Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2724
Revised: May 13, 2024
Accepted: June 7, 2024
Published online: August 27, 2024
Processing time: 133 Days and 5.6 Hours
Neuroendocrine tumors (NETs) arise from the body’s diffuse endocrine system. Coexisting primary adenocarcinoma of the colon and NETs of the duodenum (D-NETs) is a rare occurrence in clinical practice. The classification and treatment criteria for D-NETs combined with a second primary cancer have not yet been determined.
We report the details of a case involving female patient with coexisting primary adenocarcinoma of the colon and a D-NET diagnosed by imaging and surgical specimens. The tumors were treated by surgery and four courses of chemothe
Coexisting primary adenocarcinoma of the colon and D-NET were diagnosed by imaging, laboratory indicators, and surgical specimens. Surgical resection com
Core Tip: Coexisting primary adenocarcinoma of the colon and neuroendocrine tumor of the duodenum (D-NET) is a rare occurrence in clinical practice. We report the details of a case involving a female patient with coexisting primary adenocarcinoma of the colon and D-NET diagnosed by imaging and surgical specimens. The tumors were treated by surgery and four courses of chemotherapy. The patient achieved a favorable clinical prognosis. The classification and treatment criteria for D-NETs combined with a second primary cancer have not yet been determined. Our experience may help others to diagnose and manage similar patients.
- Citation: Fei S, Wu WD, Zhang HS, Liu SJ, Li D, Jin B. Primary coexisting adenocarcinoma of the colon and neuroendocrine tumor of the duodenum: A case report and review of the literature. World J Gastrointest Surg 2024; 16(8): 2724-2734
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2724.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2724
Neuroendocrine tumors (NETs) originate from endocrine organs and thus may arise from almost any location in the body. They are most commonly found in the gastrointestinal tract (i.e. GEP-NETs) and respiratory system. Neuroendocrine cells produce neuroregulators, neuropeptides, or neurotransmitter hormones. Multiple primary tumors are a unique occurrence in medical practice. The clinical features of D-NETs combined with a second primary malignant tumor lack specificity. Diagnosis of coexisting primary adenocarcinoma of the colon and NET of the duodenum (D-NET) can be diagnosed by imaging, laboratory indicators, and from surgical specimens.
Different D-NETs have different treatments, but surgery remains the best method. Endoscopic resection (ESD) is safe and effective for duodenal carcinoid tumors that are ≤ 10 mm in diameter and limited to the submucosal layer. For tumors between 10 mm and 20 mm in diameter, endoscopic or surgical treatment can be used, and surgical treatment is performed for suspected tumors > 10 mm or tumors with positive margins after resection. Surgery is the only treatment for local early colon cancer (stages I and II). Chemotherapy is the standard treatment for patients with locally advanced stage III and IV after radical surgery. For some stage-2 colon cancer patients, systemic treatment with surgery is based on risk factors and microsatellite instability (MSI) gene status.
We describe the treatment of a female patient diagnosed with coexisting primary adenocarcinoma of the colon and D-NET by imaging and examination of surgical specimens. The tumors were treated by surgery and four courses of che
| Feature | Colon adenocarcinoma | Neuroendocrine tumor |
| Tumor size | 6 cm × 4 cm × 1 cm | 9 mm |
| Lymph node invasion | - (0/20) | Not applicable |
| Fat tissue invasion | + | - |
| Perineural invasion | + | - |
| Vascular invasion | + | - |
| Lymphatic vessel invasion | + | - |
| Muscularis propria invasion | + | - |
| Serosal invasion | + | - |
| Resection margins | - | - |
| Tumor necrosis | + | - |
| P53 | 90% (+) | 10% (+) |
| Ki-67 | 70% (+) | 2% (+) |
| Mitoses/2 mm2 | > 20 | 1 |
| Immunocytochemistry | Syn (-), MLH1 (+), MSH2 (+), MSH6 (+), PMS2 (+), BRAFV600E (-), CD34 (+), D2-40 (+) | CK (+), CK7 (+), CgA (+), Syn (+), NSE (+) |
| Grade of the cancer | High grade (G3) | Low grade (G1) |
| TNM stage | pT4apN0pM0 | pT1pN0pM0 |
| Astler-Coller classification[1] | B | Not applicable |
| AJCC clinical stage[2] | IIB | I |
A 66-year-old female patient was admitted to the hospital on April 25, 2023 because of abdominal pain for 4 mon and had worsened in the previous 7 days.
Four months prior to admission, the patient experienced intermittent periumbilical pain and abdominal distension without obvious reasons, accompanied by a sense of urgency and incomplete defecation, and her stools became thinner. The symptoms worsened 7 days before admission.
The patient had no history of hypertension, diabetes, or other major organ disease such as cardiopulmonary disease.
The patient’s father had a history of nasopharyngeal carcinoma, and there was no other family history of cancer. The patient had no history of smoking or drinking.
Mild tenderness in the upper abdomen, pains around the umbilicus, no rebound pain or muscle tension, and no obvious abnormalities in the rest of the physical examination.
Except for positive fecal occult blood, the laboratory examination found no abnormalities.
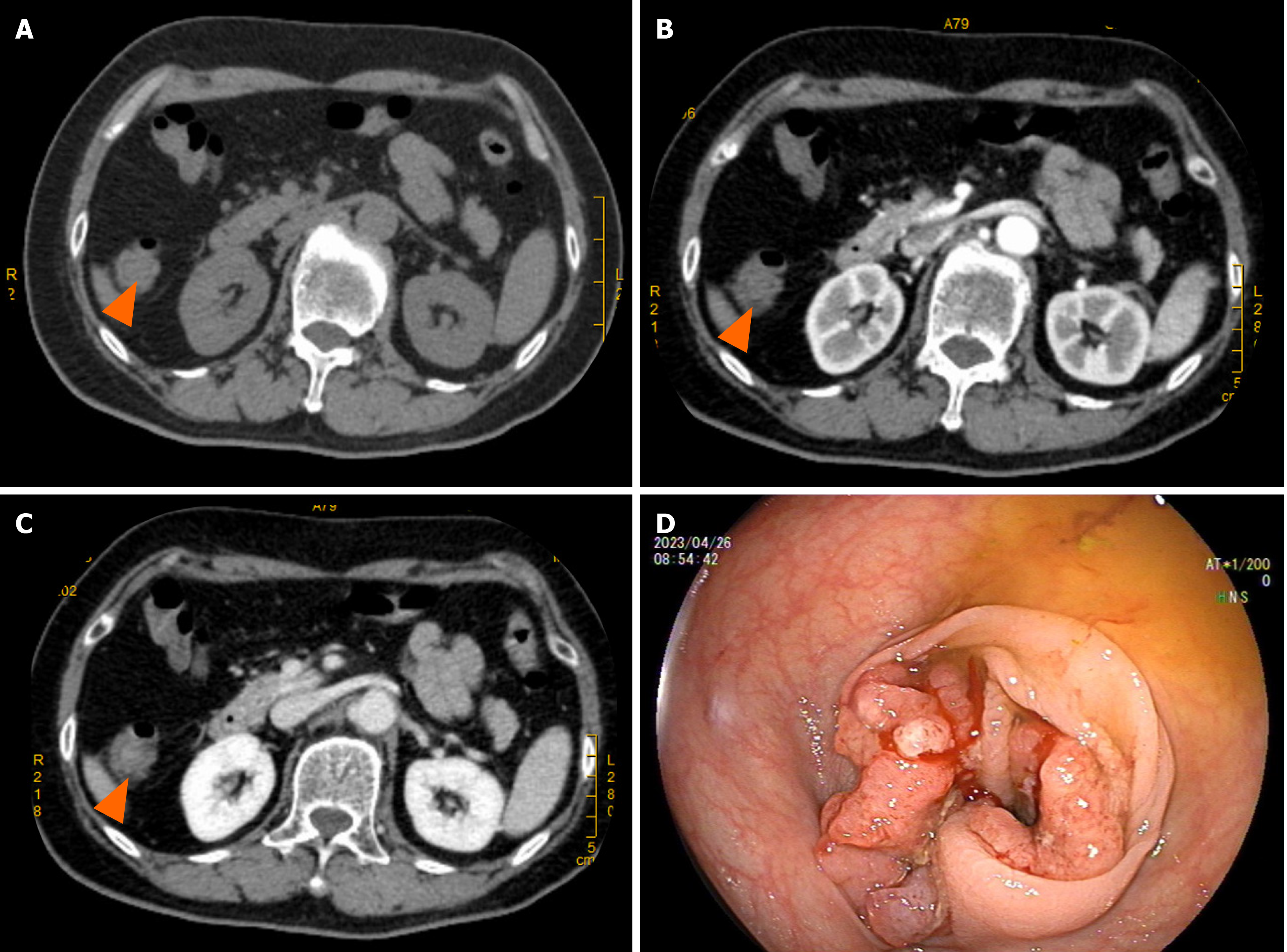
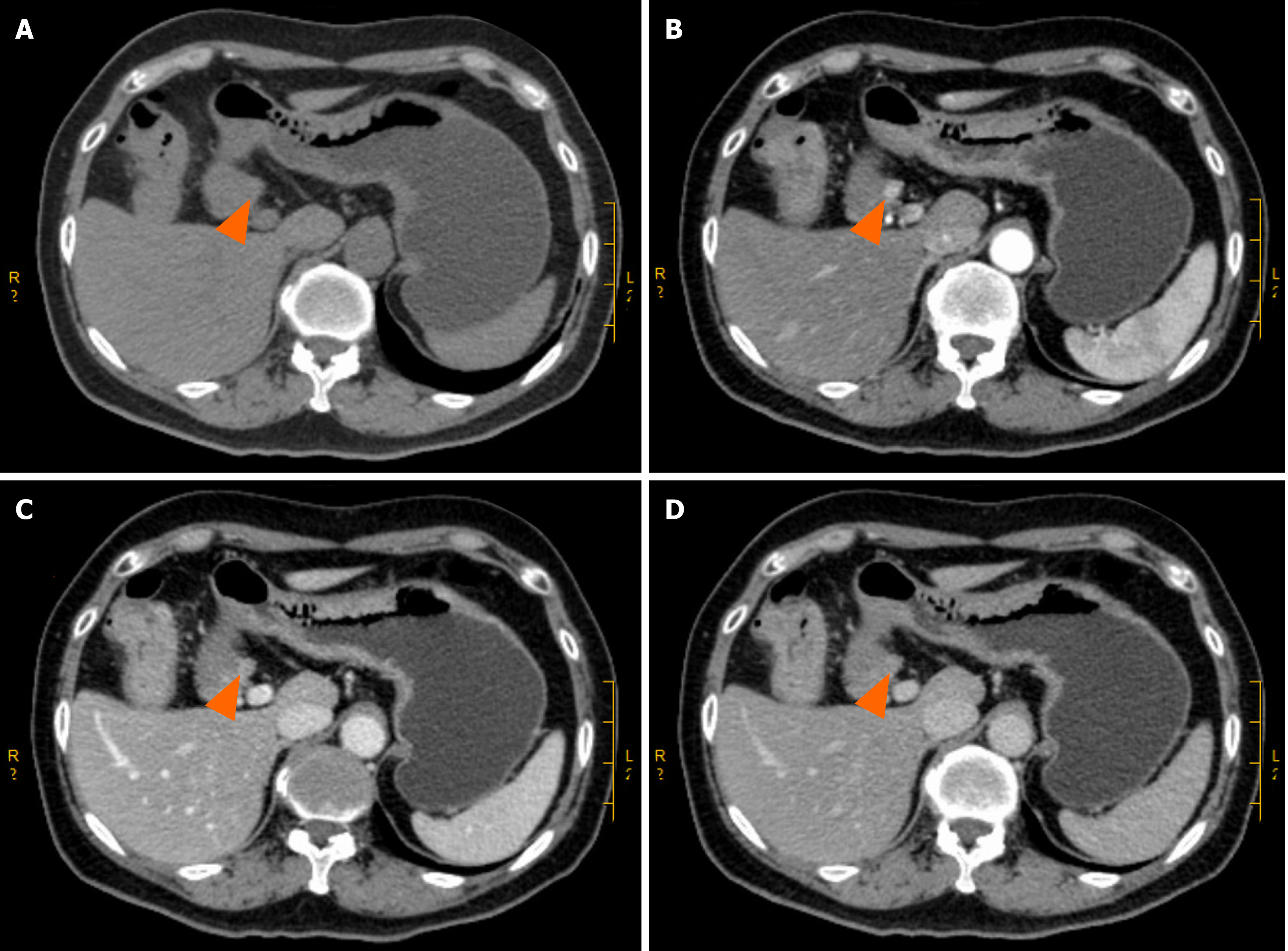
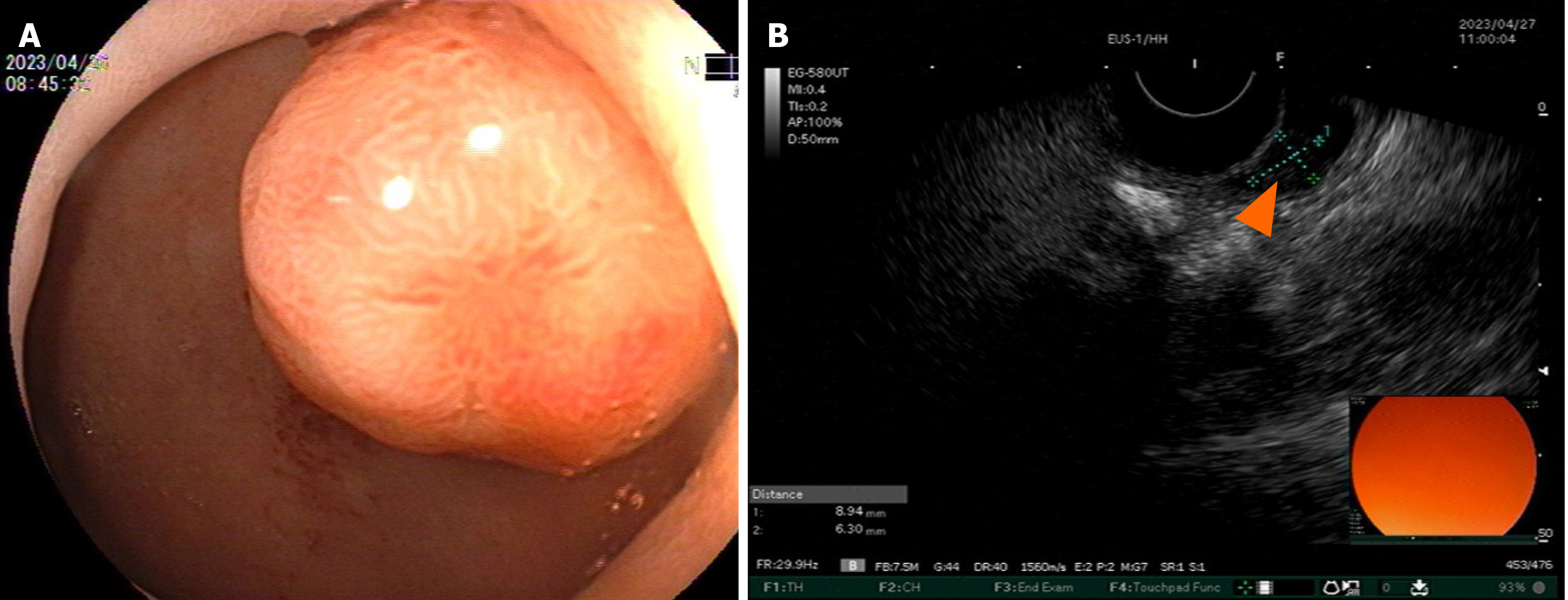
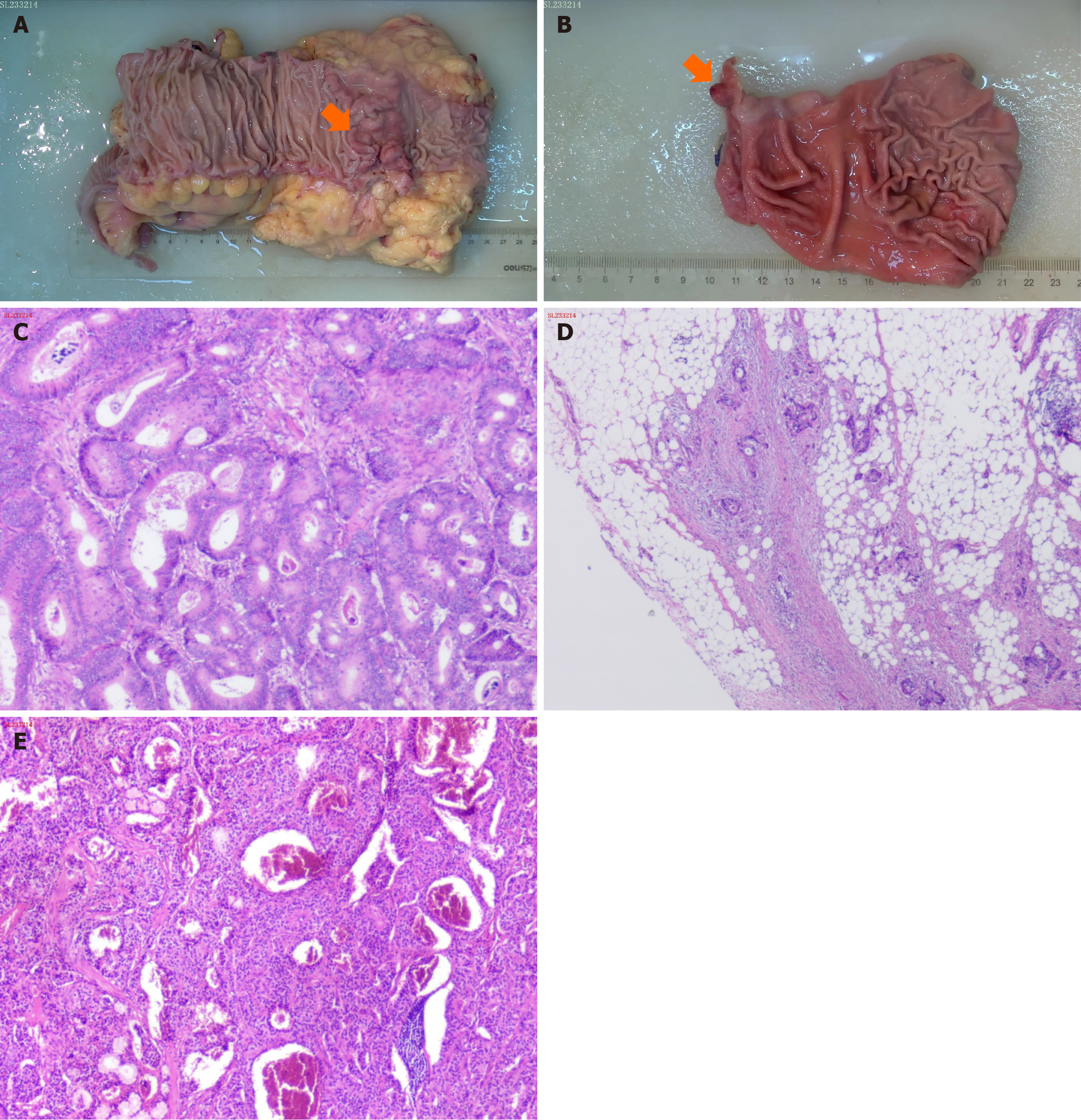
Enhanced computed tomography (CT) of the entire abdomen on admission showed intestinal cancer invading the serosal layer of transverse colon near the hepatic flexure, small mesenteric lymph node metastasis that needed to be ruled out (Figure 1A-C), and abnormal enhancement of the duodenal bulb (Figure 2). Gastroscopy and endoscopic ultrasonography showed a slightly hypoechoic lesion with a broad base measuring approximately 8.9 mm × 6.3 mm in the mucosal layer of the anterior wall of the duodenum. The remaining mucosal layer appeared normal, but a large polyp in the duodenum was of possible concern. Multiple attempts to remove the polyp by endoscopic procedures were unsuccessful. Chronic gastritis with erosion was visible (Figure 3). Colonoscopy found an irregular mass with a diameter > 2 cm located 70 cm from the anus. It had a rough surface, hard texture, bled easily, and obstructed the intestinal lumen, making it difficult for the endoscope to enter. A biopsy was taken, and the pathological diagnosis was mucosal adenocarcinoma of the trans
Considering the clinical history, CT imaging manifestations, endoscopic visualization, EUS, and the postoperative pa
The preoperative diagnosis was a malignant tumor located at the hepatic flexure of the colon and a polyp in the duodenal bulb. Intraoperative exploration revealed a mass with a hard texture and a diameter of approximately 1 cm in the duo
The patient was followed-up after discharge and given four courses of oxaliplatin plus capecitabine chemotherapy. At the time of writing, the patient has been followed-up for 11 mon, with CT scans and laboratory examination every 3 mon. The patient’s overall condition is good, with no signs of tumor progression or additional metastasis.
As NETs originate in endocrine organs, they can be found in nearly any location in the body, with most arising in the gastrointestinal tract (e.g., GEP-NETs) and respiratory system. Neuroendocrine cells produce neuroregulators, neuro
Multiple primary tumors are a unique phenomenon in medicine and are divided into two categories by their time of onset. Synchronous tumors occur simultaneously and heterochronous tumors occur in chronological order. Warren and Gates[13] conducted autopsies of 1078 cancer patients and found that 40 (3.7%) had either occult or clinically apparent second primary tumors. Some studies have reported a correlation of NETs with an increased risk of developing secon
The clinical features of GEP-NETs combined with a second primary malignant tumor lack specificity. A previous study reported that gastrin and cholecystokinin were associated with NETs and induction of tissue growth and cellular mali
Different D-NETs have different treatments, but surgery is still the best method for treat D-NETs. Endoscopic sub
In patients with NETs positive for somatostatin receptors, subcutaneous or intramuscular administration of so
With the advent of aggressive surgical intervention and second-line treatment with long-acting somatostatin agonists and targeted drugs, the prognosis and long-term survival of patients with NETs have improved. Studies have shown that in the case of malignant tumors, the 5-year survival rate can be as high as 77% to 95% following radical resection of the primary tumor and adjuvant therapy[46,47]. For localized and well-differentiated tumors treated by complete surgical resection, the 5-year survival rate of G-NETs is as high as 90%. Radical resection of the primary tumor, absence of liver metastasis, metachronous liver metastasis, and active treatment of liver metastasis are all favorable factors and improve prognosis[48,49]. However, nearly all patients diagnosed with metastatic gastric neuroendocrine cancer have a recurrence within 7 years of follow-up. Recurrence is difficult to avoid even after a complete cure[49], indicating its refractory cha
In the follow-up of NETs, early studies found that octreotide CT or octreotide SPE-CT scans have an important role in detecting the recurrence of NETs[50,51]. Frilling et al[50] found that 19 of 35 patients with NETs (54.2%) had extrahepatic tumors that were not detected by other imaging techniques, such as CT, MRI, or ultrasound. Octreo-SPECT/CT imaging can be used to detect and locate suspected NETs before their diagnosis[52,53], and can be used to follow-up and detect tumor recurrence after diagnosis or treatment. In this case, the patient was considered to have an early stage duodenal NET, and only CT and laboratory tests were used for follow-up.
Surgery is the most effective treatment for local early-stage colon cancer (stages I and II). Chemotherapy is the standard treatment for patients with locally advanced stage III and IV cancers after radical surgery. For some stage II colon cancer patients, systemic treatment with surgery is based on risk factors and MSI gene status. Commonly used drugs include capecitabine, 5-fluorouracil, irinotecan, and oxaliplatin. Biologics, including bevacizumab, cetuximab, panitumumab, regorafenib, and afatinib are important for the treatment of metastatic colon cancer. Genetic analysis of tumor patients is increasingly used role to guide the selection of treatment plans. The use of radiotherapy is currently limited to palliation of selected metastatic sites (e.g., bone or brain metastases)[44,54-57].
All patients with synchronous colorectal cancer and D-NETs undergo extensive evaluation and clinical monitoring during hospitalization and follow-up to detect disease progression or recurrence. In current practice, patients are fo
The appearance of synchronous primary tumors is of interest to surgeons and oncologists and the entire medical field. When such a phenomenon is encountered, questions invariably arise regarding common genetic pathways in the pa
There are no clear diagnostic criteria, treatment, or follow-up guidelines for patients with synchronous D-NETs and a second primary malignancy. Our experience may help to inform the diagnosis and management of similar patients. The case also highlights the advantages of HALS, which allows direct palpation of the mass and assessment of its size, texture, and mobility, and evaluation of the surrounding lymph nodes. This is of benefit to surgeons in choosing the surgical procedures. in addition, the surgical incision is small and postoperative recovery is fast. It also avoids the occurrence of large surgical incisions, incision infections, and fat liquefaction after traditional open surgery. In the setting of syn
We sincerely appreciate the patients and their families for their cooperation in information acquisition, treatment, and follow-up.
| 1. | ASTLER VB, COLLER FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954;139:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 744] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6th ed. Springer, 2022. |
| 3. | Barakat MT, Meeran K, Bloom SR. Neuroendocrine tumours. Endocr Relat Cancer. 2004;11:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2438] [Article Influence: 487.6] [Reference Citation Analysis (3)] |
| 5. | Delle Fave G, O'Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, De Herder WW, Pascher A, Ruszniewski P; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 6. | Chinese Medical Association; Digestive Disease Branch; Gastrointestinal Hormone and Neuroendocrine Tumor Study Group. Expert Consensus on the Diagnosis and Treatment of Gastrointestinal Pancreatic Neuroendocrine Tumors (2020, Guangzhou). Zhonghua Xiaohua Zazhi. 2021;41:76-87. [DOI] [Full Text] |
| 7. | Hu P, Bai J, Liu M, Xue J, Chen T, Li R, Kuai X, Zhao H, Li X, Tian Y, Sun W, Xiong Y, Tang Q. Trends of incidence and prognosis of gastric neuroendocrine neoplasms: a study based on SEER and our multicenter research. Gastric Cancer. 2020;23:591-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Yang Z, Wang W, Lu J, Pan G, Pan Z, Chen Q, Liu W, Zhao Y. Gastric Neuroendocrine Tumors (G-Nets): Incidence, Prognosis and Recent Trend Toward Improved Survival. Cell Physiol Biochem. 2018;45:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | O'Connor JM, Marmissolle F, Bestani C, Pesce V, Belli S, Dominichini E, Mendez G, Price P, Giacomi N, Pairola A, Loria FS, Huertas E, Martin C, Patane K, Poleri C, Rosenberg M, Cabanne A, Kujaruk M, Caino A, Zamora V, Mariani J, Dioca M, Parma P, Podesta G, Andriani O, Gondolesi G, Roca E. Observational study of patients with gastroenteropancreatic and bronchial neuroendocrine tumors in Argentina: Results from the large database of a multidisciplinary group clinical multicenter study. Mol Clin Oncol. 2014;2:673-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Masui T, Ito T, Komoto I, Uemoto S; JNETS Project Study Group. Recent epidemiology of patients with gastro-entero-pancreatic neuroendocrine neoplasms (GEP-NEN) in Japan: a population-based study. BMC Cancer. 2020;20:1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Gastrointestinal Pathology Study Group of Korean Society of Pathologists; Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM, Kim WH, Kim H, Kook MC, Park DY, Lee JH, Chang H, Jung ES, Kim HK, Jin SY, Choi JH, Gu MJ, Kim S, Kang MS, Cho CH, Park MI, Kang YK, Kim YW, Yoon SO, Bae HI, Joo M, Moon WS, Kang DY, Chang SJ. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study. Cancer Res Treat. 2012;44:157-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 12. | Das S, Dasari A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr Oncol Rep. 2021;23:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 210] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 13. | Warren S. Multiple primary malignant tumors. A survey of the literature and a statistical study. 1932. [cited 31 May 2024]. Available from: https://www.scienceopen.com/document?vid=c4af5a50-aa4f-438d-ba7c-69aa9e4a9481. |
| 14. | Bateni SB, Coburn NG, Law CHL, Singh S, Myrehaug S, Assal A, Hallet J. Incidence and Predictors of Second Primary Cancers in Patients With Neuroendocrine Tumors. JAMA Oncol. 2021;7:1718-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Kamp K, Damhuis RA, Feelders RA, de Herder WW. Occurrence of second primary malignancies in patients with neuroendocrine tumors of the digestive tract and pancreas. Endocr Relat Cancer. 2012;19:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Park JS, Kim L, Kim CH, Bang BW, Lee DH, Jeong S, Shin YW, Kim HG. Synchronous large-cell neuroendocrine carcinoma and adenocarcinoma of the colon. Gut Liver. 2010;4:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Baek HS, Kim SW, Lee ST, Park HS, Seo SY. Silent advanced large cell neuroendocrine carcinoma with synchronous adenocarcinoma of the colon: A case report. World J Gastrointest Oncol. 2022;14:2266-2272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Xu F, Feng GS, Wang ZJ, Zhang KN. Synchronous double cancers of colonic large cell neuroendocrine carcinoma and gastric squamous-cell carcinoma: a case report and review of literature. Int J Clin Exp Pathol. 2014;7:5177-5180. [PubMed] |
| 19. | Reim D, Weirich G, Neu B, Bajbouj M, Brücher BL. Synchronous adenocarcinoma of the lung and neuroendocrine carcinoma of the ileum. Int J Colorectal Dis. 2008;23:325-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Vilallonga R, Espín Basany E, López Cano M, Landolfi S, Armengol Carrasco M. [Neuroendocrine carcinomas of the colon and rectum. A unit's experience over six years]. Rev Esp Enferm Dig. 2008;100:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Soga J. Endocrinocarcinomas (carcinoids and their variants) of the duodenum. An evaluation of 927 cases. J Exp Clin Cancer Res. 2003;22:349-363. [PubMed] |
| 22. | Sundin A, Arnold R, Baudin E, Cwikla JB, Eriksson B, Fanti S, Fazio N, Giammarile F, Hicks RJ, Kjaer A, Krenning E, Kwekkeboom D, Lombard-Bohas C, O'Connor JM, O'Toole D, Rockall A, Wiedenmann B, Valle JW, Vullierme MP; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology. 2017;105:212-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 303] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 23. | Sahani DV, Bonaffini PA, Fernández-Del Castillo C, Blake MA. Gastroenteropancreatic neuroendocrine tumors: role of imaging in diagnosis and management. Radiology. 2013;266:38-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Levy AD, Sobin LH. From the archives of the AFIP: Gastrointestinal carcinoids: imaging features with clinicopathologic comparison. Radiographics. 2007;27:237-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Ganeshan D, Bhosale P, Yang T, Kundra V. Imaging features of carcinoid tumors of the gastrointestinal tract. AJR Am J Roentgenol. 2013;201:773-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Kim GH, Kim JI, Jeon SW, Moon JS, Chung IK, Jee SR, Kim HU, Seo GS, Baik GH, Lee YC; Korean College of Helicobacter and Upper Gastrointestinal Research. Endoscopic resection for duodenal carcinoid tumors: a multicenter, retrospective study. J Gastroenterol Hepatol. 2014;29:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Aoyama N, Wada M, Taniguchi Y, Inokuma T, Nakanishi Y, Fukuda A, Seno H. A case of neuroendocrine neoplasm of the minor duodenal papilla. Clin J Gastroenterol. 2023;16:171-179. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1851] [Article Influence: 84.1] [Reference Citation Analysis (1)] |
| 29. | Modlin IM, Shapiro MD, Kidd M. An analysis of rare carcinoid tumors: clarifying these clinical conundrums. World J Surg. 2005;29:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Strosberg JR, Coppola D, Klimstra DS, Phan AT, Kulke MH, Wiseman GA, Kvols LK; North American Neuroendocrine Tumor Society (NANETS). The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39:799-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 258] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 31. | Narayanan S, Kunz PL. Role of somatostatin analogues in the treatment of neuroendocrine tumors. J Natl Compr Canc Netw. 2015;13:109-17; quiz 117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Kos-Kudła B, Zemczak A, Foltyn W, Marek B, Strzelczyk J, Telega A, Zajecki W, Legaszewski T, Jurecka-Lubieniecka B. Octreotide suppression test in diagnosing and predicting the outcome of therapy in patients with neuroendocrine tumors. Preliminary report. Endokrynol Pol. 2007;58:123-129. [PubMed] |
| 33. | Fjallskog ML, Janson ET, Falkmer UG, Vatn MH, Oberg KE, Eriksson BK. Treatment with combined streptozotocin and liposomal doxorubicin in metastatic endocrine pancreatic tumors. Neuroendocrinology. 2008;88:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Basu B, Sirohi B, Corrie P. Systemic therapy for neuroendocrine tumours of gastroenteropancreatic origin. Endocr Relat Cancer. 2010;17:R75-R90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 584] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 36. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1829] [Article Influence: 130.6] [Reference Citation Analysis (0)] |
| 37. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2117] [Article Influence: 151.2] [Reference Citation Analysis (0)] |
| 38. | Werner RA, Weich A, Kircher M, Solnes LB, Javadi MS, Higuchi T, Buck AK, Pomper MG, Rowe SP, Lapa C. The theranostic promise for Neuroendocrine Tumors in the late 2010s - Where do we stand, where do we go? Theranostics. 2018;8:6088-6100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Voi M, Brandt U, He W, Chen D, Capdevila J, de Vries EGE, Tomassetti P, Hobday T, Pommier R, Öberg K. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers From the Randomized, Phase III RADIANT-3 Study. J Clin Oncol. 2016;34:3906-3913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 40. | Chen X, Chen Z, Wu H, Liu X, Nie F, Wang Z, Sun M. Comprehensive Genomic Characterization Analysis Identifies an Oncogenic Pseudogene RP11-3543B.1 in Human Gastric Cancer. Front Cell Dev Biol. 2021;9:743652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 903] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 42. | Steward MJ, Warbey VS, Malhotra A, Caplin ME, Buscombe JR, Yu D. Neuroendocrine tumors: role of interventional radiology in therapy. Radiographics. 2008;28:1131-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Dutton SJ, Kenealy N, Love SB, Wasan HS, Sharma RA; FOXFIRE Protocol Development Group and the NCRI Colorectal Clinical Study Group. FOXFIRE protocol: an open-label, randomised, phase III trial of 5-fluorouracil, oxaliplatin and folinic acid (OxMdG) with or without interventional Selective Internal Radiation Therapy (SIRT) as first-line treatment for patients with unresectable liver-only or liver-dominant metastatic colorectal cancer. BMC Cancer. 2014;14:497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Sofocleous CT, Garcia AR, Pandit-Taskar N, Do KG, Brody LA, Petre EN, Capanu M, Longing AP, Chou JF, Carrasquillo JA, Kemeny NE. Phase I trial of selective internal radiation therapy for chemorefractory colorectal cancer liver metastases progressing after hepatic arterial pump and systemic chemotherapy. Clin Colorectal Cancer. 2014;13:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Dworakowska D, Gueorguiev M, Laji K, Grossman AB. Multimodality palliative treatment of (111)In-pentetreotide negative/(123)I-MIBG positive metastatic carcinoid - a case report. Endokrynol Pol. 2008;59:342-347. [PubMed] |
| 46. | Hausman MS Jr, Thompson NW, Gauger PG, Doherty GM. The surgical management of MEN-1 pancreatoduodenal neuroendocrine disease. Surgery. 2004;136:1205-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Norton JA, Kivlen M, Li M, Schneider D, Chuter T, Jensen RT. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 48. | Rothenstein J, Cleary SP, Pond GR, Dale D, Gallinger S, Moore MJ, Brierley J, Siu LL. Neuroendocrine tumors of the gastrointestinal tract: a decade of experience at the Princess Margaret Hospital. Am J Clin Oncol. 2008;31:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Shebani KO, Souba WW, Finkelstein DM, Stark PC, Elgadi KM, Tanabe KK, Ott MJ. Prognosis and survival in patients with gastrointestinal tract carcinoid tumors. Ann Surg. 1999;229:815-21; discussion 822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 174] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 50. | Frilling A, Malago M, Martin H, Broelsch CE. Use of somatostatin receptor scintigraphy to image extrahepatic metastases of neuroendocrine tumors. Surgery. 1998;124:1000-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Pelaez N, Busquets J, Ortega M, Miralles EM, Puig J, Miret M, Munné A, Grande L. Intraoperative gamma probe detection of lymph node recurrence of insulinoma. J Surg Oncol. 2005;91:209-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Perri M, Erba P, Volterrani D, Lazzeri E, Boni G, Grosso M, Mariani G. Octreo-SPECT/CT imaging for accurate detection and localization of suspected neuroendocrine tumors. Q J Nucl Med Mol Imaging. 2008;52:323-333. [PubMed] |
| 53. | Dapri G, Bascombe NA. Three trocars laparoscopic right ileocolectomy for advanced small bowel neuroendocrine tumor. Surg Oncol. 2019;28:76-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Morris VK, Kennedy EB, Baxter NN, Benson AB 3rd, Cercek A, Cho M, Ciombor KK, Cremolini C, Davis A, Deming DA, Fakih MG, Gholami S, Hong TS, Jaiyesimi I, Klute K, Lieu C, Sanoff H, Strickler JH, White S, Willis JA, Eng C. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J Clin Oncol. 2023;41:678-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 311] [Article Influence: 155.5] [Reference Citation Analysis (0)] |
| 55. | Tomasello G, Petrelli F, Ghidini M, Russo A, Passalacqua R, Barni S. FOLFOXIRI Plus Bevacizumab as Conversion Therapy for Patients With Initially Unresectable Metastatic Colorectal Cancer: A Systematic Review and Pooled Analysis. JAMA Oncol. 2017;3:e170278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (1)] |
| 56. | Rako I, Jakic-Razumovic J, Katalinic D, Sertic J, Plestina S. Mutation pattern of KRAS and BRAF oncogenes in colorectal cancer patients. Neoplasma. 2012;59:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Go PH, Klaassen Z, Meadows MC, Chamberlain RS. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer. 2011;117:3630-3640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 58. | Wang YH, Yang QC, Chen MH, Chen J. Application of Serum Chromogranin A in Clinical Diagnosis and Efficacy Evaluation of Gastrointestinal Pancreatic Neuroendocrine Tumors. Zhonghua Xiaohua Zazhi. 2013;33:532-537. [DOI] [Full Text] |
| 59. | Yang XO, Li JN, Qian JM. Diagnostic Value of Plasma Chromogranin A for Multiple Neuroendocrine Neoplasms. Zhonghua Neike Zazhi. 2011;50:124-127. [DOI] [Full Text] |
| 60. | Ballinger AB, Anggiansah C. Colorectal cancer. BMJ. 2007;335:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2292] [Article Influence: 208.4] [Reference Citation Analysis (1)] |