Published online Feb 27, 2025. doi: 10.4240/wjgs.v17.i2.102444
Revised: November 16, 2024
Accepted: December 4, 2024
Published online: February 27, 2025
Processing time: 96 Days and 21.4 Hours
We discuss the findings of Wu et al on the utility of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune-inflammatory index as diagnostic markers for gastric carcinoma (GC). We commend the study's contributions to the field and suggest a prospective study to validate these markers' sensitivity and specificity for early GC detection. We also propose developing surveillance protocols that incorporate these markers with other diagnostic methods to enhance clinical decision-making. Furthermore, we highlight the need for a more diverse patient cohort to assess the generalizability of these markers across different ethnic groups and demographic factors. Our suggestions aim to refine the application of these markers in clinical practice and to understand their potential in diverse clinical scenarios.
Core Tip: This commentary highlights the potential of using neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune-inflammatory index as cost-effective early diagnostic markers for gastric carcinoma, emphasizing their significance in critical care and the need for further research to incorporate these markers into a multi-modal diagnostic approach for improved patient outcomes.
- Citation: Ding QZ, Wang RL, Xie Y. Unmasking gastric carcinoma: Unveiling diagnostic biomarkers and the role of critical care. World J Gastrointest Surg 2025; 17(2): 102444
- URL: https://www.wjgnet.com/1948-9366/full/v17/i2/102444.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i2.102444
The recent publication by Wu et al[1] offers intriguing insights into the utility of the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammatory index (SII) as diagnostic markers for gastric carcinoma (GC). As a critical care physician, I find the exploration of these immune-inflammatory markers particularly relevant in the context of early detection and intervention in GC. This letter seeks to commend the study's contributions and to propose further considerations for future research.
Firstly, the study's retrospective design, while providing a solid foundation for hypothesis generation, may not capture the full clinical spectrum of GC diagnostics. The reliance on historical data could introduce biases that a prospective study might mitigate. Future work with a longitudinal approach could offer more robust evidence of the diagnostic value of NLR, PLR, and SII.
Secondly, the study's patient cohort, though well-characterized, may not fully represent the diversity of GC patients seen in varied clinical settings. The majority of patients were of Asian descent, and the implications of these markers in different ethnicities, particularly Black and Hispanic populations, remain underexplored. Expanding the demographic scope of such studies could enhance the generalizability of findings.
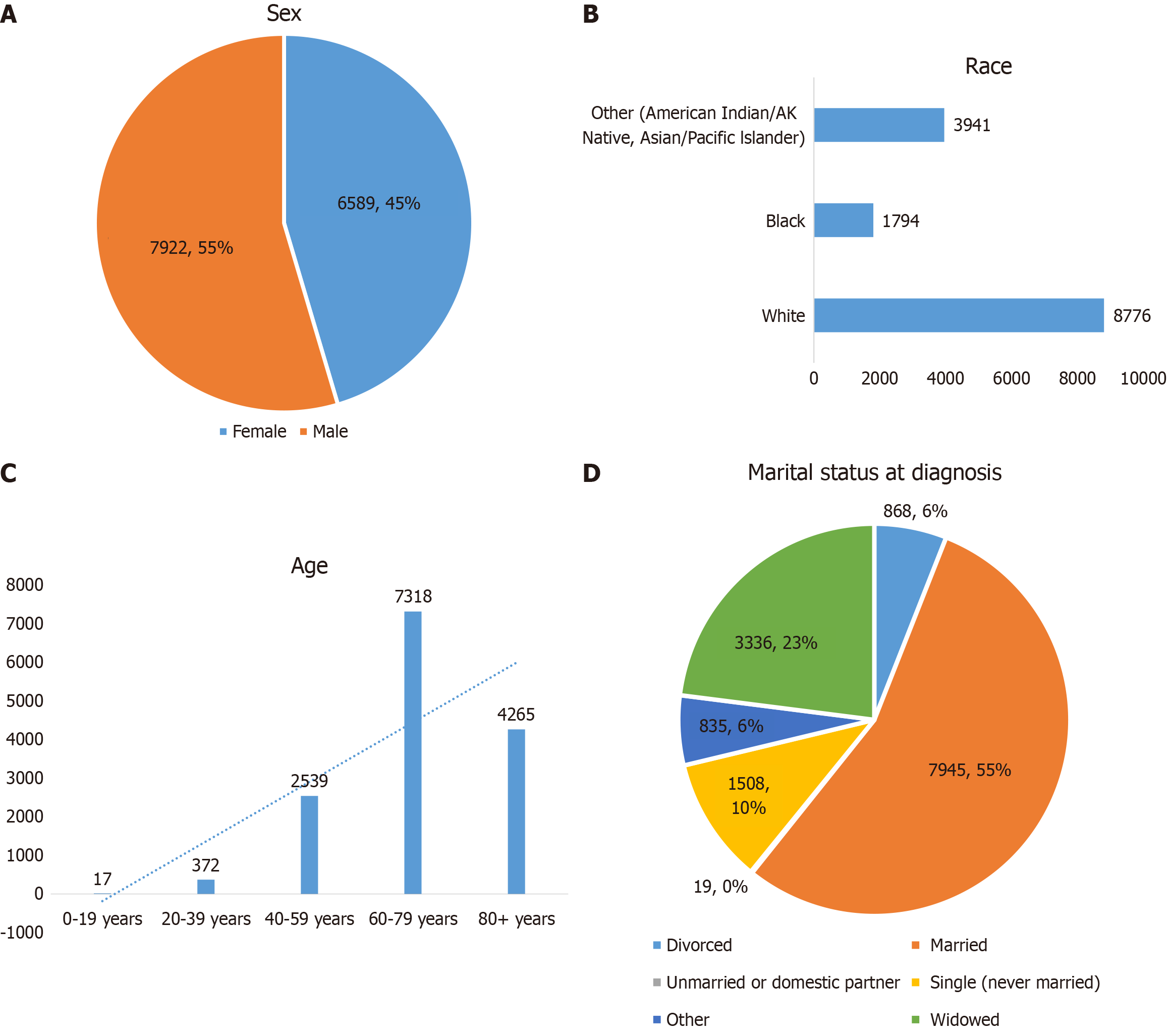
Thirdly, while the study identified gender and BMI as risk factors for GC, other potential risk factors such as racial demographics, and marital statuses were not accounted for. The SEER database provides a comprehensive dataset that can be leveraged to explore these additional risk factors. We made research on GC, Figure 1A provides the sex of the study population. Figure 1B provides insights into the racial demographics of the study population. With "White" individuals making up the largest group at 79.45%, followed by "Black" and "American Indian/AK Native" this chart underscores the need for targeted interventions in these communities. Figure 1C from the SEER data delineates the distribution of patients across various age groups. Notably, it highlights a significant number of individuals in the "20-39 years" age bracket, comprising 2.56% of the total (n = 14511). This underscores the importance of early screening and intervention in this demographic. However, the specific figures for the "40-59 years, 17.5%" "60-79 years, 50.4%" and "80+ years, 29.4%" age groups make up the largest part, suggesting a need for more detailed analysis. Furthermore, Figure 1D from the SEER data shows that illustrates the distribution of marital statuses at the time of diagnosis. This chart is particularly insightful as it reveals the proportion of patients in different marital categories, with "Married" individuals constituting the largest percentage at 55% (Figure 1). This data could be crucial in understanding the social determinants of health and how they might influence GC outcomes. In conclusion, the SEER database offers a rich source of data that can enhance our understanding of the multifaceted risk factors associated with gastric cancer. By incorporating variables such as racial demographics, and marital statuses into future analyses, we can develop a more holistic understanding of GC and devise more effective prevention and treatment strategies. Li et al[2] proposed that incorporating these variables could provide a more comprehensive understanding of the GC development.
Moreover, the study's findings on NLR, PLR, and SII could have significant implications for intensive care, particularly in the early warning of GC in patients presenting with other severe illnesses. Monitoring these markers could serve as a predictive tool for intensivists to identify patients at risk of GC who might benefit from further investigation. For example, the detection of these indicators in patients with gastric ulcer with massive gastrointestinal bleeding after admission to intensive care unit can indicate gastric cancer in advance than the pathological results of gastroscopy. To effectively leverage biomarkers such as NLR, PLR, and SII in clinical practice, it is recommended to develop specific surveillance protocols, establish threshold values for intervention, and use these markers in conjunction with other diagnostic methods to enhance clinical decision-making.
Lastly, the integration of these biomarkers into a multi-modal diagnostic approach, including endoscopy and imaging, could be a promising area of research. Just like Yao et al’s development and validation of an artificial intelligence-based system for predicting colorectal cancer invasion depth using multi-modal data[3]. The potential of these markers to stratify patients into risk categories for more targeted diagnostic testing and intervention is an exciting prospect.
In conclusion, the study by Wu et al[1] is a valuable contribution to the field of GC diagnosis. As a critical care physician, I believe that the incorporation of these immune-inflammatory markers into clinical practice could significantly enhance our ability to detect and manage GC. However, further research is needed to refine their application and to fully understand their potential in diverse and complex clinical scenarios. It is suggested to indicate more specific directions and goals for future research. For example, a prospective study plan aimed at validating the sensitivity and specificity of NLR, PLR, and SII in the early diagnosis of gastric cancer could be proposed.
We thank all participants, colleagues, and funding sources for their invaluable contributions to this research.
| 1. | Wu HM, Ying XX, Lv LL, Hu JW. Diagnostic implications of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune-inflammatory index for gastric carcinoma. World J Gastrointest Surg. 2025;17:100130. [DOI] [Full Text] |
| 2. | Li Y, Hahn AI, Laszkowska M, Jiang F, Zauber AG, Leung WK. Clinicopathological Characteristics and Risk Factors of Young-Onset Gastric Carcinoma: A Systematic Review and Meta-analysis. Clin Transl Gastroenterol. 2024;15:e1. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Yao L, Lu Z, Yang G, Zhou W, Xu Y, Guo M, Huang X, He C, Zhou R, Deng Y, Wu H, Chen B, Gong R, Zhang L, Zhang M, Gong W, Yu H. Development and validation of an artificial intelligence-based system for predicting colorectal cancer invasion depth using multi-modal data. Dig Endosc. 2023;35:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Reference Citation Analysis (0)] |