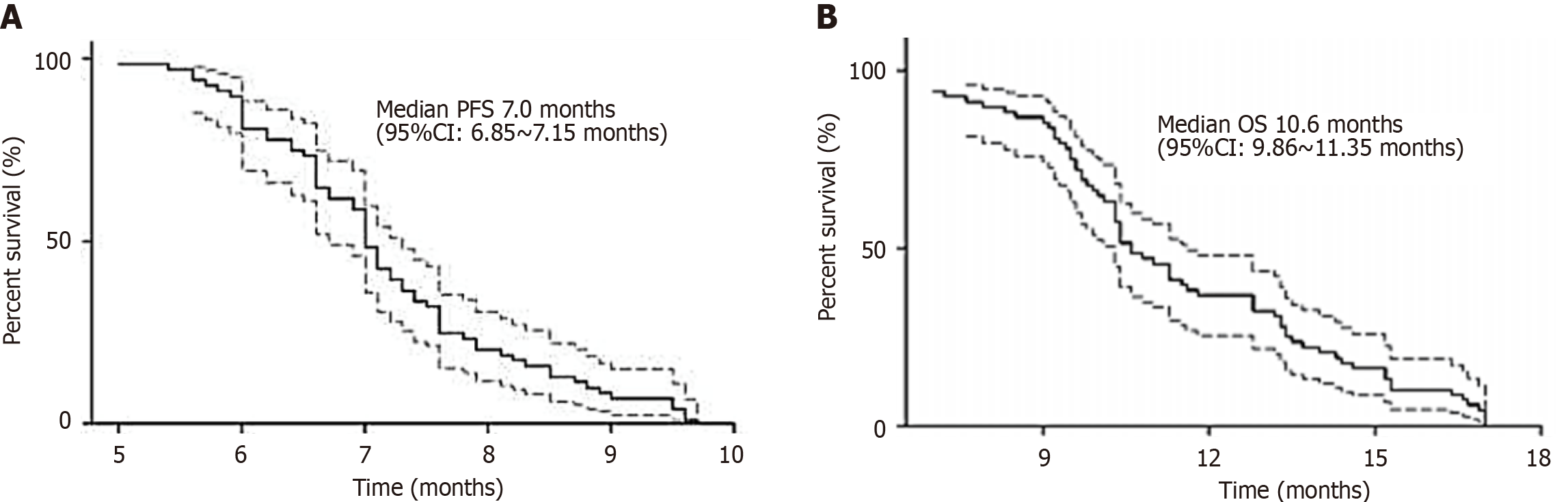
Two patients refused further chemotherapy because of grade IV neutropenia concomitant with infection, and objective efficacy was evaluated in 83 patients. Among them, 11 patients achieved CR (13.3%), 29 achieved PR (34.9%), 34 had SD (41.0%), 9 had PD (10.8%), and 48.1% had objective efficiency with 89.1% DCR (Table 5). The average follow-up time was 13.2 months, the median PFS was 7.0 months, and the median OS was 10.6 months. The PFS and OS curves are shown in Figure 1.
| Parameter | Value |
| CR | 11 (13.3) |
| PR | 29 (34.9) |
| SD | 34 (41.0) |
| PD | 9 (10.8) |
| ORR | 40 (48.1) |
| DCR | 74 (89.1) |
Safety was evaluated in all patients. Common hematological toxicities included grade I/II leukopenia (60.0%), neutropenia (38.8%), anemia (43.5%), grade III/IV neutropenia (22.4%), and total febrile neutropenia (2.9%). Common non-hematological toxicities included peripheral neurotoxicity (62.4%), liver function impairment (55.3%), and diarrhea (28.2%). The incidence rate of grade III/IV peripheral neurotoxicity was 10.6% (Table 6). No treatment-related deaths occurred.
| Toxicity (NCI-CTCAE v 3.0) | Grade I | Grade II | Grade III | Grade IV | Grade I/II (%) | Grade III/IV (%) |
| Leukopenia | 34 | 17 | 14 | 4 | 60.0 | 21.2 |
| Neutropenia | 20 | 13 | 15 | 4 | 38.8 | 22.4 |
| Anemia | 28 | 9 | 6 | 0 | 43.5 | 7.1 |
| Thrombocytopenia | 13 | 5 | 6 | 1 | 21.2 | 8.2 |
| Nausea/vomiting | 14 | 5 | 0 | 0 | 22.4 | 0 |
| Diarrhea | 20 | 4 | 0 | 0 | 28.2 | 0 |
| Oral mucositis | 5 | 3 | 5 | 0 | 9.4 | 5.9 |
| Hepatic injury | 29 | 18 | 4 | 0 | 55.3 | 4.7 |
| Peripheral neurotoxicity | 35 | 18 | 9 | 0 | 62.4 | 10.6 |
| Anaphylaxis | 0 | 0 | 0 | 0 | 0 | 0 |
The efficacy of chemotherapy in patients with SRCC remains questionable. A retrospective study showed lower response rates in SRCC patients compared to those with non-SRCC GC, although 12% of SRCC patients treated with taxane regimens had an improved outlook. In metastatic SRCC, response rates are low, with only 3% of patients responding to taxane-based chemotherapy[14]. Patients with mixed SRCC showed better survival after docetaxel combination chemotherapy. Among various regimens, FLOT (5-FU-leucovorin-oxaliplatin-docetaxel) chemotherapy provided greater benefit with fewer side effects than ECF/ECX (epirubicin + cisplatin + capecitabine) regimens, highlighting the potential of taxanes in SRCC. The TEFOX (triplet chemotherapy, with docetaxel-5FU-oxaliplatin) regimen is an effective first-line treatment for advanced gastric SRCC with tolerable side effects. Paclitaxel and docetaxel are effective taxanes with few side effects[15]. Oxaliplatin, a third-generation platinum-based drug, has shown better efficacy and safety than cisplatin in gastric SRCC. The 2-week POF regimen as first-line therapy was effective, but associated with shorter PFS and OS than outcomes observed in general advanced GC. The poor prognosis of SRCC is due to its aggressive characteristics, which require specific treatment strategies. The POF regimen is safe and effective as first-line treatment for advanced gastric SRCC[16].
Chemotherapy is currently the primary treatment option for advanced GC. Oxaliplatin combined with capecitabine and docetaxel combined with oxaliplatin and tigio (Tegafur, Gemaemine and Otiracil potassium) have become the standard first-line treatment regimens for patients with advanced GC[17]. Oxaliplatin is a stable, water-soluble third-generation platinum compound, and it has demonstrated significant advantages in treating advanced GC. Compared to cisplatin, oxaliplatin exhibits similar antitumor activity, but with significantly lower toxicity to the gastrointestinal tract and kidney, making suitable for patients with kidney disease. Tigio, an oral 5-FU formulation composed of tetrafluoride combined with gemmester and oteracil potassium has shown has a monotherapy efficacy of 26%-49%, comparable to that of continuous intravenous pumping of 5-FU. Furthermore, long-term use of centrally placed artisanal catheters can assist patients in preventing local discomfort, venous thrombosis, catheter-related infections, and the need for frequent care[18]. Presently, the evaluation of chemotherapeutic efficacy relies mainly on comparisons of tumor imaging before and after treatment. The differences in the expression of tumor-related biomarkers and the patient's nutritional status during tumor progression and stabilization can, however, provide additional insights. These parameters enable rapid and accurate evaluation of treatment efficacy, which can help in optimizing therapeutic decisions and avoid delays in patient care.
Plasma albumin, synthesized in the liver, comprises 55%-65% of the total plasma proteins, and has a half-life of 21 days. It helps to maintain osmolality, transporting various plasma substances, and reflects protein nutritional status. In malnutrition, plasma albumin levels drop, triggering various dysfunctions. Serum albumin and transfer factor concentrations are vital parameters in nutritional assessment. A decline in total protein is associated with malnutrition, cachexia, cirrhosis, malignancy, and other chronic wasting diseases[19].
Older patients with advanced GC often experience reduced body function, chronic diseases, and poor tolerance to chemotherapy. Studies indicate that palliative chemotherapy can benefit these patients by improving their symptoms and survival without significantly increasing the incidence of adverse reactions. A study found an ORR of 32.5% and an MST of 11.7 months, similar to younger patients, suggesting effectiveness of chemotherapy[20]. CEA and CA19-9 are crucial markers for monitoring prognosis in GC. CEA, an acidic glycoprotein associated with colon cancer, and CA19-9, a glycolipid tumor antigen, shown to be correlate with survival outcomes. Positivity of these tumor markers predict worse outcomes, and their combined evaluation enhances diagnostic accuracy[21]. Abnormal protein and amino acid metabolism are hallmarks of tumors, with higher rates of histone conversion observed. Post-chemotherapy, CEA and CA19-9 levels were found to decline in responders, whereas albumin levels increased. In contrast, the non-responders showed higher CEA levels and lower albumin levels. CA19-9 and total protein levels remained stable in both groups. CEA, CA19-9, and albumin levels correlate with chemotherapy efficacy, whereas changes in total protein levels do not. Thus, monitoring these markers can predict chemotherapy response, aiding in the diagnosis and prognosis in advanced GSRC[22].
This study suggests that serum CEA, CA19-9, and albumin concentrations can be used to predict the efficacy of chemotherapy in patients with advanced GSRC[23]. There was no statistically significant correlation between total serum protein concentration and the efficacy of chemotherapy. However, due to the limited number of patients in this study, it is necessary to expand the sample size for further in-depth studies.
Our study had some limitations. First, the patient population included in the study was generally in good health, with only 11.8% of patients having an ECOG score of 2, and 26.5% of patients with single-onset distant metastases. Second, this was a retrospective study in which the POF regimen was compared with other regimens (paclitaxel with oxaliplatin and cisplatin with 5-FU regimen) as the first-line treatment of advanced gastric SRCC. However, the safety and efficacy of these regimens require further validation through prospective studies.
In conclusion, our study demonstrated that the chemotherapy regimen was effective in prolonging survival and improving the prognosis of patients with advanced GSRC. Changes in serum CEA, CA19-9, and albumin concentrations were significantly correlated with the efficacy of chemotherapy. Although ADRs were observed, they were manageable, and no chemotherapy-related deaths occurred. These findings provide valuable insights into the treatment of advanced GSRC and suggest that the chemotherapy regimen may be a promising option for these patients. In recent years, the application of immunotherapy for treatment of GC has emerged as significant area of research. Notably, 40.4% of the patients with SRCC demonstrated positivity for Programmed cell death ligand 1 (PD-L1), highlighting the potential of anti-programmed cell death protein 1 (PD-1)/PD-L1 therapy as a promising approach. First-line treatment with the POF regimen is safe and effective for patients with advanced GSRC. However, the potential of combining the POF regimen with anti-human epidermal growth factor receptor 2-targeted therapy and/or anti-PD-1/PD-L1 immunotherapy in patients with advanced GSRC deserves further exploration.
| 1. | PDQ Supportive and Palliative Care Editorial Board. Cancer Pain (PDQ®): Health Professional Version. 2024 Jul 25. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US), 2002. [PubMed] |
| 2. | PDQ Supportive and Palliative Care Editorial Board. Cancer Pain (PDQ®): Patient Version. 2024 Sep 17. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US), 2002. [PubMed] |
| 3. | Chalabi M. Stomach cancer gets a triple punch of therapy. Nature. 2021;600:608-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 4. | Gotoda T, Ono H. Stomach: Endoscopic resection for early gastric cancer. Dig Endosc. 2022;34 Suppl 2:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Hsu FK, Chang WK, Lin KJ, Liu CY, Fang WL, Chang KY. The Associations between Perioperative Blood Transfusion and Long-Term Outcomes after Stomach Cancer Surgery. Cancers (Basel). 2021;13:5438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Ji H, Wu H, Du Y, Xiao L, Zhang Y, Zhang Q, Wang X, Wang W. Development and External Validation of a Nomogram for Predicting Overall Survival in Stomach Cancer: A Population-Based Study. J Healthc Eng. 2021;2021:8605869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Yu JM, Zhan ZW, Zhen JX, Wang XJ, Chen Y, Lin J, Chen L, Chen LZ, Huang YF, Guo ZQ. Clinical Characteristics and Prognostic Analysis of Patients With Signet Ring Cell Gastric Carcinoma. Technol Cancer Res Treat. 2020;19:1533033820983812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Kayapinar AK, Solakoglu D, Bas K, Oymaci E, Isbilen B, Calik B, Diniz G, Akbulut G. Relationship of prognostic factors in stomach cancer with helicobacter pylori: a retrospective study. Acta Gastroenterol Belg. 2021;84:607-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Kumar NAN, Jose A, Usman N, Rajan K, Munisamy M, Shetty PS, Rao M. Signet ring cell cancer of stomach and gastro-esophageal junction: molecular alterations, stage-stratified treatment approaches, and future challenges. Langenbecks Arch Surg. 2022;407:87-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Lee MK. Decisional balance, self-leadership, self-efficacy, planning, and stages of change in adopting exercise behaviors in patients with stomach cancer: A cross-sectional study. Eur J Oncol Nurs. 2022;56:102086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Li J, Chen W, Cao Y, Li ZR. The Identification of Alternative Polyadenylation in Stomach Adenocarcinomas Using the Genotype-Tissue Expression Project and the Cancer Genome Atlas- Stomach Adenocarcinoma Profiles. Int J Gen Med. 2021;14:6035-6045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Martimianaki G, Bertuccio P, Alicandro G, Pelucchi C, Bravi F, Carioli G, Bonzi R, Rabkin CS, Liao LM, Sinha R, Johnson K, Hu J, Palli D, Ferraroni M, Lunet N, Morais S, Tsugane S, Hidaka A, Hamada GS, López-Carrillo L, Hernández-Ramírez RU, Zaridze D, Maximovitch D, Aragonés N, Martin V, Ward MH, Vioque J, Garcia de la Hera M, Zhang ZF, Kurtz RC, Lagiou P, Lagiou A, Trichopoulou A, Karakatsani A, Malekzadeh R, Camargo MC, Curado MP, Boccia S, Boffetta P, Negri E, La Vecchia C. Coffee consumption and gastric cancer: a pooled analysis from the Stomach cancer Pooling Project consortium. Eur J Cancer Prev. 2022;31:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Moradian F, Fararouei M, Karami M, Ghelichi-Ghojogh M, Gheibi Z, Nikeghbalian Z, Akbari A, Akbari ME. Trend of geographical distribution of stomach cancer in Iran from 2004 to 2014. BMC Gastroenterol. 2022;22:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Ri M, Kumagai K, Namikawa K, Atsumi S, Hayami M, Makuuchi R, Ida S, Ohashi M, Sano T, Nunobe S. Is proximal gastrectomy indicated for locally advanced cancer in the upper third of the stomach? Ann Gastroenterol Surg. 2021;5:767-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Schneider L, Kröger A, Gubler C, The FO. Diagnosis of Gastric Cancer in the Excluded Stomach After RYGB by Jejunogastrostomy Using a LAMS. ACG Case Rep J. 2022;9:e00720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Suzuki Y, Nomura K, Kikuchi D, Hoteya S. An effective method for removing surgical staples during endoscopic submucosal dissection for early gastric cancer on the suture line of remnant stomach. VideoGIE. 2021;6:495-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Tamura T, Wakai K, Lin Y, Tamakoshi A, Utada M, Ozasa K, Sugawara Y, Tsuji I, Ono A, Sawada N, Tsugane S, Ito H, Nagata C, Kitamura T, Naito M, Tanaka K, Shimazu T, Mizoue T, Matsuo K, Inoue M; Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Alcohol intake and stomach cancer risk in Japan: A pooled analysis of six cohort studies. Cancer Sci. 2022;113:261-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Tran HH, Sengngam K, Pham PV, Le NT. Case-Control Study of Alcohol Usage and Fruit Intake and Stomach Cancer in the North Viet Nam. Asian Pac J Cancer Prev. 2021;22:2903-2908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 19. | Mengardo V, Treppiedi E, Bencivenga M, Dal Cero M, Giacopuzzi S. Tailored treatment for signet ring cell gastric cancer. Updates Surg. 2018;70:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Tuo JY, Bi JH, Yuan HY, Jiang YF, Ji XW, Li HL, Xiang YB. Trends of stomach cancer survival: A systematic review of survival rates from population-based cancer registration. J Dig Dis. 2022;23:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Yang D, Piao Y, Yuan F, Chen H, Zhang D, Li X. Gastric side effects and the stomach dosimetric analysis in left-sided breast cancer radiotherapy in free-breathing and deep inspiration breath-hold technique. Radiat Oncol. 2022;17:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Zhang Y, Zhang PS, Rong ZY, Huang C. One stomach, two subtypes of carcinoma-the differences between distal and proximal gastric cancer. Gastroenterol Rep (Oxf). 2021;9:489-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Shen Y, Li Y, Wang Z, Xu W, Wang W, Chen X. The prognostic value of FAR and a novel FAR-CA125 score in resectable gastric signet ring cell carcinoma patients. J Cancer Res Clin Oncol. 2023;149:9597-9608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |