Published online Mar 27, 2025. doi: 10.4240/wjgs.v17.i3.101786
Revised: December 13, 2024
Accepted: January 7, 2025
Published online: March 27, 2025
Processing time: 109 Days and 2.2 Hours
In-depth comparative investigations in terms of clinical efficacies of liver tumor microwave ablation (MWA) and laparoscopic hepatectomy (LH), which are both important treatment modalities for liver neoplasms, have been limited in patients diagnosed with primary small liver cancer (PSLC).
To compare and analyze the clinical efficacy of liver tumor MWA and LH for PSLC.
This study retrospectively analyzed the medical records of 123 patients with PSLC admitted to Xuzhou Central Hospital from January 2015 to November 2022 and categorized them based on treatment modalities into the LH and MWA groups. The LH group, consisting of 61 cases, received LH, and the MWA group, which included 62 cases, underwent liver tumor MWA. Basic data and various perioperative indicators were compared between the two groups, including changes in liver function indicators [alanine aminotransferase (ALT), glutamic aminotransferase (AST), and total bilirubin (TBIL)] pre- and post-treatment, and efficacy and postoperative complications were analyzed.
No statistically significant difference was observed between the two groups in terms of age, gender, tumor diameter, liver function Child-Pugh classification and number of tumors, body mass index, and educational status (P > 0.05). The overall effective rate was higher in the MWA group than in the LH group (98.39% vs 88.52%) (χ2 = 4.918, P = 0.027). The MWA group exhibited less operation time, intraoperative bleeding, defecation time, and hospital stay than the LH group (P < 0.05). No difference was found in liver function indicators between the two groups pre-treatment (P > 0.05), and ALT, AST, and TBIL levels decreased in both groups post-treatment, with the MWA group demonstrating lower levels (P < 0.05). The MWA and LH groups exhibited postoperative complication rates of 4.84% and 19.67%, respectively, with statistically significant differences between the two groups (P = 0.012, χ2 = 6.318).
MWA is more effective in treating PSLC, and it promotes faster postoperative recovery for patients, and more security improves liver function and reduces postoperative complications compared to LH.
Core Tip: This study primarily aimed to comparatively analyze the clinical effectiveness of liver tumor microwave ablation (MWA) and laparoscopic hepatectomy (LH) in treating primary small liver cancer (PSLC). We conducted a comparative analysis of the two intervention methods from multiple perspectives, including various perioperative indicators, changes in liver function indicators pre- and post-treatment, curative effects, and postoperative complications. This study confirmed that MWA demonstrated better curative effects than LH in PSLC treatment, with a reduced intraoperative blood loss level, shorter surgical procedure and hospitalization durations, rapid recovery facilitation, liver function improvement, postoperative complication reduction, and a high safety level. Therefore, selecting the appropriate surgical method is the key to achieving better clinical outcomes. Our analysis provides more reliable clinical references and options for future PSLC treatment.
- Citation: Li HS, Zhang XF, Fu J, Yuan B. Efficacy of microwave ablation vs laparoscopic hepatectomy for primary small liver cancer: A comparative study. World J Gastrointest Surg 2025; 17(3): 101786
- URL: https://www.wjgnet.com/1948-9366/full/v17/i3/101786.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i3.101786
Primary small liver cancer (PSLC), one of the most prevalent malignant tumors globally[1,2], is a digestive system malignancy characterized by high morbidity and mortality rates. According to statistics, over 90% of primary liver cancers were hepatocellular carcinomas[3], and the rest were cholangiocellular and mixed-type hepatocellular car
The etiology and pathogenesis of PSLC are currently undetermined[9]. Academic research indicated that early detection and treatment effectively save the lives of patients with PSLC[10-12]. One study[13] revealed laparoscopic hepatectomy (LH) to be less damaging to patients’ liver function, demonstrates higher liver function reserve capacity, is less invasive, causes less intraoperative bleeding, and exhibits a lower incidence of postoperative complications than open hepatectomy. The range of inactivated tissues is expanding with the continuous improvement of ablation techniques, thereby increasing definitive treatment results[14,15]. MWA is the use of physically generated high heat to coagulate and inactivate tumor tissue in the body[16,17], prompting the body to decompose, remove, or mechanize necrotic tissue, thereby destroying the lesion for eradication. MWA is less traumatic for patients and exhibits the characteristics of simple operation, quick recovery, safety, and efficiency[18]. Furthermore, it does not require tumor removal, which indicates the presence of a variety of minimally invasive methods available and a wealth of treatment opportunities and options for patients with liver cancer[19]. Moreover, it cures patients who cannot undergo surgical treatment. MWA, LH, and other surgical modalities were gaining clinical attention as one of the important treatment methods for liver tumors. A wide range of medical practitioners acknowledged its advantages and disadvantages.
However, studies comparing MWA with LH were scarce. This study aims to investigate the clinical efficacy of MWA and LH in PSLC treatment.
This study retrospectively analyzes the medical records of 123 patients with PSLC admitted to Xuzhou Central Hospital from January 2015 to November 2022, categorized based on treatment modality into the LH (61 cases) and MWA groups (62 cases).
Hepatocellular carcinoma confirmed by liver puncture histopathology or postoperative pathology; a single cancer foci of ≤ 3 cm in diameter; liver function Child-Pugh grades A or B[20].
Age of > 18 years and ≤ 69 years; normal preoperative coagulation and other indicators; no extrahepatic transfer or other surgical, chemotherapeutic, or anti-tumor treatments.
Patients with surgical contraindications; incomplete case information; cardiac, pulmonary, renal, and other vital organ insufficiency; pregnant and lactating women. The basic information of the two groups of patients.
Lost to follow-up post-discharge; automatically discharged from hospital during treatment; serious complications occurring during hospitalization (malignant arrhythmia, myocardial infarction, cardiac arrest, severe decline in muscle strength, etc.); rejecting the experimenter midway.
Those who were actively hospitalized and refused to be followed up by telephone, or those who were unreachable during the follow-up period due to force majeure factors.
The LH group received LH, with the operation as follows. The patient is positioned supine. A 1-cm sub-umbilical incision was created after general anesthesia, and pneumoperitoneum was established by puncture of pneumoperitoneum needle. A 1-cm Trocar was placed via this puncture, and a laparoscopic lens was placed to explore for abdominal fluid, tumor site, size, and number, cases with a liver texture, etc., that was compatible with surgical resection. The position of the other operating trocar was identified based on the specific tumor location. The corresponding lesion area of the liver was resected with the help of an ultrasonic knife and bipolar electrocoagulation, and the resection line was identified at 2 cm from the surface of the tumor with an electric knife. The resection line was gradually cut through the liver tissue from superficial to deep and from anterior to posterior for complete resection of the liver tumor. The large vessels and corresponding bile ducts were clamped closed with a titanium clip during excision, and hemostasis was achieved through electrocoagulation. The tumor was placed in a specimen bag, and the specimen was removed from the bag after dilatation through the sub-umbilical observation hole.
The MWA group underwent liver tumor MWA as follows. The preceding steps were performed in the supine position, under general anesthesia, with pneumoperitoneum establishment and laparoscopic lens exploration, as previously in the LH group. A thermal isolation zone was established and the MWA needle was placed in the center of the tumor under the lumpectomy after accurate exploration of the tumor location under the guidance of the lumpectomy ultrasound probe. The appropriate radiofrequency voltage and time were then set and the ablation treatment was initiated after determination. The ablation protocol was selected based on the tumor size. A one- or two-point MWA was utilized for lesions of ≤ 2 cm in diameter, and a multi-point MWA was employed for lesions of > 2 cm in diameter. The ablation output was 60 W, and each ablation cycle lasted 5 minutes. The coagulation mode was changed and the needle tract was cauterized to prevent bleeding from the needle tract and to end the procedure if the ultrasound showed complete tumor ablation post-treatment. Ablation treatment was continued if tumor ablation was incomplete, and the tumor exhibited hyperechoic changes on ultrasound post-procedure, with inactivated tissue beyond the preoperative tumor margins. The procedure was completed with the choice of whether to perform a hilar block or hemihepatic blood flow block, based on tumor location in both groups.
Efficacy criteria[21] were identified based on the patient’s peritoneal fluid into complete remission (CR), which is the complete disappearance of ascites maintained for more than one month; partial remission (PR), with over 50% reduction in ascites; stabilizing disease (SD), with no significant change in ascites and less than 50% reduction from pre-treatment; progressive disease, with a > 25% increase in ascites from pre-treatment. Total effective rate = (CR + PR + SD) / total number of cases × 100%. The extraction time, intraoperative bleeding, defecation, and length of hospital stay were recorded for both groups. Liver function indexes pre- and post-treatment were taken from an automatic biochemical analyzer, consisting of alanine aminotransferase (ALT), glutamic aminotransferase (AST), and total bilirubin (TBIL). Postoperative complications were documented in both groups, including nausea and vomiting, lung infection, incisional infection, and abdominal bleeding. All patients were followed up from December 1, 2016, to March 30, 2023.
The measurement data (including age and tumor diameter, body mass index, operation time, intraoperative bleeding, defecation time, length of hospital stay, and liver function indicators) were presented as mean ± SD using the t-test. The count data (gender, educational status, liver function Child-Pugh classification, number of tumors, efficacy, and postoperative complications) were expressed as rates and percentages (%) using χ2 tests. The test level was α = 0.05. Statistical Package for the Social Sciences version 23.0 was used for analysis.
No statistically significant difference was found between the two groups in terms of age, gender, tumor diameter, liver function Child-Pugh classification and the number of tumors, body mass index, and educational status (P > 0.05) (Table 1).
| Group | LH group (n = 61) | MWA group (n = 62) | t/χ2 | P value |
| Age (years) | 65.27 ± 16.08 | 66.18 ± 16.24 | 0.312 | 0.755 |
| Gender (M/F) | 34/27 | 33/29 | 0.078 | 0.780 |
| Tumour diameter (cm) | 4.53 ± 1.46 | 4.48 ± 1.43 | 0.192 | 0.848 |
| Liver function Child-Pugh classification (A/B) | 42/19 | 44/18 | 0.065 | 0.798 |
| Number of tumours (single/multiple) | 47/14 | 46/16 | 0.136 | 0.712 |
| Body mass index (kg/m2) | 23.16 ± 4.75 | 23.23 ± 4.84 | 0.081 | 0.936 |
| Education status (junior high school and below/high school and above) | 39/22 | 37/25 | 0.236 | 0.627 |
The total effective rate after treatment was 98.39% in the MWA group, which was significantly higher than in the LH group at 88.52%, with a statistically significant difference (P < 0.05) (Table 2).
| Group | Number of examples | CR | PR | SD | PD | Total efficiency |
| LH group | 61 | 24 (39.34) | 17 (27.87) | 13 (21.31) | 7 (11.48) | 54 (88.52) |
| MWA group | 62 | 39 (62.90) | 16 (25.81) | 6 (9.68) | 1 (1.61) | 61 (98.39) |
| χ2 | 4.918 | |||||
| P value | 0.027 |
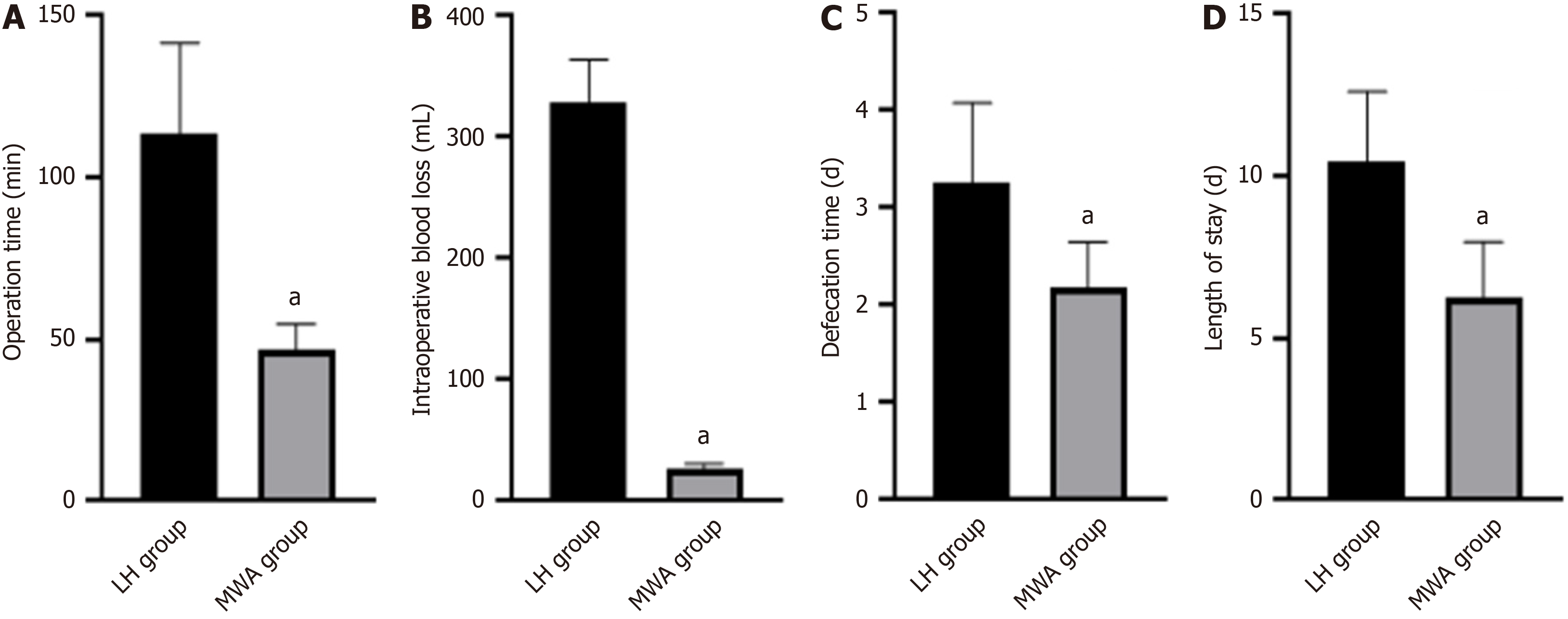
The operative time, intraoperative bleeding, defecation time, and hospital stay were less in the MWA group than in the LH group (P < 0.05) (Figure 1).
No difference was observed in the comparison of liver function indexes between the two groups pre-treatment (P > 0.05), and ALT, AST, and TBIL levels decreased in both groups post-treatment, which were lower in the MWA group than in the LH group (P < 0.05) (Figure 2).
The incidence of postoperative complications in the MWA group compared to the LH group (4.84% vs 19.67%) was statistically significant (P = 0.012, χ2 = 6.318), tolerable, and promptly managed without affecting the normal treatment course and symptom relief (Table 3).
| Group | Number of examples | Post-operative complications | Total incidence | |||
| Nausea and vomiting | Lung infections | Infection of the incision | Abdominal bleeding | |||
| LH group | 61 | 6 (9.84) | 3 (4.92) | 2 (3.28) | 1 (1.64) | 12 (19.67) |
| MWA group | 62 | 2 (3.23) | 1 (1.61) | 0 (0.00) | 0 (0.00) | 3 (4.84) |
| χ2 | 6.318 | |||||
| P value | 0.012 | |||||
Research reveals that[22] laparoscopic MWA combined with hepatic artery chemoembolization is a safe and effective treatment option for early-stage hepatocellular carcinoma, with good recent results, prolonged survival, and low incidence of serious adverse events. This study revealed that MWA demonstrated a higher overall efficiency than LH (98.39% vs 88.52%), indicating that MWA is more effective than laparoscopic liver resection for patients with PSLC. This may be attributed to the application of an ablation needle in the MWA procedure. The ablation needle is accurately inserted into the tumor through the liver tissue under ultrasonography guidance. Subsequently, the electrode needle of the ablation device is used to generate heat within the tumor tissue utilizing high-frequency alternating current, thereby achieving the ablation effect. Tumor tissues demonstrate relatively poor heat tolerance. Coagulative inactivation is induced within the tumor tissue and the adjacent parenchymal tissue once the local temperature exceeds 50°C. Irreversible impairment of tissue cells occurs when the temperature surpasses 60°C. Moreover, the increased temperature generated by the tissue can disrupt the blood vessels that supply the tumor and the surrounding tissues. Tumor tissue necrosis becomes more comprehensive as a consequence of nutrient deprivation, which consequently diminishes the probability of intrahepatic metastasis of the tumor[23,24]. Operative time, intraoperative bleeding, defecation, and hospital stay were less in the MWA group than in the LH group, indicating that MWA promotes early recovery and is more minimally invasive for patients with small liver cancers. Liver function indicators reflect liver damage in a timelier manner. Notably, ALT, AST, and TBIL hold considerable significance. High ALT, AST, and TBIL levels are closely associated with liver damage in the body. Effective treatment interventions decrease the levels of abnormally increased liver function indices[25]. ALT, AST, and TBIL levels were lower in the treated group than in the LH group post-treatment because MWA causes coagulation and necrosis of the tumor and local liver tissue, resulting in transient impairment of liver function, decrease in ALT, AST, and TBIL levels post-treatment, and a gradual return to normal liver function due to complete absorption of necrotic tissue[26]. MWA is more convenient, less damaging to liver function, and can be repeated multiple times for single hepatocellular carcinoma of ≤ 5 cm in diameter[27]. The incidences of post
In conclusion, compared with LH, MWA of PSLC is more effective, with low intraoperative bleeding, shorter operation and hospital stay, rapid recovery, improved liver function, reduced postoperative complications, and high safety; thus, selecting the appropriate procedure is the key to achieving better clinical results.
| 1. | Lee TK, Guan XY, Ma S. Cancer stem cells in hepatocellular carcinoma - from origin to clinical implications. Nat Rev Gastroenterol Hepatol. 2022;19:26-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 289] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 2. | Zhang Z, Zeng X, Wu Y, Liu Y, Zhang X, Song Z. Cuproptosis-Related Risk Score Predicts Prognosis and Characterizes the Tumor Microenvironment in Hepatocellular Carcinoma. Front Immunol. 2022;13:925618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 162] [Article Influence: 54.0] [Reference Citation Analysis (1)] |
| 3. | Donne R, Lujambio A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology. 2023;77:1773-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 323] [Article Influence: 161.5] [Reference Citation Analysis (0)] |
| 4. | De Muzio F, Cutolo C, Dell'Aversana F, Grassi F, Ravo L, Ferrante M, Danti G, Flammia F, Simonetti I, Palumbo P, Bruno F, Pierpaoli L, Fusco R, Giovagnoni A, Miele V, Barile A, Granata V. Complications after Thermal Ablation of Hepatocellular Carcinoma and Liver Metastases: Imaging Findings. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Roudi R, D'Angelo A, Sirico M, Sobhani N. Immunotherapeutic treatments in hepatocellular carcinoma; achievements, challenges and future prospects. Int Immunopharmacol. 2021;101:108322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Polychronidis G, Feng J, Murtha-Lemekhova A, Heger U, Mehrabi A, Hoffmann K. Factors Influencing Overall Survival for Patients with Fibrolamellar Hepatocellular Carcinoma: Analysis of the Surveillance, Epidemiology, and End Results Database. Int J Gen Med. 2022;15:393-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Zhang X, Wang Z, Tang W, Wang X, Liu R, Bao H, Chen X, Wei Y, Wu S, Bao H, Wu X, Shao Y, Fan J, Zhou J. Ultrasensitive and affordable assay for early detection of primary liver cancer using plasma cell-free DNA fragmentomics. Hepatology. 2022;76:317-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 8. | Zhou J, Sun H, Wang Z, Cong W, Zeng M, Zhou W, Bie P, Liu L, Wen T, Kuang M, Han G, Yan Z, Wang M, Liu R, Lu L, Ren Z, Zeng Z, Liang P, Liang C, Chen M, Yan F, Wang W, Hou J, Ji Y, Yun J, Bai X, Cai D, Chen W, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Guo Y, Hua B, Huang X, Jia W, Li Q, Li T, Li X, Li Y, Li Y, Liang J, Ling C, Liu T, Liu X, Lu S, Lv G, Mao Y, Meng Z, Peng T, Ren W, Shi H, Shi G, Shi M, Song T, Tao K, Wang J, Wang K, Wang L, Wang W, Wang X, Wang Z, Xiang B, Xing B, Xu J, Yang J, Yang J, Yang Y, Yang Y, Ye S, Yin Z, Zeng Y, Zhang B, Zhang B, Zhang L, Zhang S, Zhang T, Zhang Y, Zhao M, Zhao Y, Zheng H, Zhou L, Zhu J, Zhu K, Liu R, Shi Y, Xiao Y, Zhang L, Yang C, Wu Z, Dai Z, Chen M, Cai J, Wang W, Cai X, Li Q, Shen F, Qin S, Teng G, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2022 Edition). Liver Cancer. 2023;12:405-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 192] [Reference Citation Analysis (0)] |
| 9. | Hytiroglou P, Bioulac-Sage P, Theise ND, Sempoux C. Etiology, Pathogenesis, Diagnosis, and Practical Implications of Hepatocellular Neoplasms. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 10. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1103] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 11. | Xue T, Yam JWP. Role of Small Extracellular Vesicles in Liver Diseases: Pathogenesis, Diagnosis, and Treatment. J Clin Transl Hepatol. 2022;10:1176-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Papaconstantinou D, Tsilimigras DI, Pawlik TM. Recurrent Hepatocellular Carcinoma: Patterns, Detection, Staging and Treatment. J Hepatocell Carcinoma. 2022;9:947-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 13. | Aboudou T, Li M, Zhang Z, Wang Z, Li Y, Feng L, Chu X, Chen N, Zhou W, Yang K. Laparoscopic versus Robotic Hepatectomy: A Systematic Review and Meta-Analysis. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 14. | Yeow M, Zhao JJ, Fong KY, Wong J, Tan AYH, Kam JH, Nikfarjam M, Goh BKP, Kabir T. Radiofrequency Ablation Versus Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. World J Surg. 2022;46:2778-2787. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Mermekli A, Reddy P, McKean D, Abdelsalam H, Teh J, Mansour R. Ultrasound-guided continuous radiofrequency ablation of the suprascapular nerve for chronic shoulder pain secondary to osteoarthritis: a retrospective cohort study. Eur Radiol. 2022;32:6230-6237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Abreu de Carvalho LF, Logghe B, Van Cleven S, Vanlander A, Moura Ribeiro S, Geboes K, Lecluyse C, Smeets P, Degroote H, Van Vlierberghe H, Berrevoet F. Local control of hepatocellular carcinoma and colorectal liver metastases after surgical microwave ablation without concomitant hepatectomy. Langenbecks Arch Surg. 2021;406:2749-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Ryu T, Takami Y, Wada Y, Saitsu H. Operative Microwave Ablation for Hepatocellular Carcinoma Within 3 cm and 3 Nodules: Experience in 559 Patients. J Gastrointest Surg. 2022;26:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Wang K, Wang C, Jiang H, Zhang Y, Lin W, Mo J, Jin C. Combination of Ablation and Immunotherapy for Hepatocellular Carcinoma: Where We Are and Where to Go. Front Immunol. 2021;12:792781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 19. | Pfannenstiel A, Iannuccilli J, Cornelis FH, Dupuy DE, Beard WL, Prakash P. Shaping the future of microwave tumor ablation: a new direction in precision and control of device performance. Int J Hyperthermia. 2022;39:664-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Huang Z, Zhang G, Liu J, Huang M, Zhong L, Shu J. LRFNet: A deep learning model for the assessment of liver reserve function based on Child-Pugh score and CT image. Comput Methods Programs Biomed. 2022;223:106993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Ozen M, Birmingham E, Raissi D. Re: Liver tumor ablation in difficult locations: Microwave ablation of perivascular and subdiaphragmatic hepatocellular carcinoma. Clin Imaging. 2022;85:7. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Liu J, Zhang W, Lu H, Li H, Zhou X, Li J, Han X. Drug-eluting bead trans-arterial chemoembolization combined with microwave ablation therapy vs. microwave ablation alone for early stage hepatocellular carcinoma: a preliminary investigation of clinical value. J Cancer Res Clin Oncol. 2022;148:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Lee SK, Chung DJ, Cho SH. A Real-World Comparative Study of Microwave and Radiofrequency Ablation in Treatment-Naïve and Recurrent Hepatocellular Carcinoma. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Wang CZ, Yan GX, Dong DS, Xin H, Liu ZY. LncRNA-ATB promotes autophagy by activating Yes-associated protein and inducing autophagy-related protein 5 expression in hepatocellular carcinoma. World J Gastroenterol. 2019;25:5310-5322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Shi R, Liu Z, Liu T. The antagonistic effect of bisphenol A and nonylphenol on liver and kidney injury in rats. Immunopharmacol Immunotoxicol. 2021;43:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Chong CCN, Lee KF, Chu CM, Chan AWH, Wong J, Chan SL, Lok HT, Fung AKY, Fong AKW, Cheung YS, Yu SCH, Johnson P, Lai PBS. Microwave ablation provides better survival than liver resection for hepatocellular carcinoma in patients with borderline liver function: application of ALBI score to patient selection. HPB (Oxford). 2018;20:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Wang Z, Liu M, Zhang DZ, Wu SS, Hong ZX, He GB, Yang H, Xiang BD, Li X, Jiang TA, Li K, Tang Z, Huang F, Lu M, Chen JA, Lin YC, Lu X, Wu YQ, Zhang XW, Zhang YF, Cheng C, Ye HL, Wang LT, Zhong HG, Zhong JH, Wang L, Chen M, Liang FF, Chen Y, Xu YS, Yu XL, Cheng ZG, Liu FY, Han ZY, Tang WZ, Yu J, Liang P. Microwave ablation versus laparoscopic resection as first-line therapy for solitary 3-5-cm HCC. Hepatology. 2022;76:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |