Published online Feb 27, 2025. doi: 10.4240/wjgs.v17.i2.97862
Revised: October 25, 2024
Accepted: December 3, 2024
Published online: February 27, 2025
Processing time: 225 Days and 0.5 Hours
The magnamosis, a minimal invasive, suture-free procedure, has been used for digestive tract or vessel reconstruction, such as gastrointestinal anastomosis, bilioenteric anastomosis, and coronary artery bypass. Although some case reports have demonstrated the potential of magnamosis for the treatment of congenital rectal atresia (RA), they cannot provide strong evidence for its widespread application.
To assess the feasibility and safety of magnamosis in treating RA in dogs as compared to suturing anastomosis. The findings of this study can be beneficial in guiding the clinical application of magnamosis.
Thirty-six dogs were randomly assigned to the magnamosis group (n =18) and the suturing anastomosis group (n =18). The rectum was freed laparoscopically in all dogs. In the magnamosis group, rectal anastomosis was performed using a pair of magnetic rings, while the suturing anastomosis group underwent a straight-sighted end-to-end rectal anastomosis with 4-0 absorbable sutures. The anastomosis time was recorded, and abdominal plain film examination was performed to locate the magnets until they were expelled postoperatively. Specimens of the anastomosis were evaluated at one month, three months, and six months after surgery.
The mean time for rectal anastomosis was significantly shorter in the magnamosis group (12.22 ± 2.78 minutes) than the suturing anastomosis group (18.11 ± 1.68 minutes). There was one incidence of anastomotic bleeding in the suturing anastomosis group, whereas no complication was recorded in the magnamosis group. The magnets were discharged post-surgery in 7.17 ± 1.30 days in all the dogs. The histopathological examination revealed a smoother healing of anastomotic mucosa in the magnamosis group as compared to that in the suturing anastomosis group. Moreover, the fiber alignment was also more natural in the magnamosis group with minimal inflammation.
Rectal reconstruction using magnamosis is a feasible, safe, and effective alternative to suturing anastomosis in dogs, with the added benefit of faster and more natural healing of the anastomosis.
Core Tip: Magnamosis is a very new and effective method for treating rectal atresia in clinical, it can effectively avoid the extensive iatrogenic injury of pelvic floor muscle and nerve plexus caused by Pena operation. This is a very promising clinical innovation technology and method. At present, although there are a few clinical applications of magnetic rectal anastomosis papers, but the basic experiments of magnetic rectal anastomosis animals are blank. This study shows that rectal reconstruction using magnamosis is a feasible, safe, and effective alternative to suturing anastomosis in dogs, with the added benefit of faster and more natural healing of the anastomosis.
- Citation: Liu SQ, Zhang HK, Lv Y, Xu XH, Li YF, Quan DW. Magnamosis for rectal reconstruction in canines. World J Gastrointest Surg 2025; 17(2): 97862
- URL: https://www.wjgnet.com/1948-9366/full/v17/i2/97862.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i2.97862
Anorectal malformations include a wide range of defects, of which rectal atresia (RA) and rectal stenosis (RS) account for only 1%-2%[1,2]. Although rare, RA or RS typically require surgical intervention due to the patients' inability to pass meconium. Various surgical techniques have been utilized for RA or RS, including techniques that have been gradually phased out over time. These include the sacral-coccygeal perineal approach, sacral approach, and transanal or transcolostomy instrumentation for RA, as well as the Heineke-Mikulicz type of repair for RS[1]. Advancements in technology have led to the development of increasingly innovative techniques for managing RA or RS. Examples of such techniques include posterior sagittal anorectoplasty, abdominoperineal pull-through, laparoscopic-assisted anorectoplasty[3], and magnetic compression anastomosis (magnamosis or magnamosis)[4,5]. In a recent systematic review, de Beaufort et al[6] attempted to determine an optimal surgical approach for managing RA or RS. In their study, the posterior sagittal anorectoplasty and abdominoperineal pull-through were the most frequently used techniques amongst the patients; however, 57 out of 70 patients experienced postoperative complications. As a result, they concluded that no definitive recommendations regarding the best surgical management for RA or RS could be made.
Magnamosis is a promising technique used to restore digestive continuity by compressing tissue between two magnets using magnetic force. The technique was first reported by Obora et al[7] in 1978. They used magnetic rings for nonsuture microvascular anastomosis in animals. Jansen et al[8] popularized the use of magnetic compression colon resection in humans without postoperative complications and described the procedure in five patients in 1980. Since then, the use of magnamosis has grown dramatically, especially in gastrointestinal and bilioenteric anastomosis, as well as coronary artery bypass procedures[9-13]. The technique is preferred for its simplicity, ease of use, low technical requirements, and reliable outcomes. Additionally, studies have used magnamosis to treat congenital disorders and functional diseases such as oesophageal atresia[14,15], funnel chest[16,17], and gastroesophageal reflux disease[18-20]. Zaritzky et al[14] have successfully applied magnamosis in five patients with oesophageal atresia from 2001 to 2004, with an average anastomosis time of 4.8 days.
Our research team has an extensive background in magnetic surgical techniques. Last year, our research team reported the successful treatment of esophageal atresia in dogs using magnamosis with favorable results[21]. Similar to esophageal atresia, RA or RS is a complex congenital malformation that necessitates multiple surgical treatments. Nonetheless, traditional surgical techniques tend to be invasive, complex, and frequently result in postoperative complications. Physicians are focused on developing a minimally invasive procedure that can benefit patients. The magnetic surgical technique has been demonstrated to satisfy the requirement for minimally invasive procedures on many clinical occasions[11,12,20,22-24]. In 2014, Russell et al[4] reported a case wherein magnamosis was used to treat a patient with RA. However, due to the lack of cases, this report does not sufficiently verify the effectiveness or safety of magnamosis in treating RA. In this study, we conducted a study that aims to evaluate the feasibility, safety and efficacy of RA treatment using magnamosis compared to traditional surgical methods in dogs.
This study was approved by the Ethics Committee for Animal Experiments of Xi’an Jiaotong University Health Science Center (No. 2021-302). In this study, thirty-six dogs weighing 15-20 kg were provided by the Laboratory Animal Center of Xi’an Jiaotong University. The dogs were subjected to humane care and were acclimated for a week.
The animals were randomly divided into the magnamosis group (n = 18) and the suturing anastomosis group (n = 18). All animals underwent a 24-hours fast for food and a 6-hours fast for drinking, followed by general anesthesia with 3% sodium pentobarbital (1 mL/kg, intraperitoneal injection).
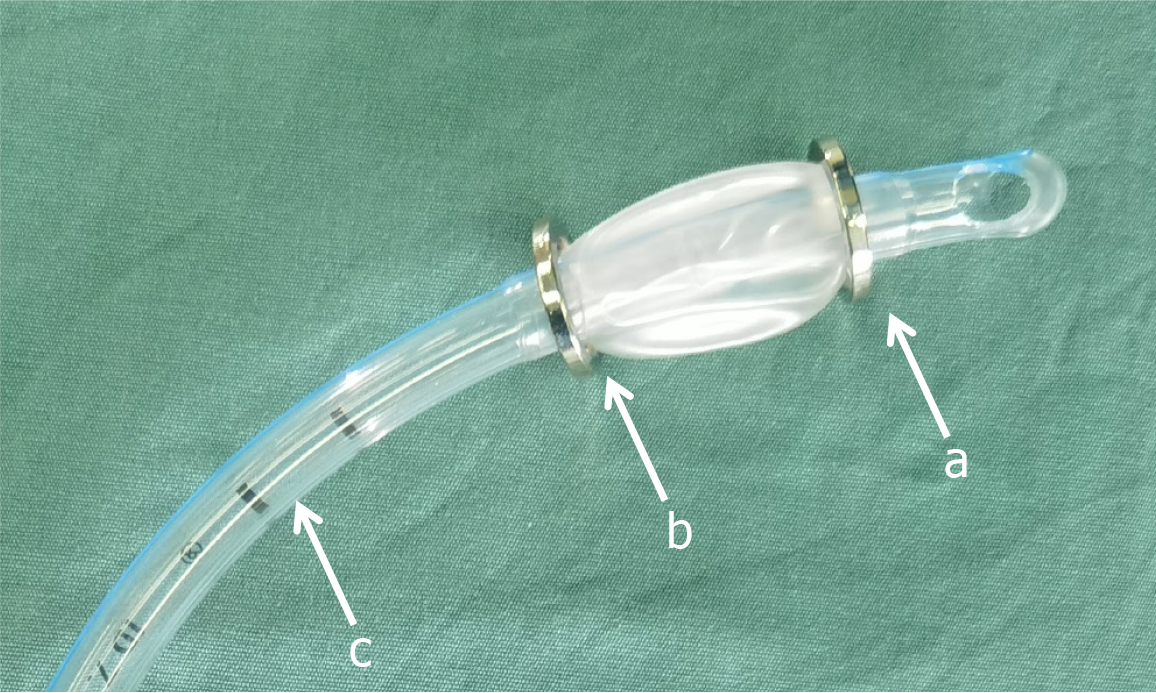
The magnetic device was composed of two magnetic rings, namely the cephalad ring and caudal ring, as well as one tracheal tube (Figure 1). These magnetic rings were made of permanent material known as Nd-Fe-B of grade N45. The magnetic rings had outer, inner diameters as well as height dimensions measuring 20 mm, 16 mm, and 2 mm, respectively. To prevent the magnetic rings from corrosion, they were all coated with a layer of nickel. The tracheal tube played the role of facilitating anastomosis via supporting and guiding the magnets during the surgery. The outer diameter and the inner diameter of the tracheal tube measured 9.6 mm and 7.0 mm, correspondingly.
The dogs were anesthetized with an intraperitoneal injection of 3% sodium pentobarbital at a dose of 1 mL/kg. After being anesthetized, they were placed in the supine position and the surgical site was prepared by shaving and sterilizing the abdominal region. The operating room was maintained at a temperature of 21 °C-25 °C. The surgical procedures were predominantly performed laparoscopically, with pneumoperitoneum pressure being maintained at 8-12 mmHg using CO2. Incisions were made at the umbilicus, the anti-McBurney’s point and the lower left abdomen. Perirectal dissection was initiated at the peritoneal reflection, with the goal of bringing the endpoint as close to the anus as possible, typically within a distance of less than 5 cm.
In the magnamosis group, two magnetic rings, namely cephalad and caudal rings, were passed through using the tracheal tube. The balloon was inflated with air to fix the cephalad ring at the distal end. Next, the cephalad ring was advanced along the anal canal. Once the cephalad ring passed the dissected rectal end, rectal ligation was carried out with a silk suture to mimic RA or RS. Subsequently, the caudal ring was pushed along the tube, causing the two magnetic rings to conjoin automatically via magnetic attraction. This process resulted in the attainment of magnamosis, after which the balloon was deflated, and the tracheal tube was extracted. A few days later, the enclosed tissue between the magnetic rings would gradually undergo necrosis, while the bonded magnets would detach and exit the body.
In the suturing anastomosis group, the anterior and posterior limits of the dissected rectal segment were marked with sutures. Subsequently, rectal transection was performed. An incision in the lower left abdomen was made, which was extended to perform a straight-sighted end-to-end rectal anastomosis using 4-0 absorbable sutures.
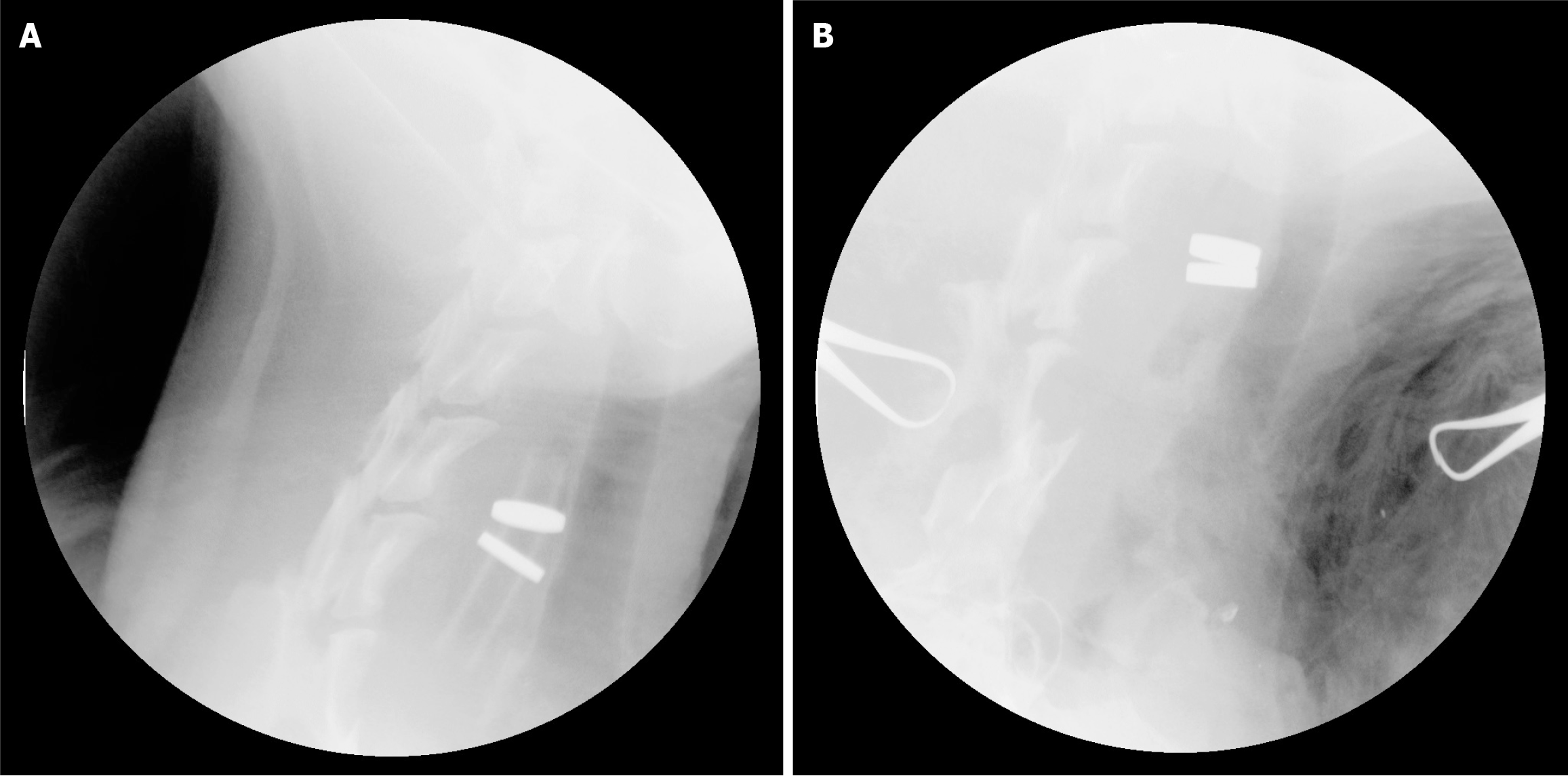
On the first postoperative day, an intramuscular injection of pethidine was administered for analgesia. The dogs underwent a 3-days fast with intravenous nutrition, and on the fourth day, they were given a liquid diet. Intravenous sodium benzylic penicillin (100000 IU/kg/day) was administered for 3 days after the surgery. An abdominal plain film examination was conducted daily (Figure 2) to determine the location of the magnets until expulsion.
Pre-operatively and 7 days post-operatively, blood samples were collected to assess the white blood cell (WBC) count and total bilirubin (TBIL) level. At 1 month, 3 months, and 6 months postoperatively, six dogs were randomly selected and euthanized with an overdose of anesthesia. Anastomotic tissues were collected for gross inspection and then fixed in 10% formalin for pathological examination using HE staining, Masson staining, and transmission electron microscopy scanning.
The data were analyzed using GraphPad Prism v7 software (GraphPad, La Jolla, CA, United States). Quantitative data are expressed as mean ± SD and analyzed using an independent samples t-test. Statistical significance was set at P < 0.05.
Anastomosis time in the magnamosis group was defined as the period between the placement of the magnetic rings and removal of the tracheal tube. In contrast, the anastomosis time in the suturing anastomosis group was measured from the beginning of the procedure until completion. The magnamosis group did not experience any perioperative complications such as anastomotic leakage, bleeding, or obstruction. Conversely, there was an occurrence of anastomosis bleeding in the suturing anastomosis group, which was treated with conservative therapy. Rectal anastomosis time averaged 12.22 ± 2.78 minutes and 18.11 ± 1.68 minutes in the magnamosis and suturing anastomosis groups. There was significant difference
| Magnamosis group | Suturing anastomosis group | P value | |
| Anastomosis time (minute) | 12.22 ± 2.78 | 18.11 ± 1.68 | < 0.01 |
| WBC count pre-op (× 109/L) | 13.60 ± 2.27 | 12.97 ± 1.96 | 0.19 |
| WBC count post-op (× 109/L) | 13.83 ± 2.36 | 14.56 ± 2.83 | 0.41 |
| TBIL pre-op (umol/L) | 18.98 ± 3.15 | 18.63 ± 2.97 | 0.73 |
| TBIL post-op (umol/L) | 19.20 ± 3.35 | 19.35 ± 3.56 | 0.89 |
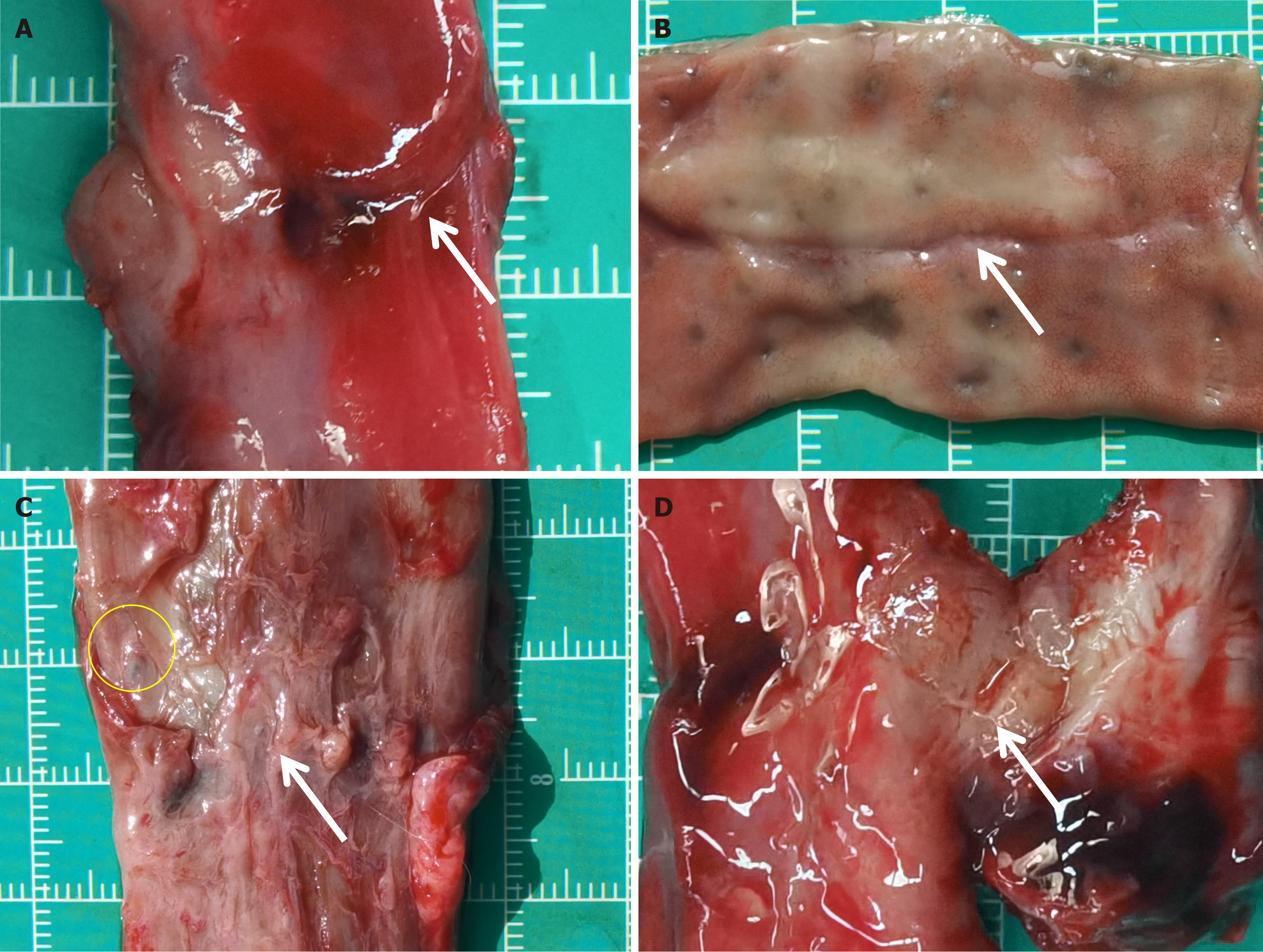
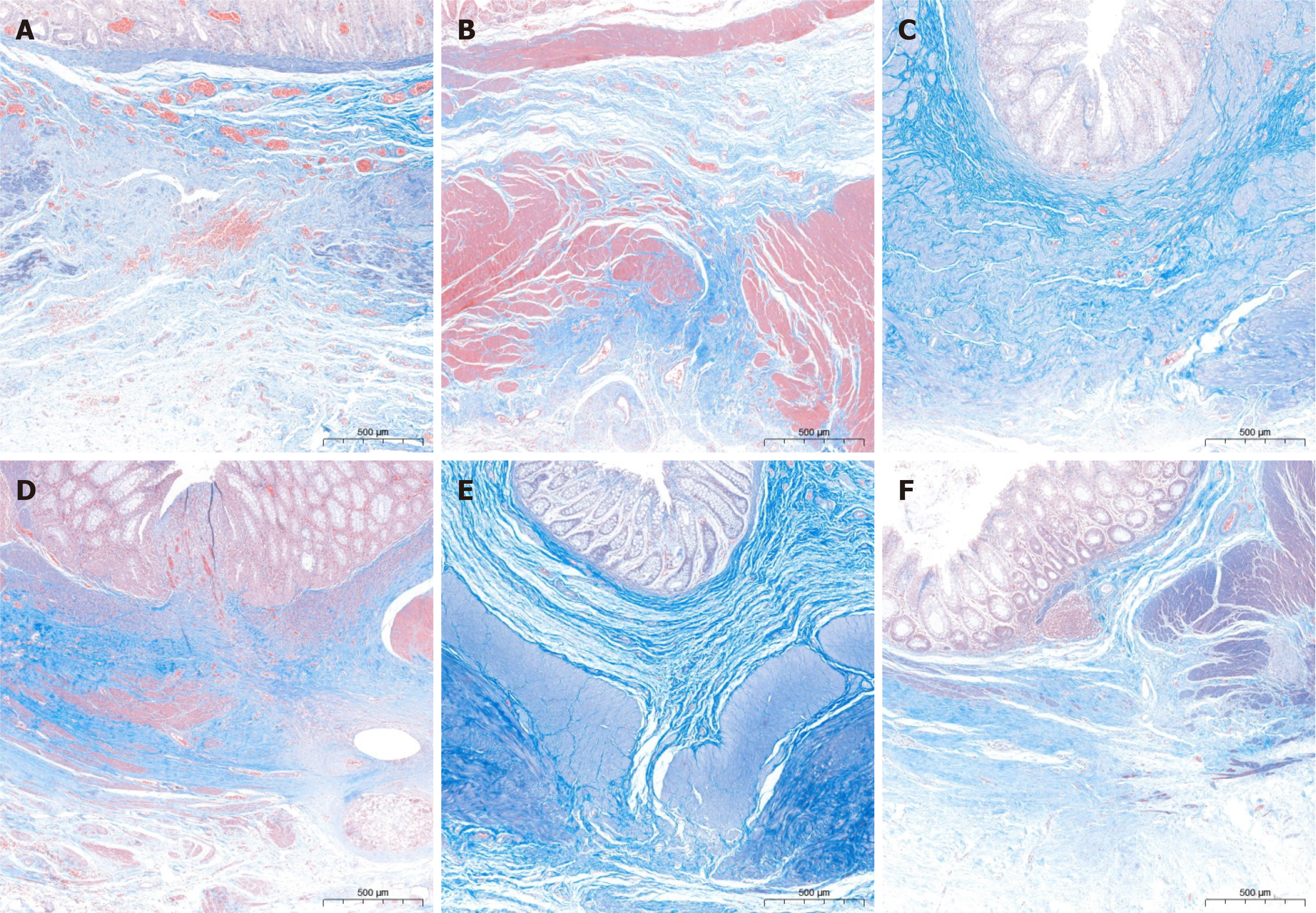
The rectal anastomosis achieved effective healing in both groups with a continuous and intact mucosal layer, and there was no evidence of ulcers or fistulas detected. However, one month post-procedure, the thickness of the anastomotic tissue was lower than the normal rectal tissue, as shown in Figure 3. Although the mucosa had properly covered the anastomosis, the muscular layer was still inadequately and discontinuously covered (Figure 3B and D). In one instance of the magnamosis group, the mucosa was so smooth that the naked eye failed to distinguish the anastomosis after a month following the procedure (Figure 3A). Unlike the magnamosis group, most of the sutures in the suturing anastomosis group were absorbed one month after the procedure, except for a few residuals (Figure 3C). Over time, the mucosa gradually covered the submucosa and muscle layer, resulting in a smoother and flatter anastomosis in both groups. Six months after the procedure, there was no significant visual difference between the groups, and it was difficult to visually identify the anastomosis in both groups.
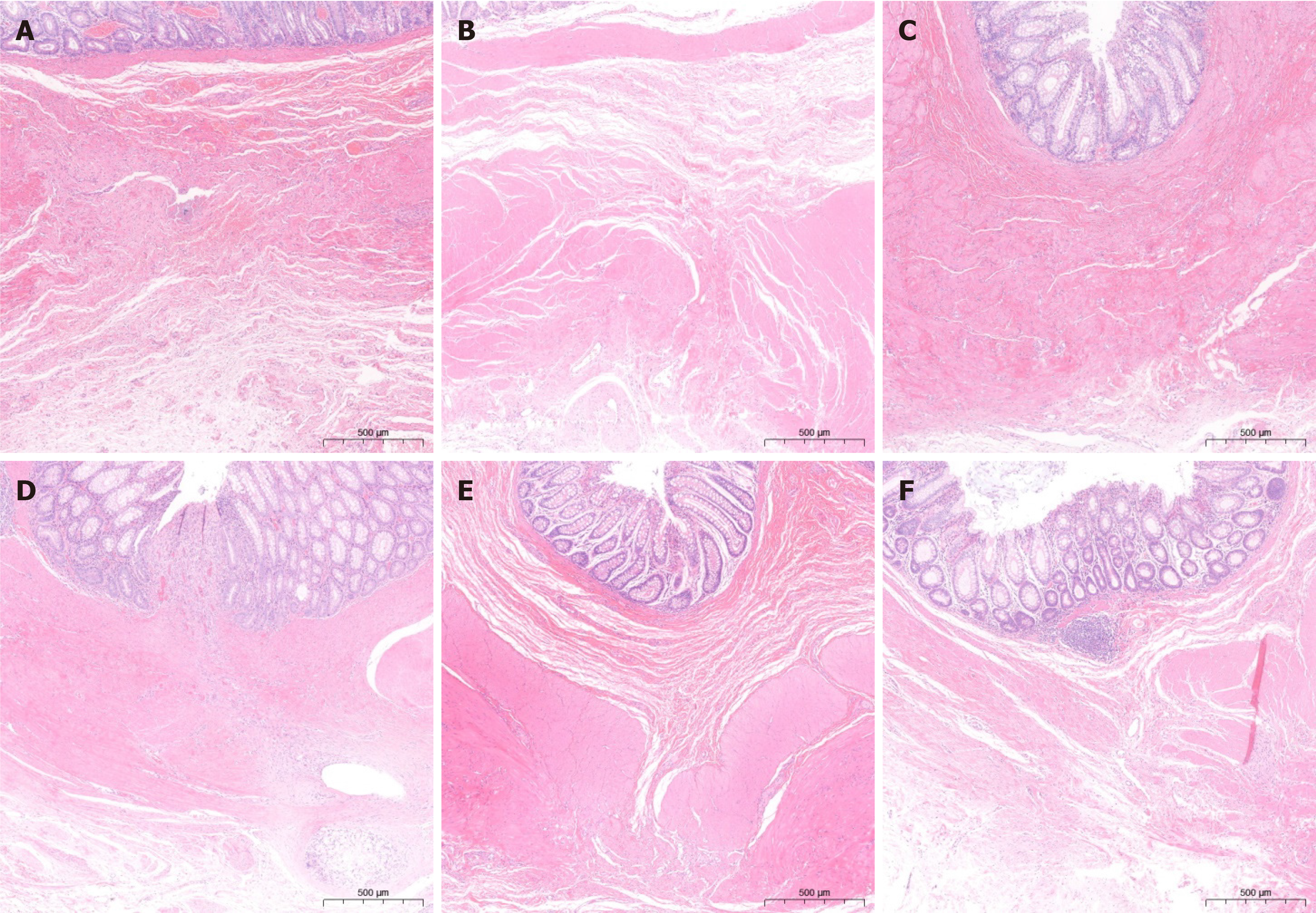
The microscopic observation revealed that the anastomosis recovered satisfactorily in both groups at 1 month postoperatively, despite poor submucosal and basal layer continuity, as shown in Figure 4A and B. In contrast, the magnamosis group exhibited a more precise fiber alignment and a lesser inflammatory reaction (Figure 5A and B). Conversely, the anastomosis alignment in the control group was disorganized and less smooth. Three months post-operatively, the inflammatory response of the anastomosis significantly diminished in both groups, and healing of the anastomosis noticeably enhanced (Figure 4C and D). However, the suturing anastomosis group had more inflammatory responses than the magnamosis group, and the tissue alignment was less accurate (Figure 5C and D). At 6 months postoperatively, there were no significant differences in inflammatory responses between the two groups, except for a slightly worse fiber alignment in the suturing anastomosis group, as seen in Figure 4E and F, and Figure 5E and F. Lastly, the anastomosis specimens of the magnamosis group underwent a transmission electron microscopic examination at 3 and 6 months postoperatively, revealing no inflammatory cell infiltration (Figure 6).
The advancement of detection and surgical methods has significantly increased the surgical success rate and post-operative survival of patients with congenital anorectal malformations. Nowadays, enhancing postoperative quality of life is a priority for surgeons. Postoperative complications, such as defecation function, fistula recurrence, anorectal stenosis or obstruction, rectal mucosal prolapse, and fecal incontinence, are significant factors that affect life quality. For "low" anorectal malformations, one-stage surgery without a protective colostomy is the standard treatment, whereas for "high" anorectal malformations, a descending colostomy is performed in the newborn period, followed by the repair of the malformation and closure of the colostomy. The complex procedures associated with these complications present a great challenge to the experience and skill of surgeons. Therefore, there is an urgent need to develop a simplified and safe surgical procedure. In 2014, Russell et al[4] reported the successful use of magnamosis in treating RA without any postoperative complications. Similarly, Lu et al[25] reported the case of rectal anastomosis atresia recanalization using magnamosis in an old man who underwent radical resection for rectal carcinoma in 2021. While these sporadic case reports demonstrate the minimal invasiveness and easy method for rectal reconstruction using magnamosis, they do not provide strong evidence for the further utilization of magnamosis in treating congenital rectal malformation.
In this study, we investigated the feasibility and safety of magnamosis in RA. Firstly, we compared the anastomotic time required for laparoscopic rectal reconstruction using the magnamosis and the traditional suturing anastomosis method. The results demonstrated that the magnamosis was easier to operate and resulted in significantly shorter anastomotic time than the suturing anastomosis. It is well established that a large number of surgical accumulations are needed for laparoscopic gastrointestinal anastomosis with sutures to achieve a satisfactory result[26,27]. As a result, the learning curve and operator experience requirements are extremely high. Nevertheless, the magnamosis can significantly reduce the learning curve and surgical skill requirements. This has been verified in other studies[28]. In actual clinical applications, in addition to the laparoscopic magnamosis discussed in this article, we have also successfully performed two surgeries using colostomy to insert magnamosis, both achieving favorable outcomes. Therefore, we have reason to believe that magnamosis can produce positive anastomotic results and simplify surgical procedures. Additionally, colonoscopy can help optimize the field of view during the insertion of magnamosis. Regarding the translation of the devices presented in this article into clinical applications, we believe that it can be achieved by inserting magnamosis into the intestine via colostomy and laparoscopy. For patients with rectal cancer, especially those undergoing anal-sparing surgery after resection of mid- and high-position cancers, we believe magnamosis can achieve satisfactory anastomotic results. We plan to carry out relevant basic research in the future.
Secondly, this study examined and compared the pathological changes of anastomoses at different time points. At one month after the operation, both groups showed inadequate recovery of anastomotic tissue. There was inadequate recovery of the muscular layer. The mucosal layer of the magnamosis group remained smoother and more intact, and the fiber arrangement of submucosal and muscular layers was better in the magnamosis group than in the suturing anastomosis group. In addition, the suturing anastomosis group displayed obvious inflammatory infiltration, and absorbable sutures could be observed.
Remarkably, in a case from the magnamosis group at one-month post-operation, the anastomosis was not visible to the naked eye. This suggests that the anastomosis in the magnamosis group had a faster initial recovery compared to the anastomosis in the suturing group. Subsequently, at three months post-operation, the anastomoses in both groups had a good recovery, with a continuous submucosa and muscle layer. However, in the suturing anastomosis group, the fiber alignment was inferior and had more infiltration of inflammatory cells.
At postoperative 6 months, both groups of specimens were largely similar, although there were slight differences in fibrous arrangement. In addition, the results of the transmission electron microscope were consistent with HE staining findings. This suggests that employing magnamosis to reconstruct the rectum can achieve faster and more natural anastomotic healing. This study provides compelling evidence that the magnamosis is superior to the suturing technique based on several reasons. Firstly, in the magnamosis group, the magnets compress anastomotic tissue in a circular pattern, causing necrosis and the shedding of compressed tissue. The anastomotic tissue does not directly expose to digestive fluid during the entire healing process. Conversely, in the suturing anastomosis group, digestive fluid directly contacts the anastomosis and penetrates into tissue through the pinhole caused by the suture, thereby significantly aggravating inflammatory reactions and increasing the risk of complications such as anastomotic infection, anastomotic leakage, and anastomotic bleeding compared to the magnamosis. Secondly, the direct pressure of magnets on the rectal anastomosis enables accurate alignment of mucosal, submucosal, and muscular layers, bringing the anastomosis closer to normal rectal structure. However, in the suturing anastomosis group, alignment of anastomotic tissue is difficult, and there is also fiber rearrangement during the healing process, thereby delaying recovery.
The present study has certain limitations which must be acknowledged. First and foremost, it needs to be mentioned that the animal model of RA utilized in this study merely approximates types 1 and 2 of congenital RA as opposed to precisely mimicking the clinical condition. As a result, this study lays emphasis on the safety, effectiveness, and simplicity of the magnamosis when it comes to performing rectal anastomosis. It is imperative to conduct studies in the future to understand better the viability of magnamosis for the treatment of other subtypes of congenital anorectal malformations. Secondly, the small sample size and the short follow-up period of this study serve as a constraint upon the extent of the results. Nonetheless, the overall results of the research are promising and warrant further investigation.
Magnamosis provides a safe, effective, and feasible alternative to the conventional suturing technique for rectal reconstruction in dogs. In addition to faster and more natural healing of the anastomosis compared to the suturing technique, magnamosis has shown promise as a treatment option for congenital rectal malformation. Therefore, additional studies on magnamosis-based rectal reconstruction are warranted.
| 1. | Sharma S, Gupta DK. Varied facets of rectal atresia and rectal stenosis. Pediatr Surg Int. 2017;33:829-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Lane VA, Wood RJ, Reck C, Skerritt C, Levitt MA. Rectal atresia and anal stenosis: the difference in the operative technique for these two distinct congenital anorectal malformations. Tech Coloproctol. 2016;20:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Han Y, Xia Z, Guo S, Yu X, Li Z. Laparoscopically Assisted Anorectal Pull-Through versus Posterior Sagittal Anorectoplasty for High and Intermediate Anorectal Malformations: A Systematic Review and Meta-Analysis. PLoS One. 2017;12:e0170421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Russell KW, Rollins MD, Feola GP, Scaife ER. Magnamosis: a novel technique for the management of rectal atresia. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Wall J, Diana M, Leroy J, Deruijter V, Gonzales KD, Lindner V, Harrison M, Marescaux J. MAGNAMOSIS IV: magnetic compression anastomosis for minimally invasive colorectal surgery. Endoscopy. 2013;45:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | de Beaufort CMC, Derikx JPM, de Jong JR, Burchell GL, Bosscha SRJ, de Beer SA, van Heurn LWE, Gorter RR. Outcomes after Surgical Treatment for Rectal Atresia in Children: Is There a Preferred Approach? A Systematic Review. Eur J Pediatr Surg. 2023;33:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Obora Y, Tamaki N, Matsumoto S. Nonsuture microvascular anastomosis using magnet rings: preliminary report. Surg Neurol. 1978;9:117-120. [PubMed] |
| 8. | Jansen A, Keeman JN, Davies GA, Klopper PJ. Early experiences with magnetic rings in resection of the distal colon. Neth J Surg. 1980;32:20-27. [PubMed] |
| 9. | Jansen A, Brummelkamp WH, Davies GA, Klopper PJ, Keeman JN. Clinical applications of magnetic rings in colorectal anastomosis. Surg Gynecol Obstet. 1981;153:537-545. [PubMed] |
| 10. | Chopita N, Vaillaverde A, Cope C, Bernedo A, Martinez H, Landoni N, Jmelnitzky A, Burgos H. Endoscopic gastroenteric anastomosis using magnets. Endoscopy. 2005;37:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | van Hooft JE, Vleggaar FP, Le Moine O, Bizzotto A, Voermans RP, Costamagna G, Devière J, Siersema PD, Fockens P. Endoscopic magnetic gastroenteric anastomosis for palliation of malignant gastric outlet obstruction: a prospective multicenter study. Gastrointest Endosc. 2010;72:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Takao S, Matsuo Y, Shinchi H, Nakajima S, Aikou T, Iseji T, Yamanouchi E. Magnetic compression anastomosis for benign obstruction of the common bile duct. Endoscopy. 2001;33:988-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Mimuro A, Tsuchida A, Yamanouchi E, Itoi T, Ozawa T, Ikeda T, Nakamura R, Koyanagi Y, Nakamura K. A novel technique of magnetic compression anastomosis for severe biliary stenosis. Gastrointest Endosc. 2003;58:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Zaritzky M, Ben R, Zylberg GI, Yampolsky B. Magnetic compression anastomosis as a nonsurgical treatment for esophageal atresia. Pediatr Radiol. 2009;39:945-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Zaritzky M, Ben R, Johnston K. Magnetic gastrointestinal anastomosis in pediatric patients. J Pediatr Surg. 2014;49:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Harrison MR, Curran PF, Jamshidi R, Christensen D, Bratton BJ, Fechter R, Hirose S. Magnetic mini-mover procedure for pectus excavatum II: initial findings of a Food and Drug Administration-sponsored trial. J Pediatr Surg. 2010;45:185-91; discussion 191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Harrison MR, Gonzales KD, Bratton BJ, Christensen D, Curran PF, Fechter R, Hirose S. Magnetic mini-mover procedure for pectus excavatum III: safety and efficacy in a Food and Drug Administration-sponsored clinical trial. J Pediatr Surg. 2012;47:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Ganz RA, Gostout CJ, Grudem J, Swanson W, Berg T, DeMeester TR. Use of a magnetic sphincter for the treatment of GERD: a feasibility study. Gastrointest Endosc. 2008;67:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Bonavina L, DeMeester T, Fockens P, Dunn D, Saino G, Bona D, Lipham J, Bemelman W, Ganz RA. Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: one- and 2-year results of a feasibility trial. Ann Surg. 2010;252:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Bonavina L, DeMeester TR, Ganz RA. LINX(™) Reflux Management System: magnetic sphincter augmentation in the treatment of gastroesophageal reflux disease. Expert Rev Gastroenterol Hepatol. 2012;6:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Xu XH, Lv Y, Liu SQ, Cui XH, Suo RY. Esophageal magnetic compression anastomosis in dogs. World J Gastroenterol. 2022;28:5313-5323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Itoi T, Yamanouchi E, Ikeuchi N, Kasuya K, Iwamoto H, Tsuchida A. Magnetic Compression Duct-to-duct Anastomosis for Biliary Obstruction in a Patient with Living Donor Liver Transplantation. Gut Liver. 2010;4 Suppl 1:S96-S98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Liu XM, Li Y, Zhang HK, Ma F, Wang B, Wu R, Zhang XF, Lv Y. Laparoscopic Magnetic Compression Biliojejunostomy: A Preliminary Clinical Study. J Surg Res. 2019;236:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Liu XM, Yan XP, Zhang HK, Ma F, Guo YG, Fan C, Wang SP, Shi AH, Wang B, Wang HH, Li JH, Zhang XG, Wu R, Zhang XF, Lv Y. Magnetic Anastomosis for Biliojejunostomy: First Prospective Clinical Trial. World J Surg. 2018;42:4039-4045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Lu G, Li J, Ren M, Ma F, Sun X, Lv Y, He S. Endoscopy-assisted magnetic compression anastomosis for rectal anastomotic atresia. Endoscopy. 2021;53:E437-E439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Wang M, Meng L, Cai Y, Li Y, Wang X, Zhang Z, Peng B. Learning Curve for Laparoscopic Pancreaticoduodenectomy: a CUSUM Analysis. J Gastrointest Surg. 2016;20:924-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 27. | Fong ZV, Chang DC, Ferrone CR, Lillemoe KD, Fernandez Del Castillo C. Early National Experience with Laparoscopic Pancreaticoduodenectomy for Ductal Adenocarcinoma: Is This Really a Short Learning Curve? J Am Coll Surg. 2016;222:209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Jamshidi R, Stephenson JT, Clay JG, Pichakron KO, Harrison MR. Magnamosis: magnetic compression anastomosis with comparison to suture and staple techniques. J Pediatr Surg. 2009;44:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |