Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.2778
Revised: May 30, 2024
Accepted: August 1, 2024
Published online: September 27, 2024
Processing time: 196 Days and 10.6 Hours
Whether hepatocellular carcinoma (HCC) with portal vein tumor thrombus (PVTT) and acute esophagogastric variceal bleeding (EGVB) can improve the success rate of endoscopic hemostasis and overall survival (OS) from transjugular intrahepatic portosystemic shunt (TIPS) remains controversial.
To compare the clinical outcomes between TIPS and standard treatment for such HCC patients.
This monocenter, retrospective cohort study included patients diagnosed as HCC with PVTT and upper gastrointestinal bleeding. Patients were grouped by the treatment (TIPS or standard conservative treatment). The success rate of en
Between July 2015 and September 2021, a total of 77 patients (29 with TIPS and 48 with standard treatment) were included. The success rate of endoscopic hemostasis was 96.6% in the TIPS group and 95.8% in the standard treatment group. All the 29 patients in TIPS group successful underwent TIPS procedure and had a better OS compared with standard treatment within the first 160 days after treatment (68 days vs 43 days, P = 0.022), but shorter OS after 160 days (298 days vs 472 days, P = 0.022). Cheng’s Classification of PVTT, total bilirubin and Child-Pugh class were independently negative associated with OS (all P < 0.05). The main causes of death were liver failure or hepatic encephalopathy (75.9%) in the TIPS group and rebleeding (68.8%) in the standard treatment.
TIPS could reduce the risk of early death due to rebleeding and prolong short-term survival in HCC patients with PVTT and acute EGVB, which deserves further investigation.
Core Tip: This study compared the clinical outcomes between transjugular intrahepatic portosystemic shunt (TIPS) treatment and standard conservative treatment in hepatocellular carcinoma patients with portal vein tumor thrombus and acute esophagogastric variceal bleeding. And showed that TIPS could reduce the risk of rebleeding by decreasing portal pressure, leading to prolonged short-term survival in hepatocellular carcinoma patients with portal vein tumor thrombus and acute esophagogastric variceal bleeding compared with standard conservative treatment. The safety of TIPS was also acceptable.
- Citation: Wu ZQ, Wang F, Wang FP, Cai HJ, Chen S, Yang JY, Guo WB. Transjugular intrahepatic portosystemic shunt for esophagogastric variceal bleeding in patients with hepatocellular carcinoma and portal vein tumor thrombus. World J Gastrointest Surg 2024; 16(9): 2778-2786
- URL: https://www.wjgnet.com/1948-9366/full/v16/i9/2778.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i9.2778
Hepatocellular carcinoma (HCC) is the sixth most commonly diagnosed cancer and the third leading cause of cancer death worldwide, with approximately 906000 new cases and 830000 deaths in 2020[1]. Portal vein tumor thrombus (PVTT) is found in 10%-40% of patients at diagnosis of HCC[2], which is one of the worst prognostic factors[3]. The possible reason is that PVTT can cause further aggravation of portal hypertension and deterioration of liver function, followed by an increased risk of acute esophagogastric variceal bleeding (EGVB)[4], which is the direct cause of death for some HCC patients especially in the decompensated stage of cirrhosis.
In recent years, with the development of targeted therapy and immunotherapy, the prognosis of HCC patients with Barcelona Clinic for Liver Cancer C stage has been significantly improved, and the median overall survival (OS) has increased from 2.7 months[5] to 19.2 months[6]. However, it should be noted that the incidence of EGVB also increased at the same time[7]. Endoscopic treatment remains the mainstay treatment for acute EGVB, usually in combination with pharmacotherapy to reduce portal pressure[8]. However, previous studies have shown that the outcomes after endoscopic treatment were unsatisfactory, with 10%-20% failure and inevitable rebleeding within 48-72 hours[9-11]. When endoscopic treatment failed to control EGVB, other non-endoscopic rescue treatment approaches (such as hemostatic drug and shunt) should be considered[8].
At present, HCC with PVTT and acute EGVB is still considered as a contraindication of transjugular intrahepatic portosystemic shunt (TIPS)[12,13]. Due to the safety concern, the evidence of TIPS in this population is still scarce. Some previous studies showed that these patients might benefit from TIPS, with a median survival of 2.6 to 12 months[14-18]. However, these studies had small sample size and did not compare with standard treatment, with a relatively short observation period. Whether HCC patients with PVTT and acute EGVB can benefit from TIPS or not is still controversial. Therefore, this aimed to compare the clinical outcomes between TIPS treatment and standard conservative treatment in HCC patients with PVTT and acute EGVB.
Patients diagnosed as HCC with PVTT and upper gastrointestinal bleeding in our hospital between July 2015 and September 2021 were analyzed in this retrospective cohort study. The inclusion criteria included: (1) Diagnosed with HCC and PVTT by computed tomography (CT) or magnetic resonance imaging[19,20]; (2) Acute EGVB for the first time, confirmed by gastroscopy; and (3) Child-Pugh score ≤ 14. The exclusion criteria included: (1) Gastroduodenal ulcer bleeding, diverticulum bleeding, or other bleeding of unknown causes by gastroscopy; (2) Uncontrolled hepatic encephalopathy of grade III or above at admission; (3) Eastern Cooperative Oncology Group performance status > 3 at admission, which could not be reduced to 2 or below after medical treatment; (4) Death within 24 hours after admission; and (5) History or plan of liver transplantation. The study was approved by the Ethics Committee of our hospital. It conformed to the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of the study.
Patients were grouped according to the treatment (TIPS or standard conservative treatment) after endoscopic treatment. All TIPS procedures were performed by experienced physicians with covered stents from Boston Science and Technology Company or Gore Company. Bare stents from Abbott or Cook Incorporated were used in the main portal vein, splenic vein and superior mesenteric vein in some patients.
Baseline clinical data were collected from medical records, including time from bleeding to admission, complications (such as hepatic encephalopathy) and comorbidities. The information of endoscopic findings (gastroscopic variceal classification[21] and degree, red-color sign or not), endoscopic treatment, blood transfusion and hospitalization days were collected.
Laboratory data included: Blood ammonia, hemoglobin, white blood cell count, platelet, creatinine, cholinesterase, prealbumin, albumin, prothrombin time, international normalized ratio, and total bilirubin (TBIL) classification (grade 1, no more than 34.1 μmol/L; grade 2, 34.2-51.2 μmol/L; grade 3, at least 51.3 μmol/L).
Imaging by CT or magnetic resonance imaging included: Tumor number, largest tumor diameter, extrahepatic metastasis, arterio-portal venous fistulas, cavernous transformation of portal vein, Cheng’s Classification[22] of PVTT, and ascites and volume. Cheng’s Classification[22] of PVTT was applied in this study by two experienced radiologists based on CT images. Tumor number, largest tumor diameter, extrahepatic metastasis, cavernous transformation of portal vein and intrahepatic arterio-portal venous fistulas were also determined by two radiologists.
Outcomes included the success rate of endoscopic hemostasis, success rate of surgical technique, surgical complications, OS, rebleeding rates, and main causes of death (liver failure, tumor progression dyscrasia, hematemesis, accident, etc.). OS was defined as the time from hospitalization due to hematemesis (PVTT was confirmed at the time of hematemesis or before hematemesis) to any-cause death. The change in portal pressure after stent placement were analyzed as well. The occurrence and episodes of rebleeding or hepatic encephalopathy requiring hospitalization were collected from follow-up records. All patients were followed once a month within half a year after discharge or when they returned to hospital for examination and treatment for other reasons.
Continuous variables were expressed as mean ± SD and t-test was used for pairwise comparison. Categorical variables were expressed as frequency (percentage) and χ2 test was used to compare the differences between groups. OS was analyzed by Kaplan-Meier method and log-rank test. Landmark and Tarone-Ware analysis was used when survival curves crossed. Multivariate Cox regression model was applied to evaluate the associated factors of OS. The variables with P < 0.10 in the univariate analysis were included in the subsequent multivariate analysis. P < 0.05 was considered statistically significant. SPSS 22.0 (IBM, Chicago, IL, United States) and R software 4.0 were used for statistical analysis.
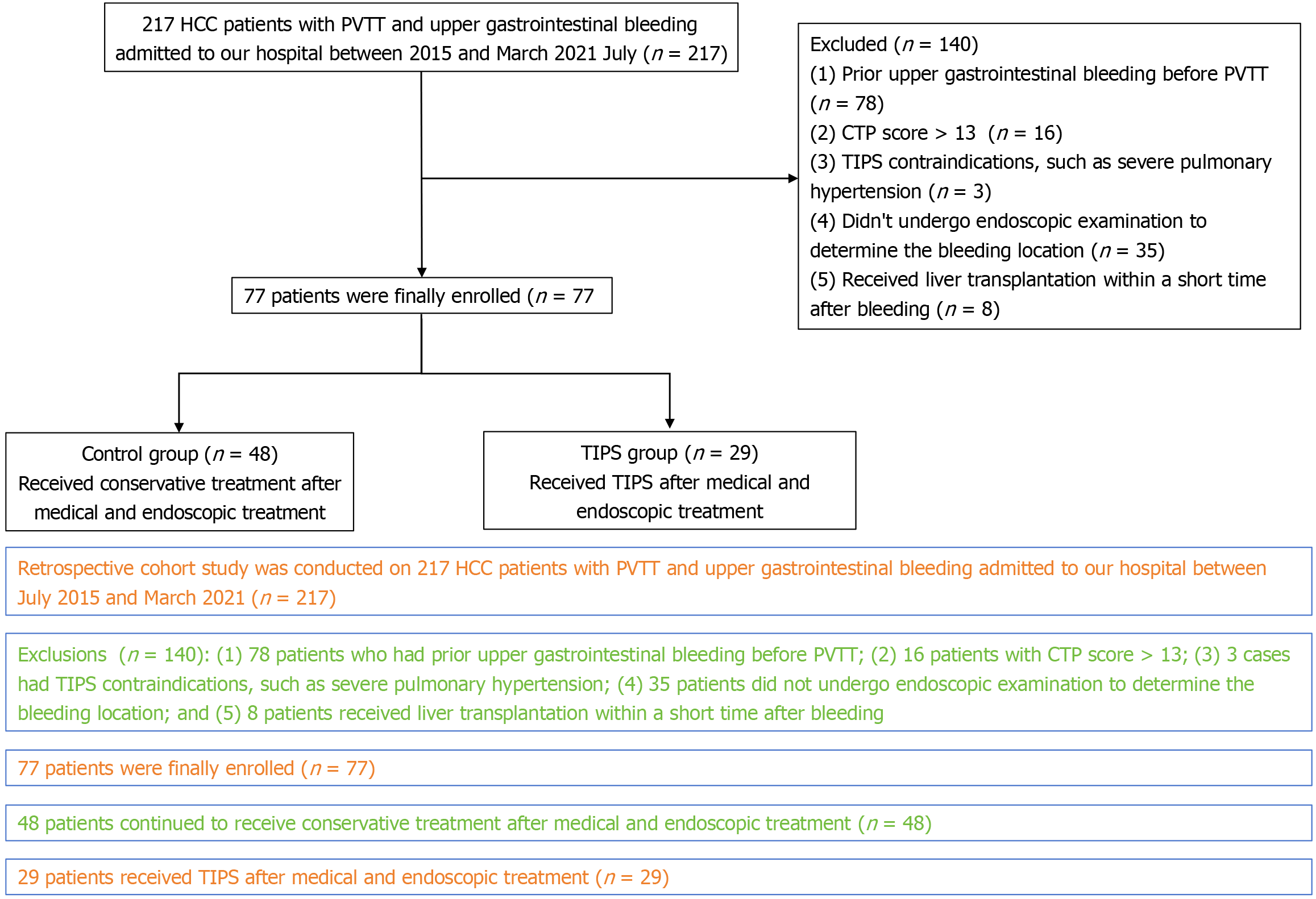
A total of 217 HCC patients with PVTT and upper gastrointestinal bleeding were screened, and 77 patients were included in this study, including 29 in the TIPS group and 48 in the standard treatment group (Figure 1). The baseline characteristics were shown in Table 1. Patients with TIPS had younger age (49.1 ± 12.0 vs 55.8 ± 11.2 years, P = 0.015), lower TBIL (30.3 ± 17.0 vs 53.1 ± 56.3 μmol/L, P = 0.038), but more HCC-related treatment (89.7% vs 68.8%, P = 0.036) than those in standard treatment group. The other clinical, laboratory, and imaging data showed no statistical differences between the two groups.
| Characteristic | Standard treatment (n = 48) | TIPS (n = 29) | P value |
| Gender, n (%) | 0.269 | ||
| Male | 43 (89.6) | 28 (96.6) | |
| Female | 5 (10.4) | 1 (3.4) | |
| Age, year, mean ± SD | 55.8 ± 11.2 | 49.1 ± 12.0 | 0.015 |
| ECOG PS, n (%) | 0.149 | ||
| 0 | 34 (70.8) | 17 (58.6) | |
| 1 | 2 (4.2) | 5 (17.2) | |
| 2 | 12 (25.0) | 7 (24.1) | |
| Hepatitis, n (%) | 42 (87.5) | 26 (89.7) | 0.775 |
| Hepatic encephalopathy, n (%) | 0.864 | ||
| No | 44 (91.8) | 26 (89.7) | |
| Mild | 2 (4.2) | 2 (6.9) | |
| Moderate and severe | 2 (4.2) | 1 (3.4) | |
| Ascites, n (%) | 0.540 | ||
| No | 2 (4.2) | 2 (6.9) | |
| Little | 9 (18.8) | 8 (27.6) | |
| Moderate and severe | 37 (77.1) | 19 (65.5) | |
| Blood transfusion, n (%) | 24 (50.0) | 11 (37.9) | 0.192 |
| Hemostatic drugs, n (%) | 0.290 | ||
| None | 24 (50.0) | 12 (41.4) | |
| Somatostatin | 4 (8.3) | 2 (6.9) | |
| Octreotide | 23 (47.9) | 15 (51.7) | |
| Terlipressin | 0 | 3 (10.3) | |
| Endoscopic treatment, n (%) | 0.733 | ||
| None | 15 (31.3) | 10 (34.5) | |
| Ligation | 27 (56.3) | 16 (55.2) | |
| Sclerosing agent | 7 (14.6) | 6 (20.7) | |
| Tissue colloid | 12 (25.0) | 8 (27.6) | |
| EGV classification, n (%) | 0.862 | ||
| EV | 17 (35.4) | 12 (41.4) | |
| EGV1 | 15 (31.3) | 7 (24.1) | |
| EGV2 | 8 (16.7) | 6 (20.7) | |
| Others | 8 (16.7) | 4 (13.8) | |
| Red-color sign, n (%) | 44 (91.7) | 26 (89.7) | 0.766 |
| EGV degree, n (%) | 0.279 | ||
| Moderate | 3 (6.3) | 3 (10.3) | |
| Severe | 31 (64.6) | 22 (75.9) | |
| Untyped | 14 (29.2) | 4 (13.8) | |
| APVF, n (%) | 2 (4.2) | 3 (10.3) | 0.286 |
| CTPV, n (%) | 10 (20.8) | 9 (31.0) | 0.314 |
| Tumor lesion number, n (%) | 0.934 | ||
| 1 | 17 (35.4) | 10 (34.5) | |
| ≥ 2 | 31 (64.6) | 19 (65.5) | |
| Largest tumor diameter, cm, mean ± SD | 87.63 ± 49.25 | 74.24 ± 39.14 | 0.264 |
| Extrahepatic metastasis | 56.3 (27) | 51.7 (15) | 0.699 |
| Cheng’s Classification, n (%) | 0.279 | ||
| I-II | 13 (27.1) | 11 (37.9) | |
| III | 28 (58.3) | 16 (55.2) | |
| IV | 7 (14.6) | 2 (6.9) | |
| ALB, g/L, mean ± SD | 30.6 ± 5.4 | 31.5 ± 4.8 | 0.450 |
| TBIL, μmol/L, mean ± SD | 53.1 ± 56.3 | 30.3 ± 17.0 | 0.038 |
| INR, mean ± SD | 1.3 ± 0.4 | 1.3 ± 0.2 | 0.442 |
| PT, mean ± SD | 15.7 ± 5.1 | 15.1 ± 2.0 | 0.521 |
| Hb, g/L, mean ± SD | 92.25 ± 22.33 | 92.41 ± 23.65 | 0.976 |
| PLT, g/L, mean ± SD | 155.04 ± 93.28 | 128.17 ± 82.53 | 0.205 |
| CREA, μmol/L, mean ± SD | 80.88 ± 47.46 | 67.17 ± 16.43 | 0.138 |
| HCC-related treatment, n (%) | 33 (68.8) | 26 (89.7) | 0.036 |
All patients were followed up to March 2021 or until their death, with a median follow-up time of 420 days. The success rate of endoscopic hemostasis was 96.6% (28/29) in the TIPS group. The only failed case survived for more than 2 years after successful hemostasis with salvage TIPS, and died of multiple organ dysfunction syndrome due to tumor progression. The success rate of surgical technique was 100% (29/29) for all patients in TIPS group, and the median time from admission to surgery was 6.2 days. The portal pressure decreased from 34.2 ± 14.4 mmHg before operation to 23.2 ± 12.3 mmHg after operation. Correspondingly, the success rate of endoscopic hemostasis in the standard treatment group was 95.8% (46/48), and the two failed cases died within 2 days after endoscopic treatment.
There were no serious surgical complications or deaths within 2 weeks after TIPS treatment.
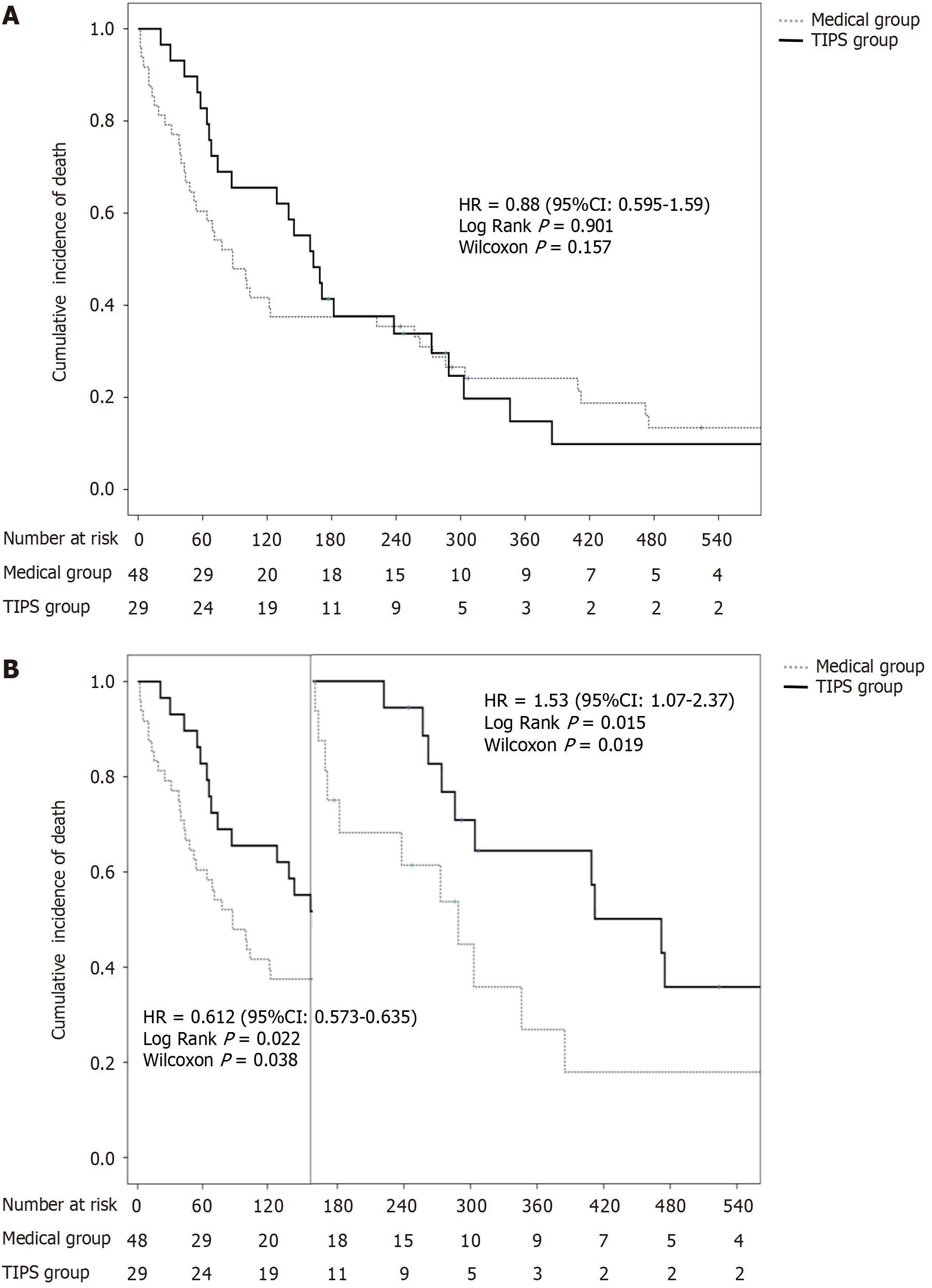
There was no significant difference in median OS between the TIPS group and standard treatment group [181 days vs 100 days, log-rank P = 0.624, Wilcoxon P = 0.157, hazard ratio (HR) = 0.880]. During the follow-up of the first 160 days, the median OS of the TIPS group was better than that of the standard treatment group (68 days vs 43 days, log-rank P = 0.022, Wilcoxon P = 0.038, HR = 0.612). After 160 days, the median OS of the TIPS group was shorter than that of the standard treatment group (298 days vs 472 days, log-rank P = 0.022, Wilcoxon P = 0.015, HR = 0.019; Figure 2).
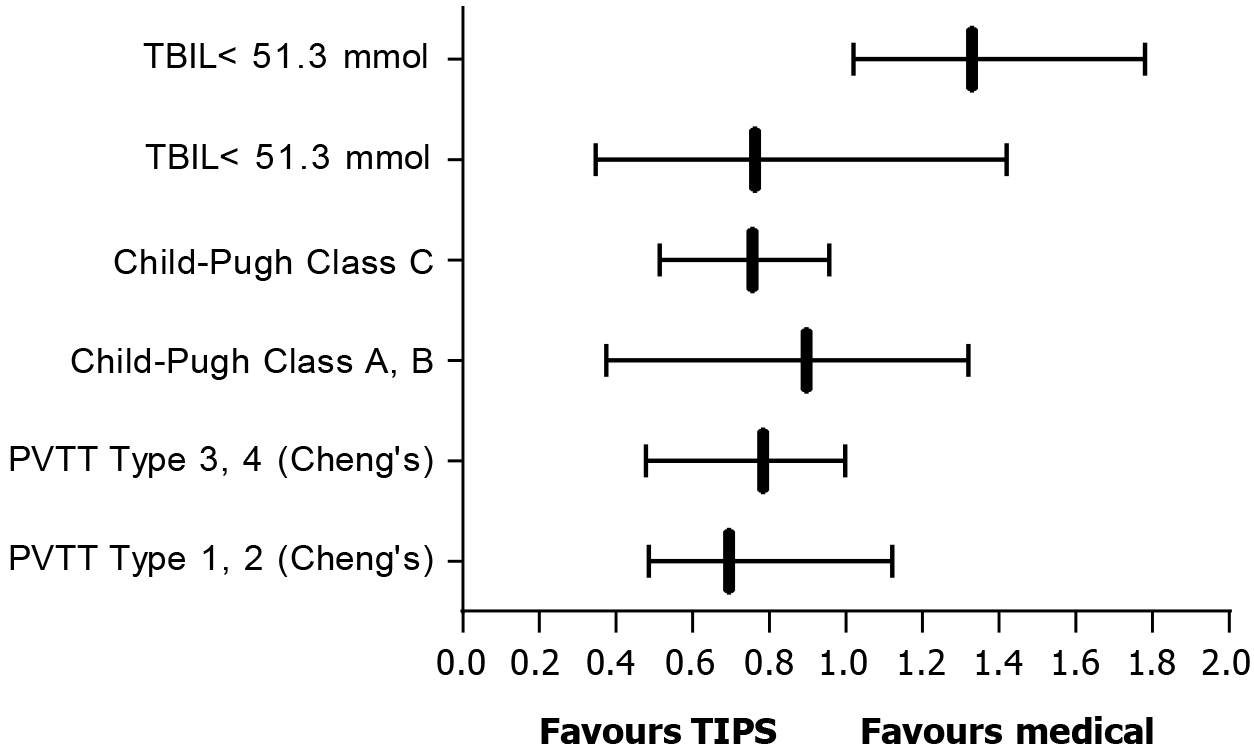
In the multivariate Cox regression analysis, it was found that Cheng’s Classification of PVTT (P = 0.011), TBIL (P = 0.015) and Child-Pugh class (P = 0.015) were independent negative associated factors of OS. Subgroup analyses showed that the median OS of patients with type III/IV PVTT and Child-Pugh C in the TIPS group was better than that in the standard treatment group, while the median OS of patients with TBIL at least 51.3 μmol/L in the TIPS group was shorter than that in the standard treatment group (Figure 3). By analyzing the main causes of death in the two groups, it was found that the death caused by liver failure or hepatic encephalopathy in the TIPS group was higher than that in the standard treatment group, while the death caused by rebleeding in the TIPS group was lower than that in the standard treatment group (Table 2).
| Causes of death | OS < 160 days | OS > 160 days | ||||
| TIPS | Standard treatment | P value | TIPS | Standard treatment | P value | |
| Rebleeding, n (%) | 2 (14.3) | 27 (90.0) | < 0.05 | 1 (6.7) | 6 (33.3) | < 0.05 |
| Liver failure or hepatic encephalopathy, n (%) | 11 (78.6) | 2 (6.7) | < 0.05 | 11 (73.3) | 5 (27.8) | < 0.05 |
| Unknown, n (%) | 1 (7.1) | 1 (3.3) | > 0.05 | 3 (20.0) | 7 (38.9) | > 0.05 |
This study compared the clinical outcomes between TIPS treatment and standard conservative treatment in HCC patients with PVTT and acute EGVB. The results showed that all patients received successful TIPS operation, with a mean portal pressure reduction of 11 mmHg. No serious surgical complications or deaths occurred within 2 weeks after TIPS treatment. TIPS treatment resulted in a better survival compared with standard treatment within the first 160 days after treatment.
The endoscopic treatment is still the mainstay treatment for HCC patients with PVTT and acute EGVB[8], which resulted in a high successful hemostasis rate in both the TIPS group (96.6%) and standard treatment group (95.8%). Notably, we found that the success rate of TIPS was 100%, with a good portal pressure-lowering effect (11 mmHg). In contrast to previous perception[12,13], TIPS treatment was safe in our study, without serious surgical complications or deaths within 2 weeks. The only patient who failed with endoscopic treatment survived for more than 2 years after successful hemostasis with salvage TIPS and died of multiple organ dysfunction syndrome due to tumor progression. This indicated the potential value of TIPS in the treatment of acute EGVB in patients with HCC and PVTT.
Our study showed that patients from the TIPS group benefited more regarding short-term survival (within 160 days after treatment) compared with the standard treatment group. The main reason might be that TIPS decreased portal pressure, thus reducing the risk of bleeding, and creating opportunities for subsequent comprehensive treatment of HCC. Previous studies showed that TIPS combined with palliative treatment could prolong survival in patients with HCC[23-26]. Our analysis of death causes supported this speculation. The majority (56.3%, 27/48) of patients in the standard treatment group died of rebleeding within 160 days, while only a small proportion (6.9%, 2/29) of patients in the TIPS group suffered rebleeding within 160 days and died. This indicated that TIPS treatment might be a better option for the treatment of acute EGVB compared with standard treatment.
Regarding that there was no statistically significant difference in the log-rank test P value (log-rank P = 0.624), although there was a large difference in median OS between the two groups (181 days vs 100 days), which was related to the crossover of the survival curves, and therefore landmark analyses were performed in this study. In terms of long-term survival, our results showed that patients with standard treatment had better OS than those with TIPS treatment. We noticed that the main cause of death in the TIPS group was liver failure or hepatic encephalopathy (37.9%, 11/29) after 160 days, while only a few (10.4%, 5/48) patients with standard treatment died of liver failure or hepatic encephalopathy after 160 days. It might be explained that the long-term reduction of hepatic perfusion caused by TIPS with covered stents led to decreased liver reserve function and subsequent liver failure and hepatic encephalopathy[27]. On the other hand, more patients with HCC-related treatment in the TIPS group might indicate the more severe disease condition compared with the standard treatment group. All these results suggested that TIPS combined with standard conservative treatment might be an optimal strategy to balance the benefit and risk. This combination could reduce the portal pressure and protect liver function, leading to better short-term and long-term survival, which deserves further investigation.
In order to ensure the comparability of survival time between the two groups of patients, this study only included patients newly diagnosed with EGVB accompanied with PVTT or with a history of PVTT, and the first bleeding was regarded as the initial follow-up time. In addition, in order to ensure baseline comparability between the two groups, patients who had absolute contraindications of TIPS surgery were excluded from the study. However, there are still some limitations in this study. As a single-center retrospective study with small sample size, potential biases were inevitable. In addition, clinical indicators for TIPS could not be found in HCC patients with PVTT and acute EGVB. It needs to be further explored by studies with larger sample size in the future.
In this retrospective cohort study, TIPS could reduce the risk of rebleeding by decreasing portal pressure, leading to prolonged short-term survival in HCC patients with PVTT and acute EGVB compared with standard conservative treatment. The safety of TIPS was also acceptable. The use of TIPS for the treatment of acute EGVB in HCC complicated with PVTT deserves further investigation.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64722] [Article Influence: 16180.5] [Reference Citation Analysis (177)] |
| 2. | Lu J, Zhang XP, Zhong BY, Lau WY, Madoff DC, Davidson JC, Qi X, Cheng SQ, Teng GJ. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4:721-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 907] [Article Influence: 34.9] [Reference Citation Analysis (1)] |
| 4. | Lim J, Kim HI, Kim E, Kim J, An J, Chang S, Kim SO, Lee HC, Lee YS, Shim JH. Variceal bleeding is aggravated by portal venous invasion of hepatocellular carcinoma: a matched nested case-control study. BMC Cancer. 2021;21:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4111] [Article Influence: 587.3] [Reference Citation Analysis (6)] |
| 6. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 963] [Article Influence: 321.0] [Reference Citation Analysis (0)] |
| 7. | Yamakado K, Nakatsuka A, Tanaka N, Fujii A, Terada N, Takeda K. Malignant portal venous obstructions treated by stent placement: significant factors affecting patency. J Vasc Interv Radiol. 2001;12:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Satapathy SK, Sanyal AJ. Nonendoscopic management strategies for acute esophagogastric variceal bleeding. Gastroenterol Clin North Am. 2014;43:819-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | García-Pagán JC, Reverter E, Abraldes JG, Bosch J. Acute variceal bleeding. Semin Respir Crit Care Med. 2012;33:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2294] [Article Influence: 229.4] [Reference Citation Analysis (3)] |
| 11. | Njei B, McCarty TR, Laine L. Early transjugular intrahepatic portosystemic shunt in US patients hospitalized with acute esophageal variceal bleeding. J Gastroenterol Hepatol. 2017;32:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Krajina A, Hulek P, Fejfar T, Valek V. Quality improvement guidelines for Transjugular Intrahepatic Portosystemic Shunt (TIPS). Cardiovasc Intervent Radiol. 2012;35:1295-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Tripathi D, Stanley AJ, Hayes PC, Travis S, Armstrong MJ, Tsochatzis EA, Rowe IA, Roslund N, Ireland H, Lomax M, Leithead JA, Mehrzad H, Aspinall RJ, McDonagh J, Patch D. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69:1173-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 212] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 14. | Jiang ZB, Shan H, Shen XY, Huang MS, Li ZR, Zhu KS, Guan SH. Transjugular intrahepatic portosystemic shunt for palliative treatment of portal hypertension secondary to portal vein tumor thrombosis. World J Gastroenterol. 2004;10:1881-1884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Zhao JB, Feng C, Zhu QH, He XF, Li YH, Chen Y. Transjugular intrahepatic portosystemic shunt with covered stents for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2014;20:1602-1607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Liu L, Zhao Y, Qi X, Cai G, He C, Guo W, Yin Z, Chen H, Chen X, Fan D, Han G. Transjugular intrahepatic portosystemic shunt for symptomatic portal hypertension in hepatocellular carcinoma with portal vein tumor thrombosis. Hepatol Res. 2014;44:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Qiu B, Li K, Dong X, Liu FQ. Transjugular Intrahepatic Portosystemic Shunt for Portal Hypertension in Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Cardiovasc Intervent Radiol. 2017;40:1372-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Chung WJ, Jang BK, Park KS, Cho KB, Hwang JS, Ahn SH, Kim YH, Kim YH, Kim YJ. [Effect of transjugular intrahepatic portosystemic shunt for variceal bleeding in hepatocellular carcinoma patients with portal vein thrombosis]. Korean J Hepatol. 2005;11:157-163. [PubMed] |
| 19. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6066] [Article Influence: 866.6] [Reference Citation Analysis (3)] |
| 20. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3032] [Article Influence: 433.1] [Reference Citation Analysis (3)] |
| 21. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 849] [Article Influence: 25.7] [Reference Citation Analysis (42)] |
| 22. | Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Guanghui D, Wenming C, Peijun W, Yuxiang Z. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology. 2007;54:499-502. [PubMed] |
| 23. | Luo SH, Chu JG, Huang H, Yao KC. Safety and efficacy of transjugular intrahepatic portosystemic shunt combined with palliative treatment in patients with hepatocellular carcinoma. World J Clin Cases. 2019;7:1599-1610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Donahue LA, Kulik L, Baker T, Ganger DR, Gupta R, Memon K, Abecassis MM, Salem R, Lewandowski RJ. Yttrium-90 radioembolization for the treatment of unresectable hepatocellular carcinoma in patients with transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2013;24:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Padia SA, Chewning RH, Kogut MJ, Ingraham CR, Johnson GE, Bhattacharya R, Kwan SW, Monsky WL, Vaidya S, Hippe DS, Valji K. Outcomes of Locoregional Tumor Therapy for Patients with Hepatocellular Carcinoma and Transjugular Intrahepatic Portosystemic Shunts. Cardiovasc Intervent Radiol. 2015;38:913-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Park JK, Al-Tariq QZ, Zaw TM, Raman SS, Lu DS. Radiofrequency Ablation for the Treatment of Hepatocellular Carcinoma in Patients with Transjugular Intrahepatic Portosystemic Shunts. Cardiovasc Intervent Radiol. 2015;38:1211-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Rajesh S, George T, Philips CA, Ahamed R, Kumbar S, Mohan N, Mohanan M, Augustine P. Transjugular intrahepatic portosystemic shunt in cirrhosis: An exhaustive critical update. World J Gastroenterol. 2020;26:5561-5596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |