Published online Feb 27, 2025. doi: 10.4240/wjgs.v17.i2.94270
Revised: November 17, 2024
Accepted: December 5, 2024
Published online: February 27, 2025
Processing time: 313 Days and 20.1 Hours
Magnetic compression anastomosis (MCA) offers a simple and reliable technique for inducing anastomoses at any point along the digestive tract. Evidence regar
To investigate any difference in the side-to-side colonic anastomosis effect achie
We designed cylindrical and circular ring magnets suitable for side-to-side colonic anastomosis in rats. Thirty Sprague-Dawley rats were randomly divided into a cylindrical group, circular ring group, and cylindrical–circular ring group (n = 10/group). Side-to-side colonic anastomosis was completed by transanal insertion of the magnets without incision of the colon. Operation time, perioperative complications, and magnet discharge time were recorded. Rats were euthanized 4 weeks post-operatively, and anastomotic specimens were obtained. The burst pressure and anastomotic diameter were mea
In all 30 rats, side-to-side colonic anastomosis was completed, for an operation success rate of 100%. No postope
This study found no significant difference in the establishment of rat side-to-side colonic anastomosis with the use of cylindrical vs circular ring magnets.
Core Tip: Magnetic compression anastomosis (MCA) is a new anastomosis approach that differs from suture and staple anastomosis. Multiple studies have demonstrated the feasibility of MCA in the digestive tract, but whether MCA establishment is affected by the shape of the magnets has not been determined. In this study, the effect of differently shaped magnets was examined in a rat model of side-to-side colonic anastomosis. The results showed no significant difference in the establishment of side-to-side colonic anastomosis with the use of circular vs cylindrical magnets. Moreover, the findings of this study support Yan-Zhang's Peripheral Decision Theory of digestive tract MCA.
- Citation: Zhang MM, Shi AH, Muensterer OJ, Uygun I, Lyu Y, Yan XP. Comparative study of cylindrical vs circular ring magnets for colonic anastomosis in rats. World J Gastrointest Surg 2025; 17(2): 94270
- URL: https://www.wjgnet.com/1948-9366/full/v17/i2/94270.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i2.94270
Magnetic compression anastomosis (MCA) is a new anastomosis mode, which differs from suture anastomosis and staple anastomosis in that the anastomosis is established by a magnetic compression technique with no foreign body retention. This leads to a better quality anastomosis effect, and current studies have shown that MCA can be effectively achieved at any point throughout the entire lumen of the digestive tract from the esophagus to rectum[1-5]. In addition, the need for anastomosis is not limited to the digestive tract, as some cases require vascular anastomosis[6,7] or ureteral anastomosis[8,9]. In some special cases, MCA combined with an endoscopic technique can be applied in place of open surgery or laparoscopic surgery for gastrointestinal anastomosis[10,11]. Therefore, to some degree, MCA can replace the traditional mode of digestive tract anastomosis. Accordingly, MCA has wide application potential due to the reliable anastomosis effect and potential for diverse clinical translations.
However, although MCA offers important advantages, it remains in the stage of technological innovation and clinical testing[12,13]. Researchers' interest continues to be focused on new technological innovations and case-by-case appli
In the existing literature regarding magnetic anastomosis, the shapes of magnets designed by researchers for digestive tract anastomosis include cylindrical[15,16], circular[17,18], oval shaped[19], and irregularly shaped[20-22]. The purpose for the design of diverse magnet shapes has been only for easy operative placement and easy discharge. However, whether the establishment of anastomosis between magnets is affected by differences in the designed shape of the magnets remains unknown. Moreover, evidence is lacking for regarding the type of magnet compression surface that will be most conducive to the establishment of anastomosis. Therefore, in the present study, we designed magnets of two different shapes, cylindrical and circular, and tested the influence of magnet shape on the anastomosis effect in a rat model of side-to-side colonic anastomosis.
A total of 30 Sprague-Dawley albino rats (n = 15 males; n = 15 females), weighing 200-250 g, were acquired from the Laboratory Animal Center of the Xi’an Jiaotong University (Xi’an, China) and used as experimental animals. The animals were acclimatized to laboratory conditions (23 °C, 50% humidity, 12-hour/12-hour light/dark cycle, and food and water provided ad libitum) for 1 week before use in experiments.
The experimental protocol was approved by the Animal Ethics Committee of Xi’an Jiaotong University (license No. 2021-1534). The research protocol and all experimental procedures were conducted strictly in accordance with the Guidelines for the Care and Use of Experimental Animals issued by the Xi’an Jiaotong University Medical Center.
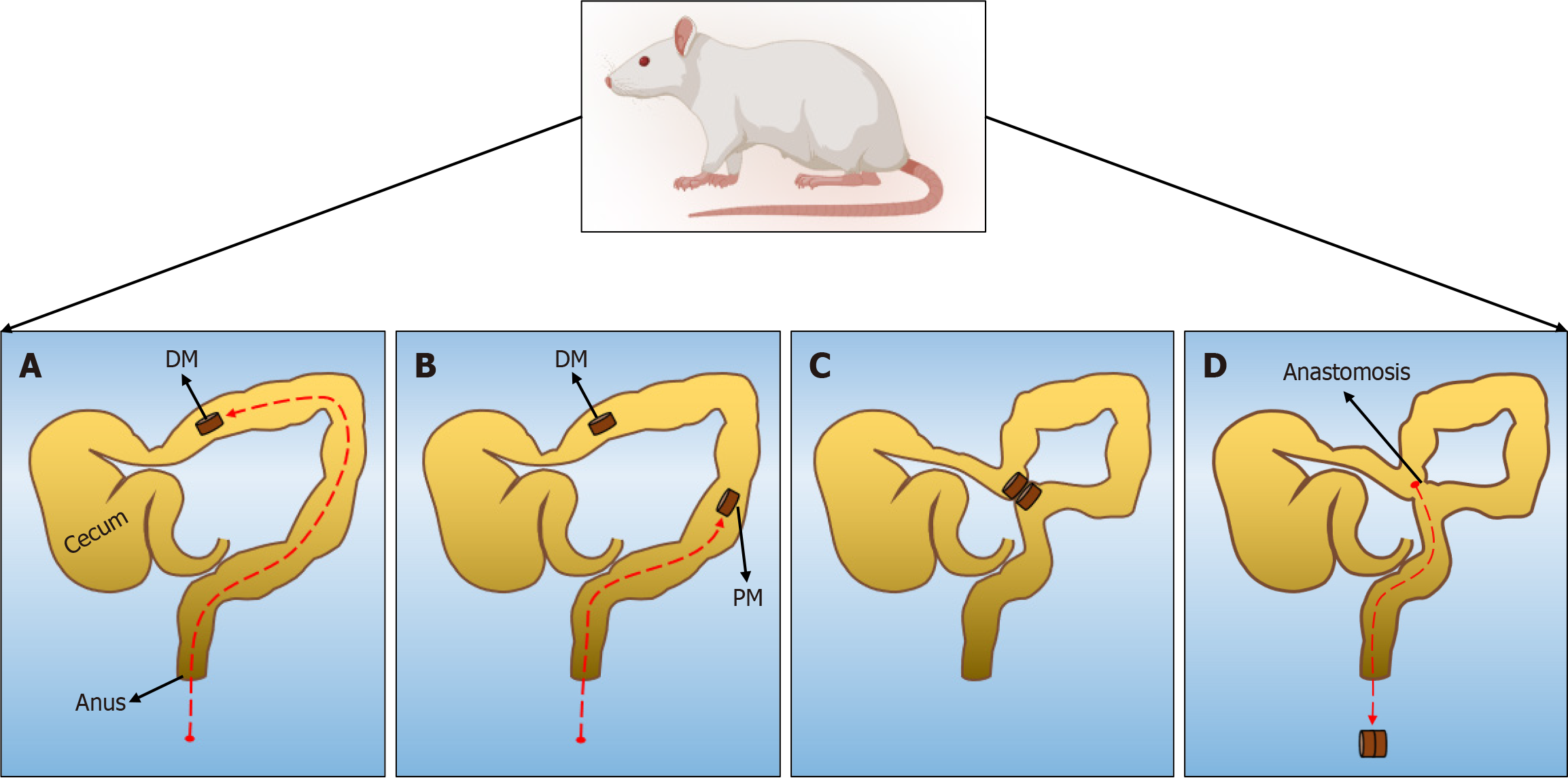
In this study, all rats were randomly divided into three groups: Cylindrical group, circular ring group, and cylindrical-circular ring group. The numbers of female and male rats in each group were ensured to be equal. In all rats, the colon was not cut open after the abdominal cavity was opened, and the daughter magnet (DM) and the parent magnet (PM) were placed into the proximal and distal ends of the colon successively through the anus. Once place, the DM and PM were attracted to each other. The operation time was recorded. After operation, the general state of rats, the time to magnet discharge, and the complications of intestinal obstruction were observed and recorded. The rats were euthanized 4 weeks after the operation, and the burst pressure and diameter of the anastomosis were measured. The formation of the anastomosis was observed by the naked eye and light microscopy.
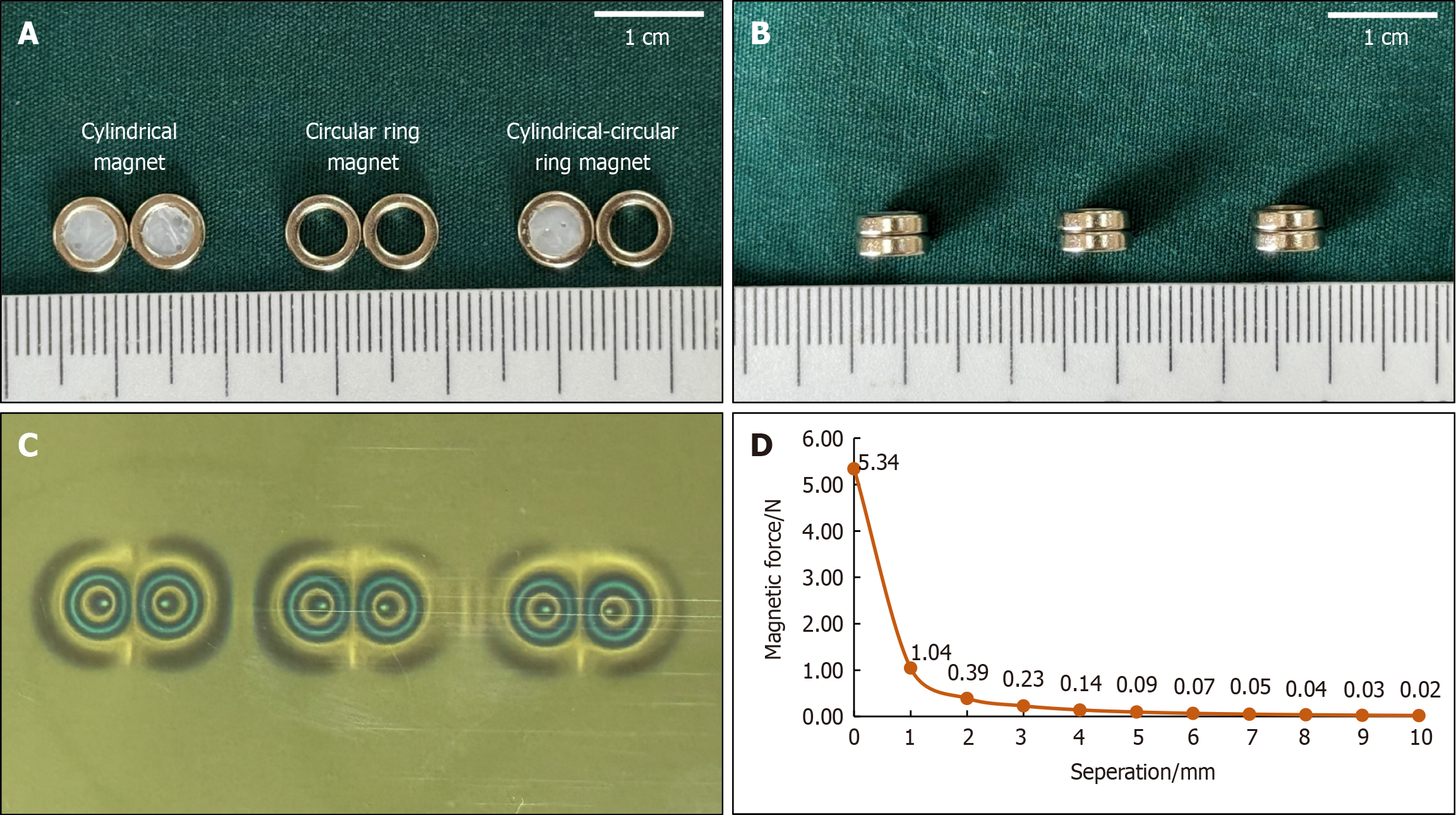
For use in this study, we designed a circular magnet with an outer diameter of 7 mm, an inner diameter of 4.2 mm, and a height of 2 mm. To simulate the bottom surface of a cylindrical magnet, the inside of a circular magnet was filled with epoxy resin glue and cured sufficiently. All magnets were made of N40 sintered-type neodymium-iron-boron (NdFeB), and the surface of the magnets was protected by nickel plating, with saturation in the height direction. The magnet designs are illustrated in Figure 1A and B. The surface magnetic flux density was 220 mT, and the magnetic force between the two magnets was 4.10 Newton at zero distance. The masses of the cylindrical magnet (the modified circular magnet) and the circular magnets were 0.36 g and 0.33 g, respectively. The magnetic field viewing film showed that the magnetic field distribution of the cylindrical magnets (modified circular magnets) was the same as that of the circular magnets (Figure 1C). The magnetic force curve for the magnets is shown in Figure 1D.
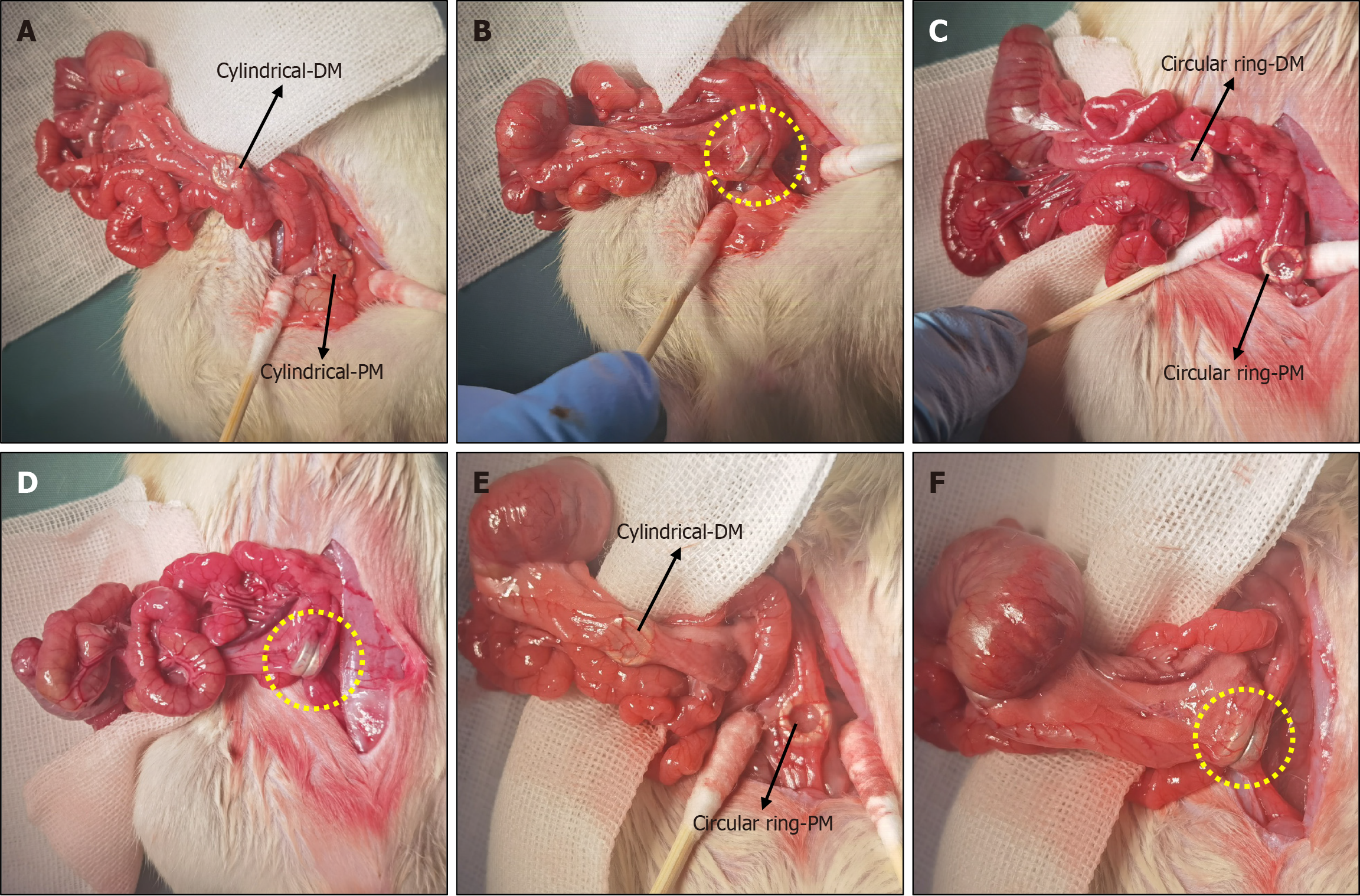
The Sprague-Dawley rats were fed a slag-free liquid diet 1 day before the surgery. They were fasted, and their water intake was restricted at 4 hours before the operation. The animals were anesthetized via intraperitoneal injection of pentobarbital sodium solution (3 mg/100 g). After confirming the loss of the paw withdrawal reflex, the animals were fixed to an operating table, and their hypogastrium was shaved. For all rats, the abdominal cavity was accessed via a 3 cm median incision in the skin of the lower abdomen and abdominal musculature. The DM was pushed through the anus into the distal colon. A cotton swab was used to push this magnet into the proximal colon under direct vision, at which time the PM was pushed through the anus into the distal colon. After adjusting both magnets in their appropriate positions, they were attracted near the anti-mesenteric portion of the colon. The colon was placed back in the abdominal cavity, which was closed with a silk suture. The surgical procedure is illustrated in Figure 2. For the cylindrical group, cylindrical magnets were used as both the DM and PM. For the circular group, circular ring magnets were used as both the DM and PM. For the cylindrical-circular ring group, a cylindrical magnet was used as the DM and a circular ring magnet as the PM.
After recovery from anesthesia, the rats were fed normally. During the first 3 days after surgery, pethidine (0.1 mg/100 g) was injected intramuscularly every 12 hours. The mental state, feeding, and defecation of rats were closely monitored after the operation. The stool was checked daily until the magnets were expelled through the anus. The time to magnet discharge was recorded for each rat.
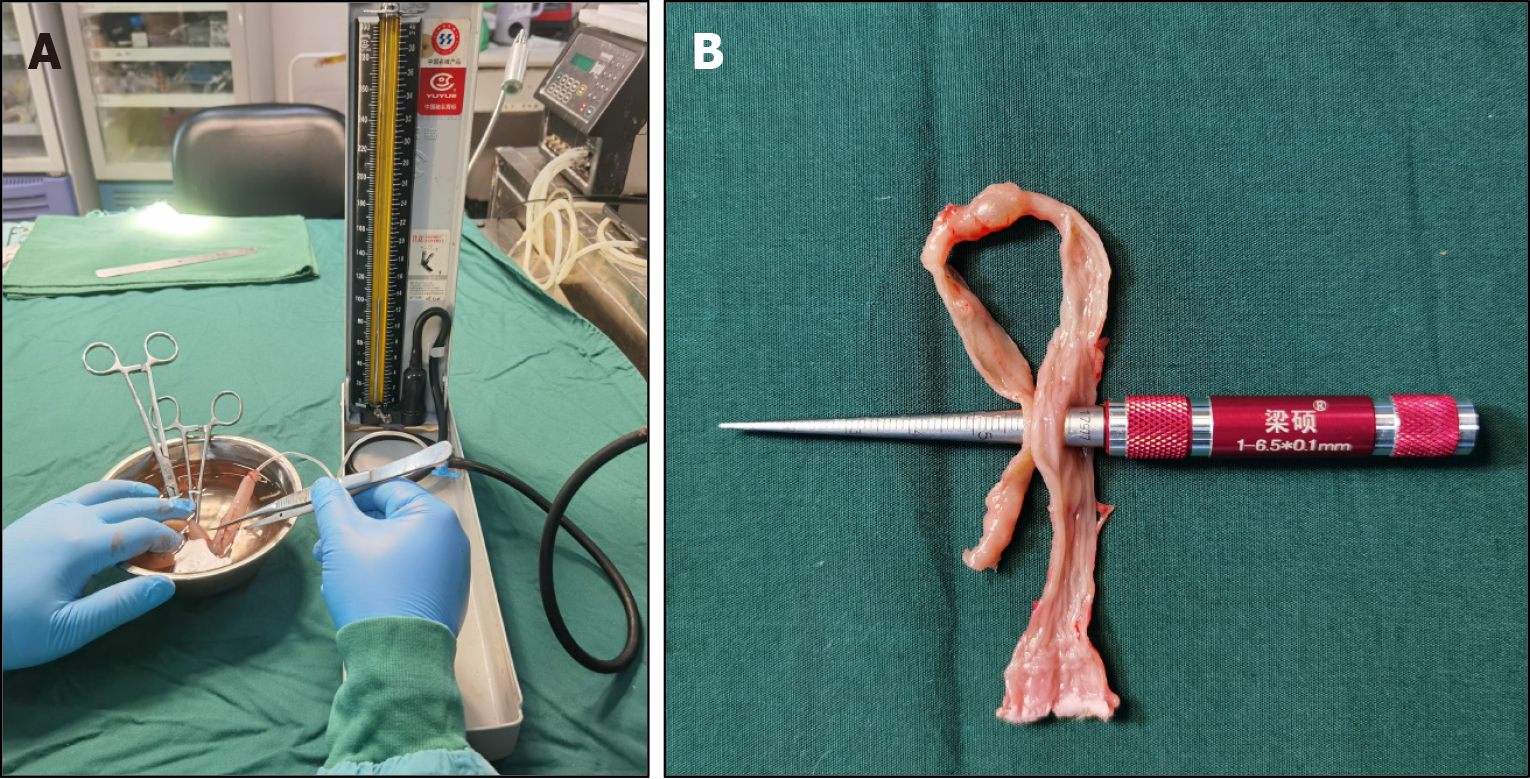
The rats were euthanized via intraperitoneal injection of pentobarbital sodium solution (6 mg/100 g) at 4 weeks after the operation, and a gross specimen of the anastomosis was obtained from each rat (the proximal end was the colon bypass anastomosis with 3-5 cm of the proximal colon, and the distal end was the anus). A catheter was introduced through the other end and ligated using a single silk suture. The entire anastomosis segment was immersed in 0.9% saline. Subse
The colon specimens were dissected longitudinally from both sides of the anastomosis, and the formation of the anastomosis was observed by naked eye. The tip of the conical aperture scale was inserted into the colon anastomosis, and the intestinal anastomosis specimen was slid toward the end of the handle with appropriate force. When the re
Each anastomotic specimen was soaked overnight in 10% formalin for fixation and embedded in paraffin before the preparation of 4 μm-thick sections. The sections were stained with hematoxylin and eosin (H&E) and Masson trichrome stain and examined under a bright-field microscope.
To model the compression stress on the intestinal wall generated by the attraction of the magnets of different shapes, we used ANSYS software (ANSYS Corporation, United States, ANSYS 2021R1) for analysis. The stress distribution on the intestinal wall between the DM and PM was calculated, and the deformation of the intestinal wall was simulated.
IBM SPSS statistical software version 20.0 was used for data analyses. Normally distributed quantitative data were described using mean and standard deviation, whereas non-normally distributed data were represented as median. Differences between the groups were compared using analysis of variance or nonparametric test. Values of P < 0.05 indicated a significant difference.
The designed magnets were inserted through the anus, and side-to-side colonic anastomosis was successfully achieved in all animals. Magnet insertion went smoothly, and attraction between the magnets was achieved once the second magnet reached the target position without perforation or bleeding. The operation times for the cylindrical group, circular ring group, and cylindrical-circular ring group were statistically similar at 12.00 ± 2.29 minutes, 11.05 ± 2.28 minutes, and 11.25 ± 2.30 minutes, respectively (P = 0.624). The intraoperative procedures for the three designs are illustrated in Figure 3.
All rats survived for 4 weeks after the surgery until the end of the experiment, for a survival rate of 100%. The animals exhibited a normal mental state and food intake postoperatively, and none of them suffered complications, such as bleeding or intestinal obstruction.
After the formation of anastomosis, the magnets were discharged without complication after similar durations among the three designs. Magnet discharge occurred after 4.60 ± 0.97 days, 4.50 ± 0.97 days, and 4.8 ± 1.14 days in the cylindrical group, circular ring group, and cylindrical-circular ring group, respectively (P = 0.760).
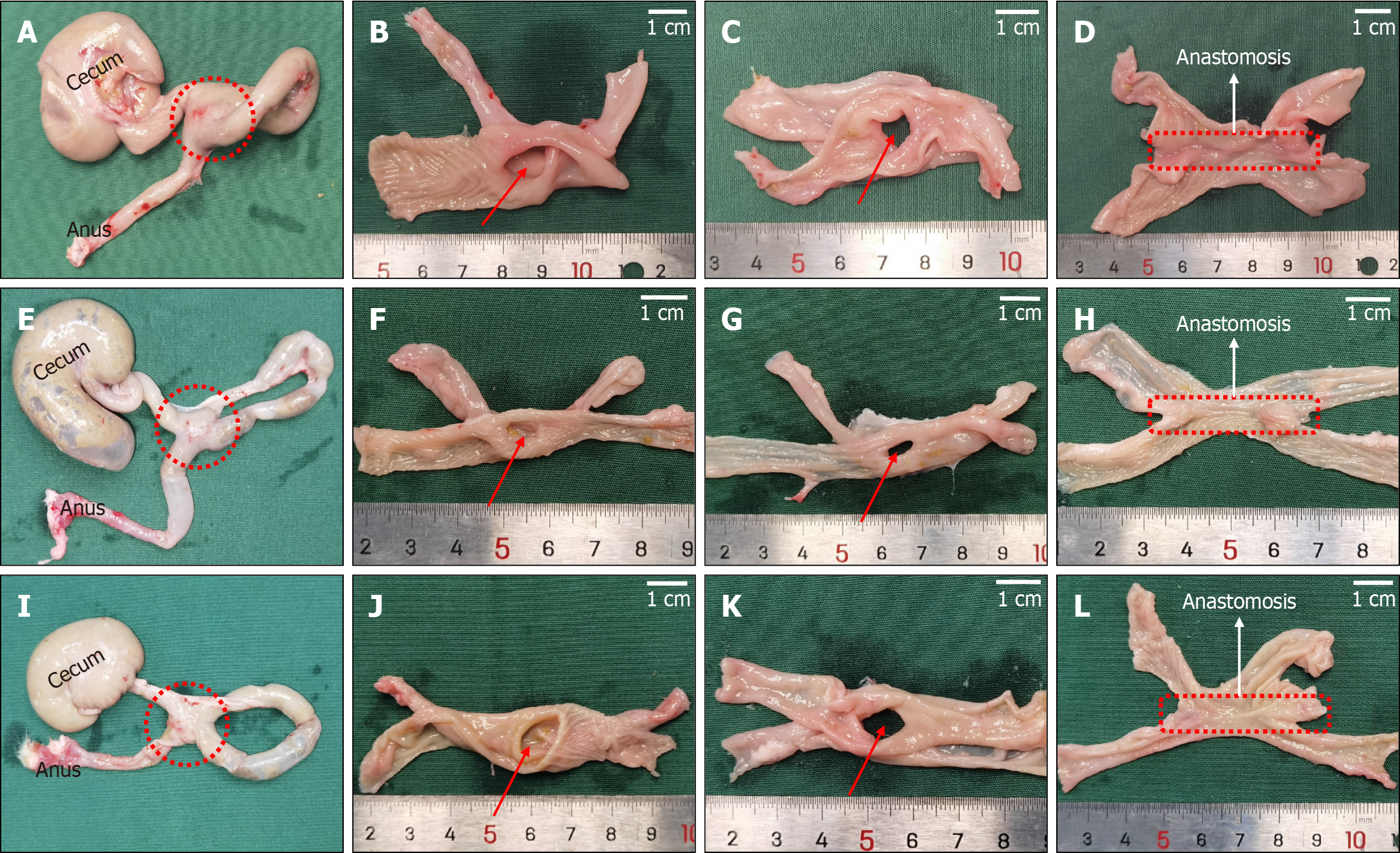
Four weeks after the operation, the rats in all groups, and colon samples covering at least 3 cm in either direction from the anastomosis were obtained. The water pumping method was used to measure the burst pressure of the anastomosis in each sample, and the burst pressure did not differ significantly among the cylindrical group, circular ring group, and cylindrical-circular ring group (212.80 ± 16.28 mmHg, 220.20 ± 14.98 mmHg, and 217.60 ± 15.51 mmHg, P = 0.567). The anastomotic diameters in the three groups were 6.94 ± 0.30 mm, 6.87 ± 0.34 mm, and 6.97 ± 0.37 mm, respectively, with no statistical difference among them (P = 0.809). Figure 4 shows the measurement process of anastomotic burst pressure and anastomotic diameter. Sample data and observation indicators are presented in Table 1.
| Cylindrical group | Circular ring group | Cylindrical-circular ring group | P value | |
| Rats, n | 10 | 10 | 10 | 1.00 |
| Gender | ||||
| Male | 5 (50) | 5 (50) | 5 (50) | 1.00 |
| Female | 5 (50) | 5 (50) | 5 (50) | |
| Operative time (minute) | 12.00 ± 2.29 | 11.05 ± 2.28 | 11.25 ± 2.30 | 0.624 |
| Time to magnet discharge (day) | 4.60 ± 0.97 | 4.50 ± 0.97 | 4.80 ± 1.14 | 0.760 |
| Bursting pressure (mmHg) | 212.80 ± 16.28 | 220.20 ± 14.98 | 217.60 ± 15.51 | 0.567 |
| Anastomotic diameter (mm) | 6.94 ± 0.30 | 6.97 ± 0.37 | 6.87 ± 0.34 | 0.809 |
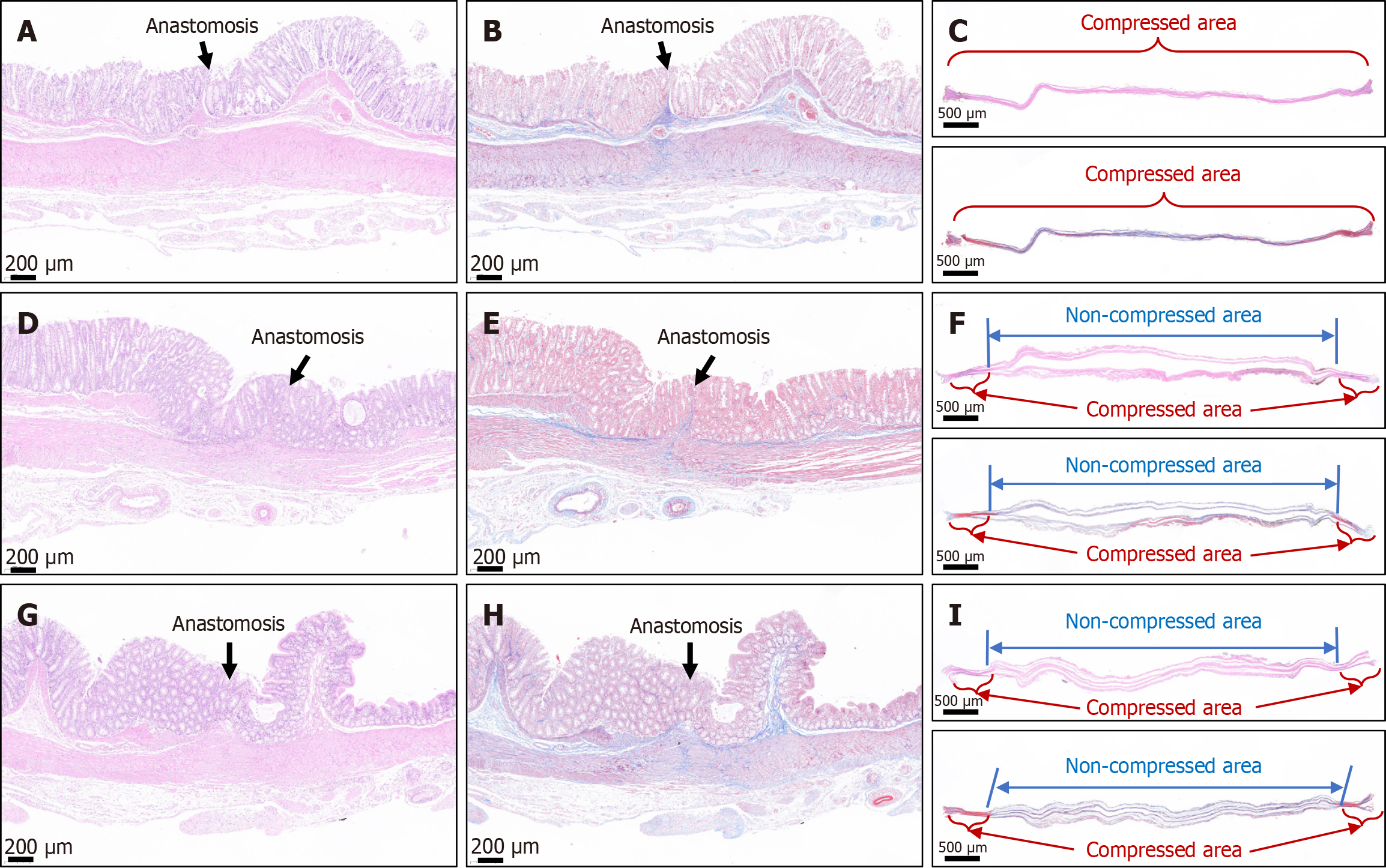
The colon portions on either side of the anastomosis were dissected longitudinally, and the mucosa at the site of the anastomosis appeared smooth and free of any foreign material (Figure 5). H&E and Masson trichrome staining showed good continuity of the mucosal layer, basal layer, and serosal layer at the anastomosis in specimens from all three groups (Figure 6). No significant difference in the appearance of the anastomosis was observed among the three groups. Necrotic tissue staining of the intestinal wall showed that in the cylindrical group, the two intestinal walls were tightly attached and fused (Figure 6C). In the circular group, the two sides of the intestinal wall tissue that had been pressed together by the magnetic ring were fused, and the two sides of the intestinal wall were separated from the area in the middle of the magnetic ring (Figure 6F). In the cylindric-circular magnet group, the uncompressed intestinal wall tissue in the middle was close, but the intestinal wall tissue on both sides could still be clearly distinguished (Figure 6I).
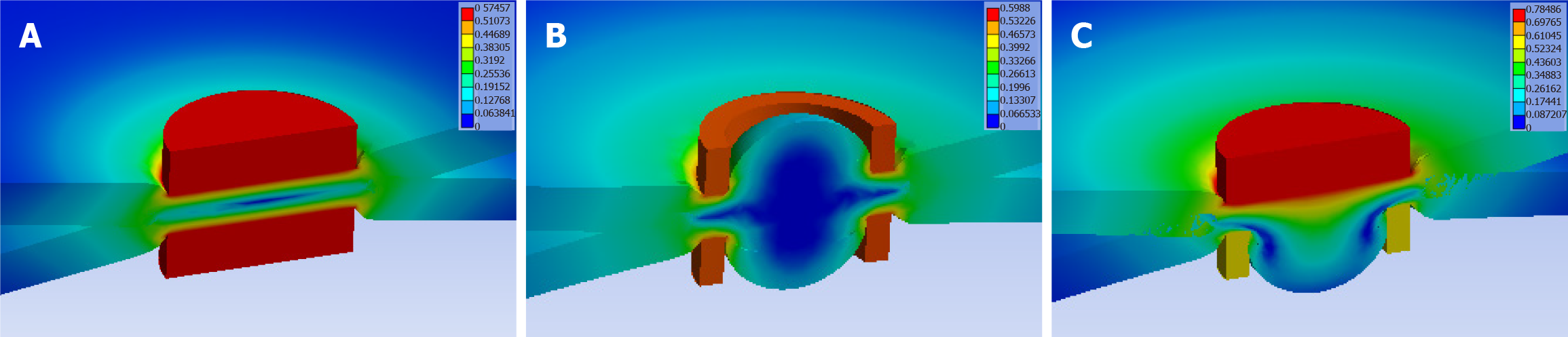
We used finite element simulation analysis, and the results showed that the compression stress on the intestinal wall generated by the surrounding area of the magnet during the establishment of magnetic anastomosis was similar in the three groups (Figure 7).
In previous basic studies on MCA, researchers have discussed the influence of magnetic force on the establishment of digestive tract anastomosis and proposed the most suitable magnetic forces for bilioenteric anastomosis and enteroenteric anastomosis[23,24]. However, few investigators have examined the influence of the structure of the magnets’ compre
In the present study, the influence of the difference in the compression surface structures of cylindrical and circular ring magnets used for anastomosis on the effect of magnetic anastomosis of the digestive tract was investigated for the first time. The results showed no significant differences in the time to magnet discharge, anastomotic burst pressure, and anastomotic diameter among the three groups. In all groups, the mucosal layer was smooth and healed well. Histological staining of necrotic intestinal wall tissue from the three groups of magnets showed no significant difference in the intestinal walls of the fully compressed areas. Necrotic changes occurred in the circular group and the uncompressed area of the cylindric-circular group due to ischemia, and no obvious fusion was observed between the two intestinal walls in these areas. However, H&E and Masson trichrome staining showed good continuity of the mucosal layer of the anastomosis in all three groups. The results of this study showed that there was no significant difference in the estab
Further analysis showed that although the true compressed area of the circular ring magnet to the intestinal wall tissue was smaller than that of the cylindrical magnet, the uncompressed intestinal wall tissue in the center of the circular ring magnet also experienced ischemia and necrosis. Because the circular ring magnet creates a circular ischemic area, in this case, the intestinal wall tissue in the middle of the circular ischemic area will undergo tissue ischemia even if not compressed. Therefore, under the premise that the magnetic force meets the anastomosis demand, the peripheral area of the magnet plays a decisive role in the establishment of the magnetic anastomosis, which is consistent with the Yan-Zhang’s Peripheral Decision Theory of digestive tract MCA. The results of finite element simulation analysis showed that the compression stress on the intestinal wall generated by the area of the magnets during the establishment of magnetic anastomosis was the same in all three groups, further verifying the rationality of Yan-Zhang’s Peripheral Decision Theory.
In the present study, no significant difference in the anastomosis effect within the digestive tract was observed with or without holes in the press surface of the magnet. Therefore, in the design of a digestive tract magnetic anastomosis device, the decision to use either a cylindrical or circular magnet depends on the convenience of magnet placement and magnetic force. This finding has important clinical implications.
This study has two major advantages. First, we construct cylindrical magnets by cementing on the basis of the structure of circular magnets, the purpose of which was to eliminate the influence of magnetic differences between different groups on the establishment of anastomosing. This method was simple and effective. Secondly, the magnets were inserted through the anus with the assistance of laparotomy, which can avoid colon incision, simplify the operation, and eliminate the influence of colon incision and suturing on the observation indicators. A disadvantage of the study is that rats were used as the model animals, and the conclusions would be more convincing conclusions if the results were obtained in larger animal models (dogs or pigs), which have a gut anatomy more similar to that of humans.
With application of the same magnetic force, cylindrical magnets and circular ring magnets showed no significant differences in the establishment of side-to-side colonic anastomosis in rats. Based on the results of this study, the Yan-Zhang’s Peripheral Decision Theory is reasonable.
| 1. | Lee WG, Evans LL, Chen CS, Fuchs JR, Zamora IJ, Bruzoni M, Harrison MR, Muensterer OJ. Lessons Learned From the First-In-Human Compassionate Use of Connect-EA™ in Ten Patients With Esophageal Atresia. J Pediatr Surg. 2024;59:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Evans LL, Lee WG, Karimzada M, Patel VH, Aribindi VK, Kwiat D, Graham JL, Cummings DE, Havel PJ, Harrison MR. Evaluation of a Magnetic Compression Anastomosis for Jejunoileal Partial Diversion in Rhesus Macaques. Obes Surg. 2024;34:515-523. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Gagner M, Krinke T, Lapointe-Gagner M, Buchwald JN. Side-to-side duodeno-ileal magnetic compression anastomosis: design and feasibility of a novel device in a porcine model. Surg Endosc. 2023;37:6197-6207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 4. | Li GZ, Ryou M, Thompson CC, Wang J. A Preclinical Study of an Esophagojejunal Compression Anastomosis After Total Gastrectomy with Self-Forming Magnets. J Gastrointest Surg. 2023;27:1710-1712. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Marchegiani F, Noll E, Riva P, Kong SH, Saccomandi P, Vita G, Lindner V, Namer IJ, Marescaux J, Diemunsch P, Diana M. Effects of Warmed and Humidified CO(2) Surgical Site Insufflation in a Novel Experimental Model of Magnetic Compression Colonic Anastomosis. Surg Innov. 2021;28:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Shi Y, Zhang W, Deng YL, Zhang YM, Zhang QS, Zhang WY, Zheng H, Pan C, Shen ZY. Magnetic ring anastomosis of suprahepatic vena cava: novel technique for liver transplantation in rat. Transpl Int. 2015;28:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Vicol C, Eifert S, Oberhoffer M, Boekstegers P, Reichart B. Mid-term patency after magnetic coupling for distal bypass anastomosis in coronary surgery. Ann Thorac Surg. 2006;82:1452-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Shlomovitz E, Copping R, Swanstrom LL. Magnetic Compression Anastomosis for Recanalization of Complete Ureteric Occlusion after Radical Cystoprostatectomy. J Vasc Interv Radiol. 2023;34:1640-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Ünal E, Çiftçi TT, Akinci D. Magnetic Compression Anastomosis of Benign Short-Segment Ureteral Obstruction. J Vasc Interv Radiol. 2024;35:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 10. | Zhang M, He S, Sha H, Xue H, Lv Y, Yan X. A novel self-shaping magnetic compression anastomosis ring for treatment of colonic stenosis. Endoscopy. 2023;55:E1132-E1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (1)] |
| 11. | Gomes GF, Noda RW, Lima TMDC, Kashiwagui LY, Nakadomari TS. Magnetic compression anastomosis for the treatment of complete gastric outlet obstruction due to corrosive injury. VideoGIE. 2022;7:223-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Pérez-Bertólez S, Godoy-Lenz J. Primary repair of esophageal atresia Gross type C via thoracoscopic magnetic compression anastomosis: Is it the best option? World J Gastrointest Surg. 2024;16:1474-1481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Gagner M, Krinke T, Lapointe-Gagner M, Buchwald JN. Magnetic compression anastomosis gastrojejunostomy: feasibility and efficacy of a novel device in a swine model. Surg Obes Relat Dis. 2024;20:1098-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Zhang M, Lyu X, Zhao G, An Y, Lyu Y, Yan X. Establishment of Yan-Zhang's staging of digestive tract magnetic compression anastomosis in a rat model. Sci Rep. 2022;12:12445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Kamada T, Ohdaira H, Takeuchi H, Takahashi J, Ito E, Suzuki N, Narihiro S, Yoshida M, Yamanouchi E, Suzuki Y. New Technique for Magnetic Compression Anastomosis Without Incision for Gastrointestinal Obstruction. J Am Coll Surg. 2021;232:170-177.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Kawabata H, Nakase K, Okazaki Y, Yamamoto T, Yamaguchi K, Ueda Y, Miyata M, Motoi S. Endoscopic ultrasonography for pre-operative local assessment and endoscopic ultrasonography-guided marking before gastrojejunostomy for duodenal obstruction using magnetic compression anastomosis. J Clin Transl Res. 2021;7:621-624. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Bruns NE, Glenn IC, Craner DR, Schomisch SJ, Harrison MR, Ponsky TA. Magnetic compression anastomosis (magnamosis) in a porcine esophagus: Proof of concept for potential application in esophageal atresia. J Pediatr Surg. 2019;54:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Graves CE, Co C, Hsi RS, Kwiat D, Imamura-Ching J, Harrison MR, Stoller ML. Magnetic Compression Anastomosis (Magnamosis): First-In-Human Trial. J Am Coll Surg. 2017;225:676-681.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Gagner M, Almutlaq L, Cadiere GB, Torres AJ, Sanchez-Pernaute A, Buchwald JN, Abuladze D. Side-to-side magnetic duodeno-ileostomy in adults with severe obesity with or without type 2 diabetes: early outcomes with prior or concurrent sleeve gastrectomy. Surg Obes Relat Dis. 2024;20:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Reich C, Weigl E, Holler AS, Lee W, Harrison M, Muensterer OJ. Repair of complex esophageal atresia with tracheobronchial remnant using special magnets. European J Pediatr Surg Rep. 2024;12:e33-e37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Ore AS, Askenasy E, Ryou M, Baldwin T, Thompson CC, Messaris E. Evaluation of sutureless anastomosis after ileostomy takedown using the self-forming magnet anastomosis system in a porcine model. Surg Endosc. 2022;36:7664-7672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 22. | Ryou M, Cantillon-Murphy P, Azagury D, Shaikh SN, Ha G, Greenwalt I, Ryan MB, Lang JH, Thompson CC. Smart Self-Assembling MagnetS for ENdoscopy (SAMSEN) for transoral endoscopic creation of immediate gastrojejunostomy (with video). Gastrointest Endosc. 2011;73:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Xue F, Guo HC, Li JP, Lu JW, Wang HH, Ma F, Liu YX, Lv Y. Choledochojejunostomy with an innovative magnetic compressive anastomosis: How to determine optimal pressure? World J Gastroenterol. 2016;22:2326-2335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Lambe T, Ríordáin MG, Cahill RA, Cantillon-Murphy P. Magnetic compression in gastrointestinal and bilioenteric anastomosis: how much force? Surg Innov. 2014;21:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Jamshidi R, Stephenson JT, Clay JG, Pichakron KO, Harrison MR. Magnamosis: magnetic compression anastomosis with comparison to suture and staple techniques. J Pediatr Surg. 2009;44:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Mascagni P, Tringali A, Boškoski I, Bove V, Schepis T, Perri V, Costamagna G. Magnetic kissing for the endoscopic treatment of a complete iatrogenic stenosis of the hypopharynx. Endoscopy. 2023;55:E499-E500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |