Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.2942
Revised: May 24, 2024
Accepted: July 17, 2024
Published online: September 27, 2024
Processing time: 176 Days and 22.1 Hours
Gastrointestinal stromal tumors (GISTs) vary widely in prognosis, and traditional pathological assessments often lack precision in risk stratification. Advanced imaging techniques, especially magnetic resonance imaging (MRI), offer potential improvements. This study investigates how MRI imagomics can enhance risk assessment and support personalized treatment for GIST patients.
To assess the effectiveness of MRI imagomics in improving GIST risk stratification, addressing the limitations of traditional pathological assessments.
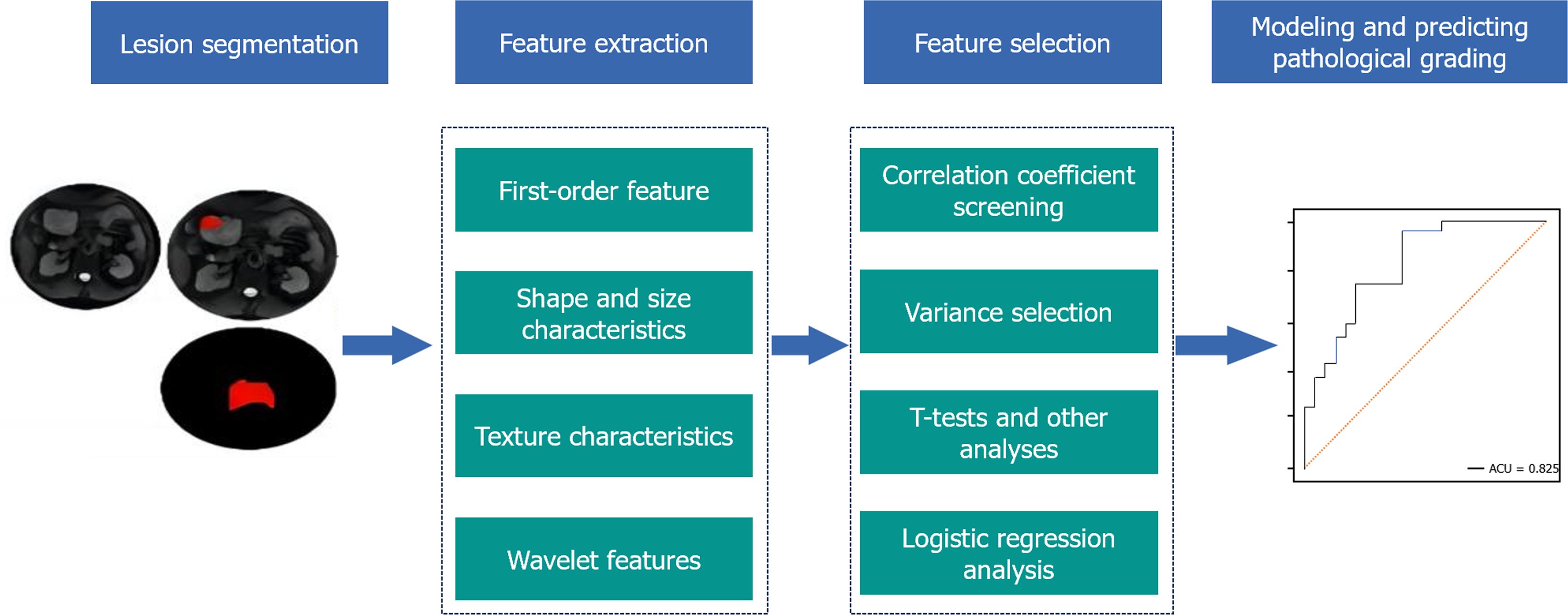
Analyzed clinical and MRI data from 132 GIST patients, categorizing them by tumor specifics and dividing into risk groups. Employed dimension reduction for optimal imagomics feature selection from diffusion-weighted imaging (DWI), T1-weighted imaging (T1WI), and contrast enhanced T1WI with fat saturation (CE-T1WI) fat suppress (fs) sequences.
Age, lesion diameter, and mitotic figures significantly correlated with GIST risk, with DWI sequence features like sphericity and regional entropy showing high predictive accuracy. The combined T1WI and CE-T1WI fs model had the best predictive efficacy. In the test group, the DWI sequence model demonstrated an area under the curve (AUC) value of 0.960 with a sensitivity of 80.0% and a specificity of 100.0%. On the other hand, the combined performance of the T1WI and CE-T1WI fs models in the test group was the most robust, exhibiting an AUC value of 0.834, a sensitivity of 70.4%, and a specificity of 85.2%.
MRI imagomics, particularly DWI and combined T1WI/CE-T1WI fs models, significantly enhance GIST risk stratification, supporting precise preoperative patient assessment and personalized treatment plans. The clinical implications are profound, enabling more accurate surgical strategy formulation and optimized treatment selection, thereby improving patient outcomes. Future research should focus on multicenter studies to validate these findings, integrate advanced imaging technologies like PET/MRI, and incorporate genetic factors to achieve a more comprehensive risk assessment.
Core Tip: This study unveils the transformative potential of magnetic resonance imaging (MRI) imagomics in the preoperative risk stratification of gastrointestinal stromal tumors (GISTs), challenging traditional, pathology-based assessments. By analyzing imagomics features from MRI sequences, particularly diffusion-weighted imaging and a combined T1-weighted imaging (T1WI)/contrast enhanced T1WI with fat saturation fat suppress model, we have demonstrated unprecedented accuracy in predicting GIST risk levels. These findings not only enhance the precision of preoperative evaluations but also pave the way for more personalized treatment plans, aligning closely with the goals of precision medicine in oncology.
- Citation: Pan GH, Zhou F, Chen WB, Pan ZJ. Advancing gastrointestinal stromal tumor management: The role of imagomics features in precision risk assessment. World J Gastrointest Surg 2024; 16(9): 2942-2952
- URL: https://www.wjgnet.com/1948-9366/full/v16/i9/2942.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i9.2942
Gastrointestinal stromal tumors (GISTs) are tumors originating from the interstitial cells of the gastrointestinal tract, exhibiting a wide range of clinical manifestations from benign or low-grade malignancy to highly malignant[1-3]. The risk assessment of GISTs is crucial for determining the most appropriate treatment plan and predicting patient prognosis[4]. Traditional risk assessment methods are primarily based on pathological features such as tumor size, location, and mitotic rate[5-7]. However, these methods have limitations in predicting tumor behavior and guiding treatment choices, especially for tumors in borderline areas where accurate risk assessment remains challenging[8].
In recent years, conventional imaging techniques have played a significant role in the diagnosis and risk assessment of GISTs, but these methods rely on the interpretation of radiologists and may be subject to subjective judgment, needing more quantitative data support. To overcome these limitations, imagomics has emerged as a novel solution, transforming imaging data into high-throughput, quantifiable biomarkers[9]. By analyzing imaging characteristics of tumors, such as shape, texture, and signal intensity variations, imagomics can reveal tumors' microstructure and biological behavior[10,11].
Magnetic resonance imaging (MRI), compared to computed tomography, offers a unique and rich data source for imagomics studies of GISTs due to its superior soft tissue contrast and diverse imaging sequences[12-14]. MRI not only provides information on tumor size and location but also reveals the internal structure and blood supply of the tumor through various sequences such as T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), and diffusion-weighted imaging (DWI)[15-18]. This multidimensional information positions MRI as an ideal tool for assessing GIST heterogeneity and predicting tumor behavior[19,20].
Despite the demonstrated utility of MRI imagomics in pathological grading and risk classification prediction in other cancer types, research on preoperative risk assessment for GISTs using MRI is relatively scarce. This gap underscores an urgent scientific need and clinical opportunity to explore the potential application of MRI imagomics in preoperative risk stratification prediction for GISTs.
This study aims to bridge this gap by developing an accurate and reliable GIST risk assessment model by using multi-sequence MRI imagomics features. The detailed insights from MRI imagomics influence surgical and pharmacological treatment strategies. Specifically, the integration of T1WI and contrast enhanced T1WI with fat saturation (CE-T1WI) fat suppress (fs) sequences offers high-resolution images that aid in identifying precise tumor margins, facilitating surgical decisions about tumor resection extents. This capability is crucial for optimizing surgical outcomes and selecting appropriate adjuvant therapies, such as targeted therapies for high-risk patients, which could significantly impact patient survival and recurrence rates[21]. Our goal is to offer an objective risk assessment method through quantitative analysis of tumor imaging characteristics, thus assisting clinicians in formulating more personalized treatment plans and improving patient prognosis. Through this research, we anticipate enhancing the precision of GIST risk assessment and providing strong scientific and technological support for the implementation of precision medicine. Applying MRI imagomics in GIST management will optimize treatment options for patients, promote the development of personalized medicine, and ultimately improve patients' quality of life and prognosis.
In summary, this study seeks not only to innovate scientifically in the method of GIST risk assessment but also to realize its clinical application, offering new perspectives and tools for diagnosing and treating GIST patients. By delving into the potential of MRI imagomics, this research aims to contribute new knowledge and technology to the precision diagnosis and treatment of GISTs, advancing personalized treatment strategies and bringing greater hope to patients.
This retrospective study analyzed 132 cases of GISTs diagnosed between January 2014 and March 2023 at the Affiliated Hospital of Guangdong Medical University, where patients underwent MRI abdominal-pelvic examinations and were confirmed by surgical and post-operative histopathological data. Disease risk stratification was categorized into four levels-deficient, low, intermediate, and high risk-according to the modified criteria of the National Institute of Health 2008. Following previous literature[22], cases were grouped into low-risk (very low, low, and intermediate risk) and high-risk categories. Inclusion criteria were: (1) Confirmed diagnosis of GIST with MRI examination; (2) No prior anti-tumor treatment; (3) MRI conducted within two weeks before surgery; and (4) Pathological examination determining tumor mitotic figures and grading. Exclusion criteria included: (1) Images assessed by two radiologists with over ten years of experience as poor quality affecting tumor margin delineation; and (2) Tumor maximum diameter < 1 cm or only visible on one MRI image.
The study design and execution strictly adhered to international and national medical ethics standards and regulations. Before participation, all GIST patients were fully informed about the study's purpose, potential risks, benefits, and possible alternative treatments. Stringent confidentiality measures were taken to protect participants' privacy and personal information. After fully understanding the study content and procedures, all participants or their legal representatives voluntarily signed written informed consent forms. The study protocol was approved by the Ethics Review Committee of the Affiliated Hospital of Guangdong Medical University (Approval No. KT2023-087), ensuring compliance with the Declaration of Helsinki and other relevant medical ethics guidelines. Patient welfare was prioritized throughout the research, maintaining the highest ethical standards. Data collection and analysis were conducted with the utmost respect for patient privacy and dignity, ensuring appropriate de-identification of patient information to prevent personal information breaches. This study aimed to enhance the risk stratification assessment of GISTs, aiming to improve clinical treatment strategies through advanced MRI imagomics techniques. The results are expected to provide a more effective scientific basis for the early diagnosis and treatment of GISTs, thereby improving patient treatment outcomes and quality of life.
MRI scans were conducted using a MR750 3.0T scanner (General Electric, United States) with a body surface phased-array abdominal coil. Patients were positioned supine, with the scanning range determined by the tumor lesion. The scanning sequences included axial T2WI fs, axial T1WI with M3D/LABA sequences, and axial DWI with SE/EPI sequences; b-values were set at 0 and 800 s/mm2 for DWI and T1WI fs contrast-enhanced (CE-T1WI fs) was performed, selecting axial images from the venous phase.
DICOM images, including T2WI fs, T1WI, DWI, and CE-T1WI fs venous phase axial sequences, were exported from the PACS system (Version 6.5.2, Global Imaging Technologies, United States) and imported into the A.K. Imagomics system (Version 2.3.1, Radiomics Solutions Inc., United States) for preprocessing, which included resampling and normalization. Two radiologists with 11 and 16 years of experience in abdominal magnetic resonance diagnostics, respectively, manually delineated the target lesions under appropriate window width and level settings, carefully avoiding calcification, hemorrhage, necrosis, and cystic changes within the tumor. The A.K. software then extracted relevant imagomic characterization data from the delineated regions of interest, including first-order statistics, shape, signal intensity, texture features, and higher-order characteristics.
Imagomic characterization data were filtered and dimensionally reduced using the A.K. software through the following methods: (1) Correlation screening, where only one of the imaging characteristics with a correlation coefficient exceeding 0.7 among independent variables was retained; (2) Variance threshold method, retaining independent variables with variance above the threshold of 1.0; (3) Univariate analysis, utilizing t-tests and rank-sum tests for comprehensive data analysis, retaining data with P < 0.05; and (4) Univariate logistic regression, retaining data with P < 0.05 for further analysis. Multivariate logistic regression was employed to model the filtered imagomics characteristics, constructing three single-sequence predictive models for DWI, T1WI, and T2WI fs, and four combined models for T1WI, T2WI fs, DWI, CE-T1WI fs.
After reduction analysis, DWI, T1WI, and T2WI fs yielded 3, 9, and 3 imagomics characteristics respectively; the combined models of T1WI with CE-T1WIfs, T2WI with CE-T1WI fs, DWI with CE-T1WI fs, and the comprehensive T1WI + T2WIfs + DWI + CE-T1WI fs resulted in 5, 9, 4, and 5 remaining characteristics respectively (Figure 1).
Clinical data were analyzed using SPSS software version 25.0 (IBM, United States). The consistency between features and observers was assessed using interclass and intraclass correlation coefficients (ICC), with an ICC > 0.80 indicating good agreement. All metric data were first subjected to normality testing using the Kolmogorov-Smirnov test, with normally distributed data presented as mean ± SD and skewed data described using the median. Comparison of categorical data was performed using the χ2 test or Fisher's exact test, while metric data were compared using the independent samples t-test (for normally distributed data with equal variances) or the Mann-Whitney U test (for skewed distributions or unequal variances), with P < 0.05 considered statistically significant. The area under the curve (AUC) was used as a metric for evaluating predictive efficacy in training and testing groups, using receiver operating characteristic (ROC) curves, where P < 0.05 indicated statistical significance. AUC values are interpreted as follows: 0.500-0.590 is poor; 0.600-0.690 is fair; 0.700-0.790 is good; 0.800-0.890 is very good; 0.900-1.00 is excellent.
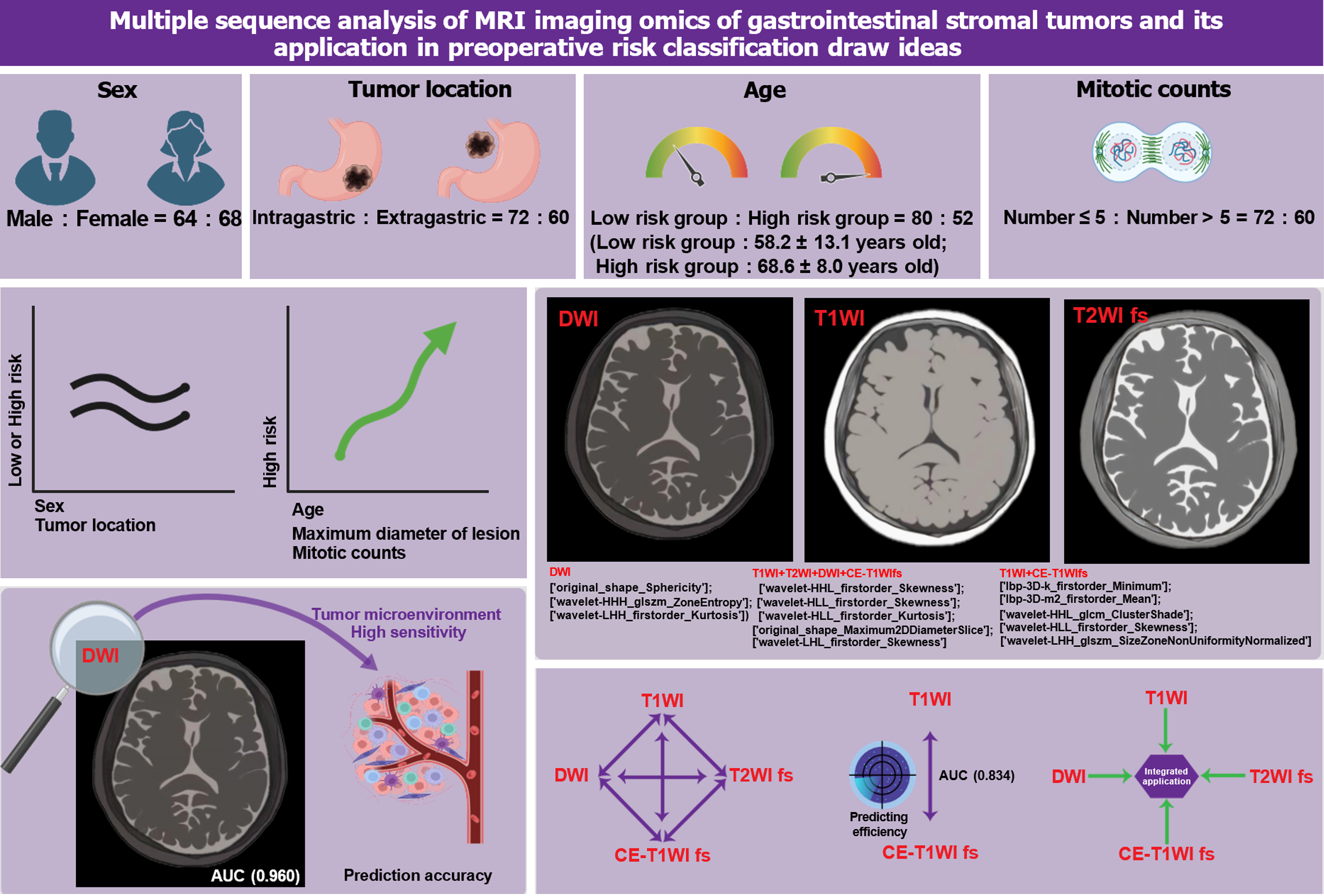
This study meticulously analyzed the data of 132 patients with GISTs to delve deeper into the clinical and disease characteristics of these patients. The sample comprised 64 males and 68 females, showing a relatively balanced distribution across age and gender. Based on tumor location, the cases were further categorized into gastric (72 cases) and extra-gastric (60 cases). Moreover, patients were divided into a low-risk group (80 cases, average age 58.2 ± 13.1 years) and a high-risk group (52 cases, average age 68.6 ± 8.0 years) according to disease risk level. Regarding mitotic figures, 82 patients had ≤ 5, while 50 had > 5. Statistical analysis revealed significant differences between the low and high-risk groups in age, the maximum diameter of the lesion, and several mitotic figures (P < 0.001), indicating their close association with the disease risk level. However, differences in gender (P = 0.482) and tumor location (P = 0.27) were not significant between the different risk groups (Table 1).
| | Low-risk group (n = 80) | High-risk group (n = 52) | P value |
| Age (years) | 58.2 ± 13.1 | 68.6 ± 10.0 | < 0.001 |
| Gender | 0.482 | ||
| Female | 44 (55.0) | 24 (46.2) | |
| Male | 36 (45.0) | 28 (53.8) | |
| Maximal longitudinal diameter (cm) | 5.8 ± 2.5 | 10.2 ± 2.8 | < 0.001 |
| The position of the tumor | 0.270 | ||
| Stomach | 48 (60.0) | 24 (46.2) | |
| Gastric extracorporeal | 32 (40.0) | 28 (53.8) | |
| Mitotic figures in pathology (50/HPF) | < 0.001 | ||
| ≤ 5 | 72 (90.0) | 10 (23.8) | |
| > 5 | 8 (10.0) | 42 (76.2) |
In summary, through a comprehensive comparative analysis of the clinical and disease characteristics of 132 GIST patients, this study identified a significant correlation between age, the maximum diameter of the lesion, and the number of mitotic figures with the disease risk level, while gender and tumor location had minimal impact on the disease risk level.
In the preoperative risk assessment of GISTs, accurately predicting the disease risk level using imagomics features from MRI sequences is crucial. This study aimed to select the optimal set of imagomics features from various MRI sequences through dimension reduction techniques, thereby enhancing the accuracy of GIST risk stratification predictions. Representative imagomics features were selected from DWI, T1WI combined with CE-T1WI fs, and a combination of T1WI, T2WI, DWI, and CE-T1WI fs.
Our detailed analysis identified the following optimal imagomics feature set: For the DWI sequence, features include sphericity of the original shape, regional entropy in the high-high-high frequency after wavelet transformation, and kurtosis in the low-high-high frequency first-order statistics. In the T1WI and CE-T1WI fs sequences, selected features comprise the three-dimensional minimum and average values of the local binary pattern, cluster shade and skewness in the high-high-low and high-low-low frequencies after wavelet transformation, and size-zone non-uniformity normalization in the low-high-high frequency. For the combined T1WI, T2WI, DWI, and CE-T1WI fs set, identified features include skewness and kurtosis in the high-high-low and high-low-low frequencies after wavelet transformation, the maximum two-dimensional diameter slice of the original shape, and skewness in the low-high-low frequency.
In summary, by filtering and analyzing imagomics features across different MRI sequences, this study successfully established an optimal set of features demonstrating significant correlation and predictive value in GIST risk stratification (Table 2). These findings further validate the potential application of MRI imagomics in precision medicine and provide clinicians with a more accurate and reliable tool to assess the preoperative risk level of GIST patients, thereby facilitating more personalized treatment plans.
| Sequence screening | Imaging omics features |
| DWI (3) | [['original_shape_Sphericity'] |
| 'wavelet-HHH_glszm_ZoneEntropy'] | |
| ['wavelet-LHH_firstorder_Kurtosis'] | |
| T1WI + CE-T1WI fs (5) | [['lbp-3D-k_firstorder_Minimum'] |
| ['lbp-3D-m2_firstorder_Mean'] | |
| ['wavelet-HHL_glcm_ClusterShade'] | |
| ['wavelet-HLL_firstorder_Skewness'] | |
| ['wavelet-LHH_glszm_SizeZoneNonUniformityNormalized'] | |
| T1WI + T2WI + DWI +; CE-T1WI fs (5) | ['wavelet-HHL_firstorder_Skewness'] |
| ['wavelet-HLL_firstorder_Skewness'] | |
| ['wavelet-HLL_firstorder_Kurtosis'] | |
| ['original_shape_Maximum2DDiameterSlice'] | |
| ['wavelet-LHL_firstorder_Skewness'] |
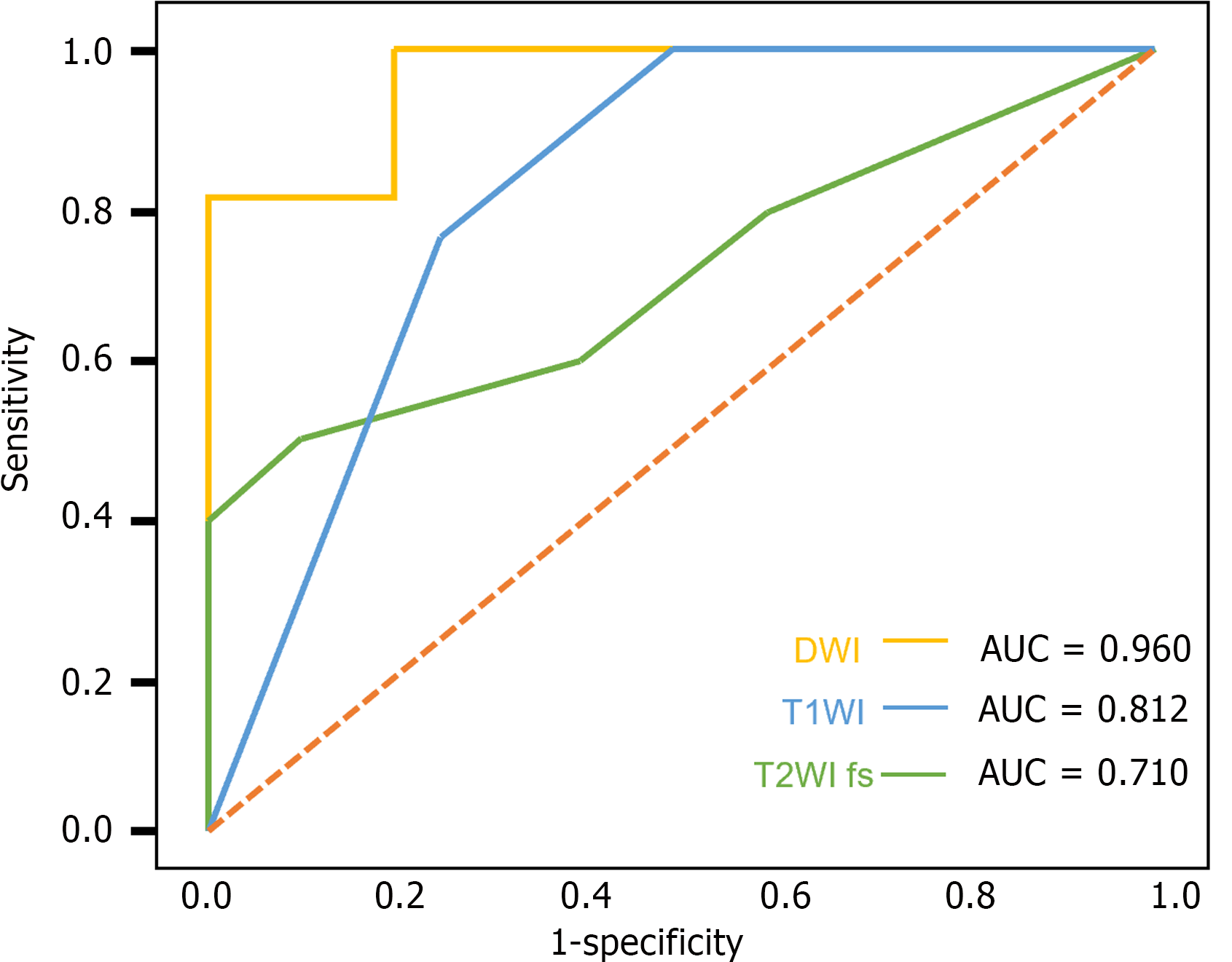
This study further investigated the performance of single-sequence predictive models from different MRI sequences in predicting the risk stratification of GISTs, with a specific focus on DWI, T1WI, and T2WI fs. The aim of analyzing the AUC under the ROC, sensitivity, and specificity of these sequences was to assess their efficacy in predicting GIST risk levels.
The results demonstrated that the DWI sequence had the highest performance in predicting GIST risk stratification, with an AUC value of 0.960 [95% confidence interval (CI): 0.840 to 1.000] in the test group, along with a sensitivity of 80.0% and a specificity of 100.0%. The T1WI and T2WI fs sequences also showed predictive capabilities, but their AUC values, sensitivity, and specificity were lower than those of the DWI sequence. Particularly in the training group, the DWI sequence had an AUC of 0.889 (95%CI: 0.736 to 1.000), a sensitivity of 88.9%, and a specificity of 66.7%, further confirming its superiority in predicting GIST risk stratification (Table 3; Figure 2).
| | Test group | Training group | ||||
| AUC (95%CI) | Sensitivity (%) | Specificity (%) | AUC (95%CI) | Sensitivity (%) | Specificity (%) | |
| DWI | 0.960 (0.840-1.000) | 80.0 | 100.0 | 0.889 (0.736-1.000) | 88.9 | 66.7 |
| T1WI | 0.812 (0.467-1.000) | 75.0 | 100.0 | 0.864 (0.677-1.000) | 66.7 | 100.0 |
| T2WI fs | 0.710 (0.495-0.894) | 60.0 | 60.0 | 0.828 (0.727-0.917) | 82.6 | 65.2 |
In summary, the DWI sequence demonstrated significant performance in the risk stratification prediction of GISTs, particularly noted for its high specificity and AUC values in the test group.
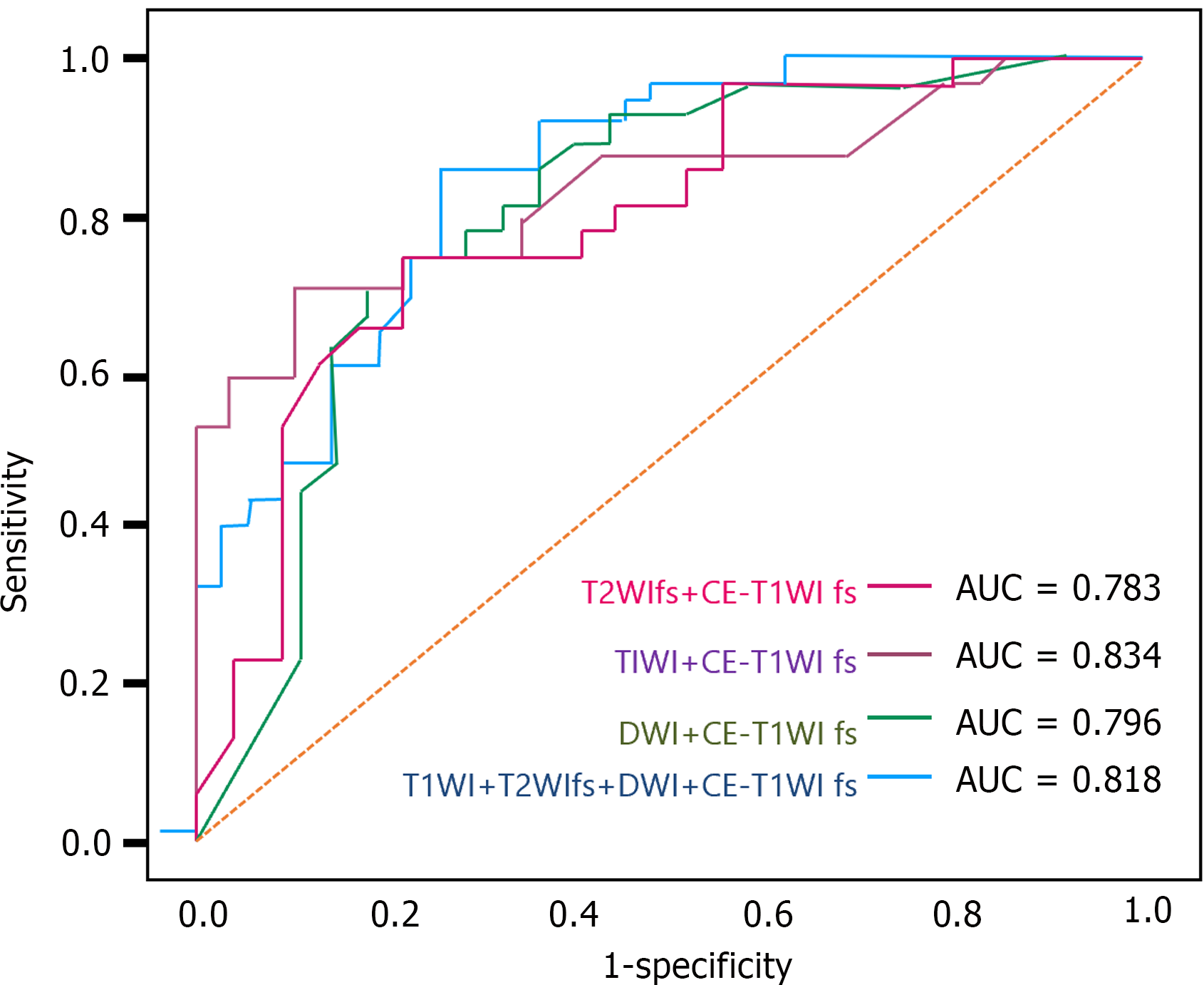
Considering the potential limitations of relying on a single MRI sequence for specific types of GISTs, we further developed and analyzed predictive models based on multiple sequences. The results indicated that the combined multi-sequence predictive model, utilizing T1WI with CE-T1WI fs, demonstrated the best performance in the test group, with an AUC of 0.834 (95%CI: 0.736 to 0.922), a sensitivity of 70.4%, and a specificity of 85.2%. In the training group, this model's AUC increased to 0.917 (95%CI: 0.825 to 0.984), with a sensitivity of 77.8% and a specificity of 88.9%. Other multi-sequence models also showed good predictive performance, although slightly inferior to the T1WI and CE-T1WI fs combined models. The model integrating all sequences achieved an AUC of 0.818 in the test group, indicating good predictive accuracy, albeit slightly lower sensitivity, but demonstrating reasonable specificity. Unlike single-sequence models, multi-sequence models offer a more comprehensive risk assessment, potentially enhancing prediction precision and reliability (Table 4; Figure 3). In summary, predictive models constructed from integrating multiple MRI sequences exhibit higher predictive efficacy in GIST risk stratification, particularly the T1WI and CE-T1WI fs combined model, which maintains good sensitivity while ensuring high specificity.
| | Test group | Training group | ||||
| AUC (95%CI) | Sensitivity (%) | Specificity (%) | AUC (95%CI) | Sensitivity (%) | Specificity (%) | |
| TIWI + CE-T1WI fs | 0.834 (0.736-0.922) | 70.4 | 85.2 | 0.917 (0.825-0.984) | 77.8 | 88.9 |
| T2WIfs + CE-T1WI fs | 0.783 (0.661-0.893) | 73.9 | 73.0 | 0.866 (0.769-0.949) | 73.9 | 91.3 |
| DWI + CE-T1WI fs | 0.796 (0.684-0.899) | 70.4 | 77.8 | 0.825 (0.714-0.917) | 70.8 | 79.2 |
| T1WI + T2WIfs + DWI + CE-T1WI fs | 0.818 (0.727-0.897) | 60.0 | 77.1 | 0.770 (0.567-0.938) | 70.0 | 80.0 |
Risk assessment of GISTs is crucial for guiding treatment decisions[23,24]. MRI imagomics substantially enhances the precision of preoperative risk assessments by providing detailed morphological and textural analysis of tumors. For example, features such as sphericity and heterogeneity derived from DWI sequences correlate strongly with tumor aggressiveness, influencing risk stratification and surgical planning[25]. By incorporating these features, clinicians can better determine the risk category of GISTs, potentially avoiding over-treatment for low-risk cases and under-treatment for high-risk cases. Traditional risk assessments rely on clinical and pathological indicators such as tumor size and mitotic figures, which significantly predict tumor behavior[26,27]. Previous literature has confirmed that larger tumor volumes are associated with higher malignant potential, and the count of mitotic figures is closely related to the tumor's proliferation, invasion, and metastasis capabilities[28,29]. Our findings support this, showing significant differences in the maximum tumor diameter and mitotic figure count between high and low-risk GISTs, consistent with previous research[5,30,31].
However, the predictive value of tumor location has been contentious in prior studies. Some research suggests significant risk level differences in GISTs based on location, while other studies indicate a weaker correlation between tumor location and invasiveness[1,32,33]. In our study, the difference in tumor location between different risk level groups was not statistically significant, aligning with the findings of Mao et al[34], suggesting that evaluating GIST risk levels based solely on tumor location may not provide a comprehensive assessment.
This study established multi-sequence predictive models using MRI imagomics and evaluated their predictive performance through ROC curves. All single and multi-sequence models achieved good to excellent predictive efficacy, especially the DWI sequence model, which demonstrated superior performance with an AUC value exceeding 0.9. This outcome underscores the significant application value of the DWI sequence in GIST risk stratification, supporting the ability of DWI and its ADC values to assess tumor cell density and growth rates. These findings align with previous research, confirming DWI sequence models' high reliability and accuracy in GIST risk assessment[22].
Furthermore, this study explored imagomics models incorporating venous phase enhanced scan images and found these models also achieved good to excellent predictive efficacy. Notably, the model combining T1WI and CE-T1WI fs sequences performed best in the test group, with an AUC value reaching 0.834. This discovery highlights the importance of combining MRI sequences in GIST risk assessment, offering new avenues to enhance predictive accuracy.
This study demonstrates significant clinical value by predicting the preoperative risk levels of GISTs using MRI imagomics. Notably, the high predictive performance of the DWI sequence model (AUC > 0.9) indicates its potential to provide clinicians with critical information on the malignant potential of GISTs before surgery. This is crucial for formulating personalized treatment plans, selecting the most appropriate surgical strategies, and determining the necessity for adjuvant therapy, such as imatinib. Additionally, the application of imagomics models combining different MRI sequences further improves the accuracy of risk assessments, enabling more precise clinical decision-making, optimizing treatment outcomes, reducing unnecessary interventions, and achieving the goal of precision medicine in patient management. By enabling a more accurate prediction of tumor behavior, MRI imagomics contributes to personalized patient management and improved outcomes. High predictive accuracy, particularly in models incorporating multiple MRI sequences, offers clinicians tools to tailor follow-up and treatment strategies effectively[35]. This approach helps in reducing unnecessary interventions for patients with lower-risk GISTs and intensifying monitoring for those at higher risk, thus potentially improving overall survival and quality of life. Therefore, the findings of this study offer new perspectives and tools for the clinical management of GISTs, potentially improving patient prognosis and quality of life (Figure 4).
Despite significant progress in GIST risk assessment, this study has limitations. First, its retrospective design may introduce selection bias, particularly because high-grade tumors are more likely to be confirmed by surgical pathology, potentially leading to a sample bias that includes more high-grade tumors. Secondly, the relatively small sample size might limit the generalizability of the results. These limitations could impact the precision of the model's predictive performance. Second, manual region of interest (ROI) delineation could affect the precision of imagomics feature extraction, impacting model predictive performance. Additionally, selecting only venous phase images for enhanced scans might only partially represent some tumor information. Furthermore, being a single-center study with a relatively small sample size might limit the generalizability of the results. Finally, not considering genetic and gene mutation factors such as KIT and PDGFRA mutations could affect the diagnosis and prediction of complex cases.
Future research should adopt multicenter, large-scale prospective designs to enhance the findings' general applicability and accuracy. Developing and applying automatic or semi-automatic ROI delineation methods could improve the precision and repeatability of imagomics feature extraction. Future studies should also consider different phases of enhanced scans and possibly integrate other advanced imaging technologies, like PET/MRI, to provide more comprehensive biological information on tumors. Moreover, considering the significant role of genetic and gene mutations in GIST pathogenesis, future imagomics studies should incorporate these molecular biological parameters into risk assessment models to explore the correlation between imaging phenotypes and genotypes. Through these efforts, future research could further enhance the accuracy and clinical value of GIST risk assessment, offering patients more precise and personalized treatment strategies.
In conclusion, this study highlights the significant potential of MRI imagomics in enhancing the precision of GIST risk stratification. The high predictive performance of the DWI sequence model, along with the combined T1WI and CE-T1WI fs models, underscores the value of integrating advanced imaging features into clinical practice. These findings support the use of MRI imagomics for more accurate preoperative assessments, aiding in personalized treatment planning and improving patient outcomes. Despite limitations, this study paves the way for future research to further refine these models and incorporate genetic factors, ultimately contributing to more effective and individualized GIST management.
| 1. | Blay JY, Kang YK, Nishida T, von Mehren M. Gastrointestinal stromal tumours. Nat Rev Dis Primers. 2021;7:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 248] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 2. | Casali PG, Blay JY, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brodowicz T, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dufresne A, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Frezza AM, Gasperoni S, Gelderblom H, Gouin F, Grignani G, Haas R, Hassan AB, Hindi N, Hohenberger P, Joensuu H, Jones RL, Jungels C, Jutte P, Kasper B, Kawai A, Kopeckova K, Krákorová DA, Le Cesne A, Le Grange F, Legius E, Leithner A, Lopez-Pousa A, Martin-Broto J, Merimsky O, Messiou C, Miah AB, Mir O, Montemurro M, Morosi C, Palmerini E, Pantaleo MA, Piana R, Piperno-Neumann S, Reichardt P, Rutkowski P, Safwat AA, Sangalli C, Sbaraglia M, Scheipl S, Schöffski P, Sleijfer S, Strauss D, Strauss SJ, Hall KS, Trama A, Unk M, van de Sande MAJ, van der Graaf WTA, van Houdt WJ, Frebourg T, Gronchi A, Stacchiotti S; ESMO Guidelines Committee, EURACAN and GENTURIS. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 325] [Article Influence: 108.3] [Reference Citation Analysis (1)] |
| 3. | Jacobson BC, Bhatt A, Greer KB, Lee LS, Park WG, Sauer BG, Shami VM. ACG Clinical Guideline: Diagnosis and Management of Gastrointestinal Subepithelial Lesions. Am J Gastroenterol. 2023;118:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 69] [Reference Citation Analysis (0)] |
| 4. | Song DJ, Yang LT. [Advances in recurrence risk assessment of gastrointestinal stromal tumor]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Nowak K, Formenti K, Huang J, Bigras G, Chu Q, Adam BA, Izevbaye I. Risk stratification of gastrointestinal stromal tumors by Nanostring gene expression profiling. J Cancer Res Clin Oncol. 2022;148:1325-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 6. | Grazzini G, Guerri S, Cozzi D, Danti G, Gasperoni S, Pradella S, Miele V. Gastrointestinal stromal tumors: relationship between preoperative CT features and pathologic risk stratification. Tumori. 2021;107:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Webb EM, Mongan J. Gastrointestinal Stromal Tumors: Radiomics may Increase the Role of Imaging in Malignant Risk Assessment. Acad Radiol. 2022;29:817-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Suarez-Ibarrola R, Basulto-Martinez M, Heinze A, Gratzke C, Miernik A. Radiomics Applications in Renal Tumor Assessment: A Comprehensive Review of the Literature. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Shah J, Rahman Siddiquee MM, Krell-Roesch J, Syrjanen JA, Kremers WK, Vassilaki M, Forzani E, Wu T, Geda YE. Neuropsychiatric Symptoms and Commonly Used Biomarkers of Alzheimer's Disease: A Literature Review from a Machine Learning Perspective. J Alzheimers Dis. 2023;92:1131-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Zheng D, He X, Jing J. Overview of Artificial Intelligence in Breast Cancer Medical Imaging. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Zheng D, Grandgenett PM, Zhang Q, Baine M, Shi Y, Du Q, Liang X, Wong J, Iqbal S, Preuss K, Kamal A, Yu H, Du H, Hollingsworth MA, Zhang C. radioGWAS: link radiome to genome to discover driver genes with somatic mutations for heterogeneous tumor image phenotype in pancreatic cancer. medRxiv. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Omeh DJ, Shlofmitz E. Angiography. 2023 Aug 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 13. | Özdemir M, Das JM. Skull Imaging. 2023 Jan 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 14. | Dobaria DG, Cohen HL. Osteomyelitis Imaging. 2023 Aug 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 15. | Das IJ, McGee KP, Tyagi N, Wang H. Role and future of MRI in radiation oncology. Br J Radiol. 2019;92:20180505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Bai Y, Zhang R, Zhang X, Wang X, Nittka M, Koerzdoerfer G, Gong Q, Wang M. Magnetic Resonance Fingerprinting for Preoperative Meningioma Consistency Prediction. Acad Radiol. 2022;29:e157-e165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Wang H, van der Velden BHM, Ragusi MAA, Veldhuis WB, Viergever MA, Verburg E, Gilhuijs KGA. Toward Computer-Assisted Triaging of Magnetic Resonance Imaging-Guided Biopsy in Preoperative Breast Cancer Patients. Invest Radiol. 2021;56:442-449. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Huang X, Shu J, Yan Y, Chen X, Yang C, Zhou T, Li M. Feasibility of magnetic resonance imaging-based radiomics features for preoperative prediction of extrahepatic cholangiocarcinoma stage. Eur J Cancer. 2021;155:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Lyu Q, Lin D, Tang M, Liu D, Zhang J, Wang Y, Shelat VG, Raissi D, Ostwal V, Chen X, Li S. (18)F-FDG PET/CT and MR imaging features of liver metastases in gastrointestinal stromal tumors: a cross-sectional analysis. Ann Transl Med. 2022;10:1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Nagano H, Ohyama S, Sato A, Igarashi J, Yamamoto T, Kadoya M, Kobayashi M. Jejunal gastrointestinal stromal tumor that developed in a patient with neurofibromatosis type 1: a case report. Diagn Pathol. 2023;18:110. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Ibrahim AM, Le May M, Bossé D, Marginean H, Song X, Nessim C, Ong M. Imaging Intensity and Survival Outcomes in High-Risk Resected Melanoma Treated by Systemic Therapy at Recurrence. Ann Surg Oncol. 2020;27:3683-3691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Yang L, Zheng T, Dong Y, Wang Z, Liu D, Du J, Wu S, Shi Q, Liu L. MRI Texture-Based Models for Predicting Mitotic Index and Risk Classification of Gastrointestinal Stromal Tumors. J Magn Reson Imaging. 2021;53:1054-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Hemming ML, Coy S, Lin JR, Andersen JL, Przybyl J, Mazzola E, Abdelhamid Ahmed AH, van de Rijn M, Sorger PK, Armstrong SA, Demetri GD, Santagata S. HAND1 and BARX1 Act as Transcriptional and Anatomic Determinants of Malignancy in Gastrointestinal Stromal Tumor. Clin Cancer Res. 2021;27:1706-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Husain NE, Osman IM, Khalid A, Satir AA, Stoehr R, Agaimy A. Clinicopathological, immunohistochemical, molecular-genetic and risk profiles of gastrointestinal stromal tumors in a cohort of Sudanese patients. Afr Health Sci. 2023;23:444-458. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Avanzo M, Wei L, Stancanello J, Vallières M, Rao A, Morin O, Mattonen SA, El Naqa I. Machine and deep learning methods for radiomics. Med Phys. 2020;47:e185-e202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 321] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Wang Y, Ren J, Jia L, Ma L, Yin X, Yang F, Gao BL. Malignancy risk of gastrointestinal stromal tumors evaluated with noninvasive radiomics: A multi-center study. Front Oncol. 2022;12:966743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 27. | Cannella R, Tabone E, Porrello G, Cappello G, Gozzo C, Incorvaia L, Grignani G, Merlini A, D'Ambrosio L, Badalamenti G, Regge D, Bartolotta TV. Assessment of morphological CT imaging features for the prediction of risk stratification, mutations, and prognosis of gastrointestinal stromal tumors. Eur Radiol. 2021;31:8554-8564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Serrano C, García-Del-Muro X, Valverde C, Sebio A, Durán J, Manzano A, Pajares I, Hindi N, Landolfi S, Jiménez L, Rubió-Casadevall J, Estival A, Lavernia J, Safont MJ, Pericay C, Díaz-Beveridge R, Martínez-Marín V, Vicente-Baz D, Vivancos A, Hernández-Losa J, Arribas J, Carles J. Clinicopathological and Molecular Characterization of Metastatic Gastrointestinal Stromal Tumors with Prolonged Benefit to Frontline Imatinib. Oncologist. 2019;24:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Lino-Silva LS, Segales-Rojas P, Aguilar-Cruz E, Salcedo-Hernández RA, Zepeda-Najar C. Gastrointestinal Stromal Tumors Risk of Recurrence Stratification by Tumor Volume is a Best Predictor Compared with Risk Based on Mitosis and Tumor Size. J Gastrointest Cancer 2019; 50: 513-518 [PMID: 29766411 DOI: 10.1007/s12029-018-0115-2] 30 Delvaux M, Hagué P, Craciun L, Wozniak A, Demetter P, Schöffski P, Erneux C, Vanderwinden JM. Ferroptosis Induction and YAP Inhibition as New Therapeutic Targets in Gastrointestinal Stromal Tumors (GISTs). Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 30. | Delvaux M, Hagué P, Craciun L, Wozniak A, Demetter P, Schöffski P, Erneux C, Vanderwinden JM. Ferroptosis Induction and YAP Inhibition as New Therapeutic Targets in Gastrointestinal Stromal Tumors (GISTs). Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 31. | Erhan SS, Sensu S, Keser SH, Kangal E, Gul AE, Gundogan GA, Sakin A. Mitotic Activity in Gastrointestinal Stromal Tumors: Can we use Phosphohistone H3 Immunohistochemistry Instead of Hematoxylin and Eosin for Mitotic Count? Sisli Etfal Hastan Tip Bul. 2022;56:276-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 32. | Kelly CM, Gutierrez Sainz L, Chi P. The management of metastatic GIST: current standard and investigational therapeutics. J Hematol Oncol. 2021;14:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 165] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 33. | Deprez PH, Moons LMG, OʼToole D, Gincul R, Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M, Vieth M, Borbath I, Moreels TG, Nieveen van Dijkum E, Blay JY, van Hooft JE. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:412-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 197] [Article Influence: 65.7] [Reference Citation Analysis (1)] |
| 34. | Mao H, Zhang B, Zou M, Huang Y, Yang L, Wang C, Pang P, Zhao Z. MRI-Based Radiomics Models for Predicting Risk Classification of Gastrointestinal Stromal Tumors. Front Oncol. 2021;11:631927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Yang G, Bai J, Hao M, Zhang L, Fan Z, Wang X. Enhancing recurrence risk prediction for bladder cancer using multi-sequence MRI radiomics. Insights Imaging. 2024;15:88. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |