Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2679
Revised: May 20, 2024
Accepted: June 17, 2024
Published online: August 27, 2024
Processing time: 163 Days and 6 Hours
Growth hormone (GH) plays a crucial role in wound healing and tissue repair in postoperative patients. In particular, colonic anastomosis healing following colorectal surgery is impaired by numerous chemotherapy agents.
To investigate whether GH can improve the healing of a colonic anastomosis following the adverse effects of intraperitoneal administration of 5-fluorouracil (5-FU), bleomycin and cisplatin.
Eighty Wistar rats underwent laparotomy and a 1 cm-resection of the transverse colon, followed by an end-to-end anastomosis under general anesthesia. The rats were blindly allocated into four equal groups and administered a different daily intraperitoneal therapeutic regimen for 6 days. The control group (A) received normal saline. Group B received chemotherapy with 5-FU (20 mg/kg), bleomycin (4 mg/kg) and cisplatin (0.7 mg/kg). Group C received GH (2 mg/kg), and group D received the aforementioned combination chemotherapy and GH, as described. The rats were sacrificed on the 7th postoperative day and the anastomoses were macroscopically and microscopically examined. Body weight, bursting pressure, hydroxyproline levels and inflammation markers were measured.
All rats survived until the day of sacrifice, with no infections or other complications. A decrease in the body weight of group D rats was observed, not statistically significant compared to group A (P = 1), but significantly different to groups C (P = 0.001) and B (P < 0.01). Anastomotic dehiscence rate was not statistically different between the groups. Bursting pressure was not significantly different between groups A and D (P = 1.0), whereas group B had a significantly lower bursting pressure compared to group D (P < 0.001). All groups had significantly more adhesions than group A. Hydroxyproline, as a measurement of collagen deposition, was significantly higher in group D compared to group B (P < 0.05), and higher, but not statistically significant, compared to group A. Significant changes in group D were recorded, compared to group A regarding inflammation (3.450 vs 2.900, P = 0.016) and fibroblast activity (2.75 vs 3.25, P = 0.021). Neoangiogenesis and collagen deposition were not signifi
Intraperitoneal administration of chemotherapy has an adverse effect on the healing process of colonic anastomosis. However, GH can inhibit the deleterious effect of administered chemotherapy agents and induce colonic healing in rats.
Core Tip: The present study was designed to investigate the effect of growth hormone (GH) on the healing of colonic anastomoses in rats after intraperitoneal administration of 5-fluorouracil, bleomycin and cisplatin. The application of GH was found to have a protective role in the healing of colonic anastomoses in rats by effectively reversing the detrimental effects of chemotherapy.
- Citation: Lambrou I, Mantzoros I, Ioannidis O, Tatsis D, Anestiadou E, Bisbinas V, Pramateftakis MG, Kotidis E, Driagka B, Kerasidou O, Symeonidis S, Bitsianis S, Sifaki F, Angelopoulos K, Demetriades H, Angelopoulos S. Effect of growth hormone on colonic anastomosis after intraperitoneal administration of 5-fluorouracil, bleomycin and cisplatin: An experimental study. World J Gastrointest Surg 2024; 16(8): 2679-2688
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2679.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2679
Colectomy and subsequent anastomosis are among the most common procedures performed by general and colorectal surgeons, and remain the treatment of choice in colon cancer, the third most common cancer worldwide[1,2]. Uncom
Healing of the colonic anastomosis can be disrupted by several factors, such as operative technique, preoperative bowel preparation, blood flow and inflammation[3]. In addition, systemic factors, such as age, medical history and nutritional status can also profoundly affect successful healing of the anastomosis[4,5]. Subsequent complications such as anastomotic bleeding, stenosis and dehiscence can occur when one or more of the aforementioned factors are present. These can lead to increased postoperative morbidity and mortality[6].
Anastomotic dehiscence can cause up to 35% of postoperative mortality[3,7]. Dehiscence is usually observed between the fifth and seventh postoperative day. An anastomotic leak after dehiscence is a major complication, more frequently observed in rectal anastomoses, and is observed in up to 21% of anastomoses[8]. Complete rupture of the anastomosis is less frequent, observed in less than 4%, but does not always lead to leakage and dispersal of enteral contents into the peritoneum, as adjacent tissues may cover the deficit[6,8].
Chemotherapy after tumor resection is a common adjuvant therapy in the management of colon cancer. Therapeutic agents such as 5-fluorouracil (5-FU), bleomycin and cisplatin are commonly administered, and have been proven to increase the long-term survival of patients[9,10]. Intraperitoneal administration of the abovementioned regimen has been proven to decrease local recurrence rates, with a lower incidence of systemic side effects[11-19]. Nevertheless, their adverse effects are evident at the local tissue level, since, like all antineoplastic drugs, they interfere with the processes of healthy tissues and can inhibit postoperative healing[20-24].
Growth hormone (GH), a natural anabolic substance, can positively affect the healing process both directly, as it increases tissue response to stress and oxidative factors via increased collagen deposition, and indirectly, as it affects somatomedins which are produced in the liver, reducing inflammation and antioxidant effects[25,26]. These result in weight gain and increased metabolic rate which are beneficial in patients who are expected to lose weight postoperatively[27].
The aim of this study was to investigate whether GH can improve the healing process of colonic anastomosis following the adverse effects of intraperitoneal administration of 5-FU, bleomycin and cisplatin. The healing process was evaluated clinically by measuring anastomotic ruptures and adhesions, mechanically by measuring the bursting pressure, biochemically by measuring tissue hydroxyproline, and histologically[28,29].
This study was a double-blind, randomized, prospective experimental study. All necessary approved protocols for laboratory animal care were ensured. The experiment was performed and results were published in accordance with the ARRIVE guidelines 2.0.
A total of 80 male Wistar rats, 2-3 months old and weighing 242 ± 6.5 g, were included in the present study. Male rats were chosen to avoid overlapping hormone-related confounding factors in the experiment. An experimental animal protocol was designed to minimize pain and discomfort in the animals. The experimental animals were from a colony established in the Biology Laboratory, School of Medicine, Aristotle University of Thessaloniki. They were acclimatized in the Experimental Laboratory of the Hospital "G. Papanikolaou" in ideal living conditions for approximately 7 days before the start of the experiment. The environment included a constant temperature, humidity, ventilation and hygiene with a 12-hour normal day-night cycle. The animals were kept in groups of five in cages of similar dimensions and had unrestricted access to standard food and water throughout the experimentation period.
The experiments were performed in accordance with the provisions of the European Convention for the protection of animals used for experimental and research purposes (Law 1197/81 Art. 4, Law 2015/92, PD 160/91). The study was conducted under the supervision and contribution of a veterinarian, who was responsible for observance of the rules of hygiene in the premises and the rules of acclimatization, protection and nutrition of the experimental animals.
For the above experimental study, the Directorate of Veterinary Medicine of the Prefecture of Thessaloniki with No. Prot. issued a special approval: 13/10767 /15-9-2003.
The 80 rats were equally randomized into four groups (A, B, C and D) of 20 experimental animals, with the use of electronic randomization software. Each experimental animal was given a unique code number.
Group A (Control): The rats were given intraperitoneal saline (2 mL) intraoperatively, as well as postoperatively daily for six days. In addition, the rats were administered 1 mL of normal saline subcutaneously in the neck daily, in two equal doses, until the 6th postoperative day.
Group B (5-FU, bleomycin and cisplatin): The rats were administered a solution of 5-FU (20 mg/kg body weight), bleomycin (4 mg/kg body weight) and cisplatin (0.7 mg/kg body weight), in 2 mL intraperitoneally, as well as each day postoperatively until the 6th postoperative day.
Group C (GH): The rats were administered daily 1 mL of GH solution (2 mg/kg body weight) subcutaneously in the neck, in two equal doses per day, until the 6th postoperative day.
Group D (GH, 5-FU, bleomycin and cisplatin): The rats were administered a solution of 5-FU (20 mg/kg body weight), bleomycin (4 mg/kg body weight) and cisplatin (0.7 mg/kg body weight) in 2 mL intraperitoneally, as well as each day postoperatively until sacrifice. Postoperatively, 1 mL of GH solution (2 mg/kg body weight) daily was administered subcutaneously in the neck, in two equal doses, until the 6th postoperative day.
For the administration of each of the above substances the experimental animals received light anesthesia with ether. They were placed in ether cages for a few seconds to achieve sufficient anesthesia for the injection and then returned to their cages.
The operation was performed under general anesthesia. Initially the experimental animals were placed in a bell jar with ether for initiation of anesthesia. Pentothal solution was then injected intraperitoneally at a dose of 50 mg/kg body weight. Approximately 30 seconds after the intraperitoneal administration of pentothal, satisfactory anesthesia and analgesia of the experimental animals occurred, which lasted 50-60 minutes.
Following induction of anesthesia, the abdomen was shaved and antisepsis using a 10% povidone iodine solution was performed. The experimental animal was then placed on a specially designed and disinfected surgical table, its limbs were immobilized, and a sterile surgical field was placed on the abdominal wall.
Access was gained through a 3 cm long midline laparotomy. The cecum was identified and retracted outside the peritoneal cavity. A 1 cm section of the transverse colon was subsequently resected 10 cm distal to the ileocecal valve. After bowel resection, the central and peripheral parts of the intestine were mechanically cleaned of fecal content and an end-to-end colo-colic anastomosis was performed with interrupted 6/0 polypropylene sutures. Initially, two guide sutures were placed at the mesenteric and antimesenteric edges of the intestine with three single sutures on each side involving the serosa and submucosa. Eight sutures were required to complete the anastomosis. The peritoneal cavity was then irrigated with saline (NaCl 0.9%) and the abdominal wall was closed with 3-4 sutures of 3/0 silk, followed by local antisepsis of the surgical incision. No antibiotics were administered. Net operative time ranged from 15 to 25 minutes.
After the operation, the experimental animals were kept in pairs in cages where they were monitored for an hour until they fully recovered and regained normal mobility. All experimental animals had free access to food and water from the day of surgery until sacrifice.
Planned sacrifice took place on the 7th postoperative day. Animals were pre-anesthetized with ether and then administered an intracardial injection of 10% KCl. After sacrifice of the experimental animals, the abdominal wall was opened and macroscopic examination of the anastomosis was performed to determine rupture, presence of abscess or peritonitis and evaluation of adhesions according to the scale of Van der Hamm (0 = no adhesions, 1 = minimal adhesions between the anastomosis and small bowel, 2 = moderate adhesions between the anastomosis and small bowel, 3 = severe and extensive adhesions with development of peri-anastomotic abscess).
A 2.5 cm section of the colon was excised on either side of the anastomosis, without resolution of adhesions and diligent cleaning of feces followed.
Measurement of the anastomosis rupture pressure was performed with a manometer which actively records intraluminal pressure to the distal end of the edge of the large intestine containing the anastomosis. A pump for continuous administration of saline at a rate of 1 mL/minute was attached to its proximal edge. During this process, the pressure at which saline was observed to exudate from the anastomosis or other point of the intestine was noted. Burst pressure was recorded in mmHg.
After measuring the burst pressure, the bowel segment containing the anastomosis was placed in 4% formalin solution and sent for histological analysis with hematoxylin-eosin staining solution.
Microscopic findings were classified according to the Erlich and Hunt scale as modified by Phillips[30]. Inflammatory reaction (white blood cell count), fibroblasts, neo-angiogenesis, and collagen growth were assessed and scored on a quantitative scale from 0 to 4 (0 = no data, 1 = occasional evidence, 2 = slightly increased evidence, 3 = abundant evidence, 4 = confluent cells or fibers).
Hydroxyproline is an indicator of the total amount of collagen in a tissue. For the determination of hydroxyproline levels at the site of the anastomosis, a sample of the anastomosis stored at -20°C was used, after being completely dried for 24 hours and weighed with a precision scale. The sample was then placed in 30 μL of concentrated caustic sodium solution (NaOH) at a concentration of 10.125 N which was made by dissolving 0.86 g of NaOH in 2 mL of distilled water. This was carried out to extract the tissue collagen and convert it into a solution. The resulting solution was incubated at a temperature of 1200°C for 20 minutes using an autoclave.
35.5 μL of concentrated hydrochloric acid (HCl) at a concentration of 8 N was added to the resulting solution. This was prepared by adding distilled water to a solution of 1.578 mL of 37% HCl, reaching a volume of 2 mL. This procedure was undertaken to achieve tissue NaCl binding, in order to avoid interference with the results.
450 μL of a freshly prepared alcoholic solution of T-chloramine at a concentration of 0.056 M was added in order to dissolve the tissue collagen and homogenize the solution. This solution was obtained by dissolving 0.635 g of chloramine in 10 mL of 50% propanol which was then diluted until it reached a volume of 50 mL with the addition of acetyl-citric acid buffer solution.
Finally, 500 μL of 1 M Erlich reagent was added, and the end solution was incubated for 20 minutes at 650°C. A spectrophotometer was used to measure absorbance of the solution at 550 nm. Hydroxyproline absorption was determined with the help of an absorption curve and a pre-approved mathematical formula.
Quantitative variables were summarized using means with 95% confidence interval (CI). The normality of quantitative variables was tested with the Kolmogorov-Smirnov test and they were expressed as mean values with 95%CI. Also, for the graphical representation of data, bar charts were used for each treatment group, where error bars represent the variability in each group. The median has an advantage over the mean as it is not affected by the presence of extreme values. Comparison of proportions between groups was assessed by Analysis of variance (ANOVA).
Body weight changes in the rats before and after the experiment were recorded in each group using the student's t-test for paired samples. ANOVA was applied for the overall comparison of weight changes between groups. If there was an indication of a statistically significant difference (P < 0.05) between the groups with the analysis of variance, the specific categories were identified. This identification was achieved by comparing all combinations of the categories pairwise, using the student's-test. A simple Bonferroni adjustment was used for multiple comparisons. For variables that were not normally distributed, non-parametric Kruskal-Wallis analysis of variance was applied for overall comparison of medians between groups. If there was an indication of a statistically significant difference (P < 0.05) between the groups with the Kruskal-Wallis analysis of variance, the specific categories were identified. This was achieved by comparing all combinations of categories pairwise, using a non-parametric Mann-Whitney test.
In all statistical analyses, the significance level was set at 0.05 and was two-sided. Statistical analyses were performed with the SPSS 13.0. statistical package.
In the following section, results concerning the combined (D) group will be presented and compared to the control group (A). Groups B and C have already been published and will not be further analyzed in this publication[31,32]. No deaths or surgical site infections were noted before euthanasia day. All animals were sacrificed on the 7th postoperative day. Macroscopic inspection then took place to check for anastomotic dehiscence.
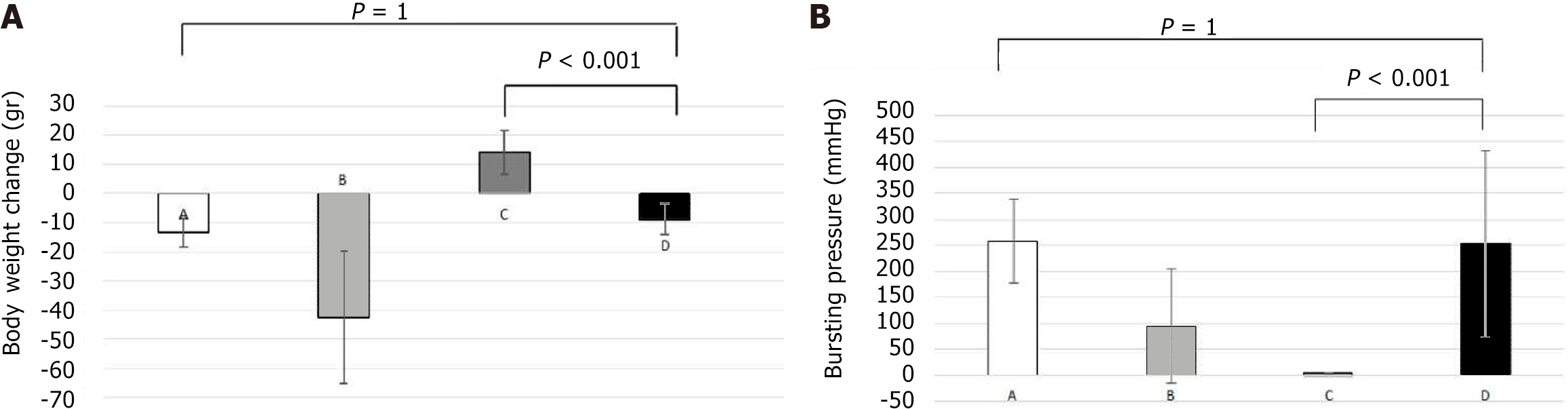
Body weight was measured with precision scales in all rats following anesthetization of the experimental animals pre-operatively and on the day of euthanasia. In group A, there was a statistically significant reduction in mean body weight during the experiment and until euthanasia (P = 0.005). In the combined (D) group, a decrease in mean body weight was observed during the experiment, but was not statistically significant (P = 0.181). No statistically significant decrease in body weight was observed between groups D and A (P = 1.0). Statistical significance was also recorded in the weight difference between groups B and D (P = 0.001) and C and D (P < 0.001). Figure 1A presents the mean values of body weights and body weight changes.
No anastomotic dehiscence was noted in the control group, while 2 rats (10%) were noted to have anastomotic dehiscence in group D. The difference between groups A, B and C, compared to group D is not statistically significant (P = 0.4).
Group D did not show statistically significant bursting pressure values compared to the control group (P = 1.0), but presented statistically significant increased values compared to group B (P < 0.001). In contrast, group D presented statistically significant lower bursting pressure values, when compared to group C (P < 0.001).
In numerous experimental animals, a bowel perforation was observed away from the anastomosis during the process of bursting pressure measurement. A rupture was observed in 50% of the rats in group D, compared to 60% in the control group, but this difference was not statistically significant (P = 0.745). In addition, rupture rate at the site of the anastomosis was significantly increased in group B compared to group D (64% vs 50%, P = 0.490). The sites of rupture among the groups when assessing the anastomotic bursting pressure are presented in Figure 1B.
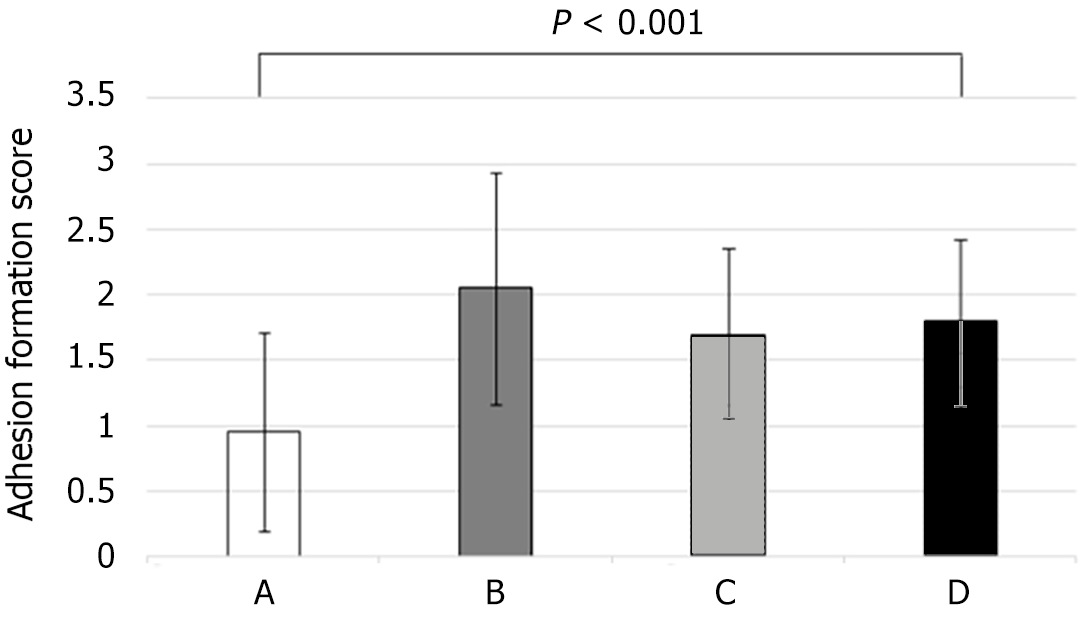
In group A, no adhesions were noted in 30% of the rats and no rats showed adhesions postoperatively graded with score 3 according to Van der Hamm’s scale. Adhesions were present in all animals in group D. Specifically, 30% of the rats had an adhesion score of 1, 60% had an adhesion score of 2 and 10% had an adhesion score of 3. Figure 2 presents the incidence of adhesion formation scores. Group A had statistically significant lower adhesion formation than group D (P = 0.001). Group D had fewer adhesions than group B, but the difference was not statistically significant (P = 1.0). When group B was compared with group D, it was found that group B presented a higher adhesion score, but with no statistically significant difference (P = 1.0).
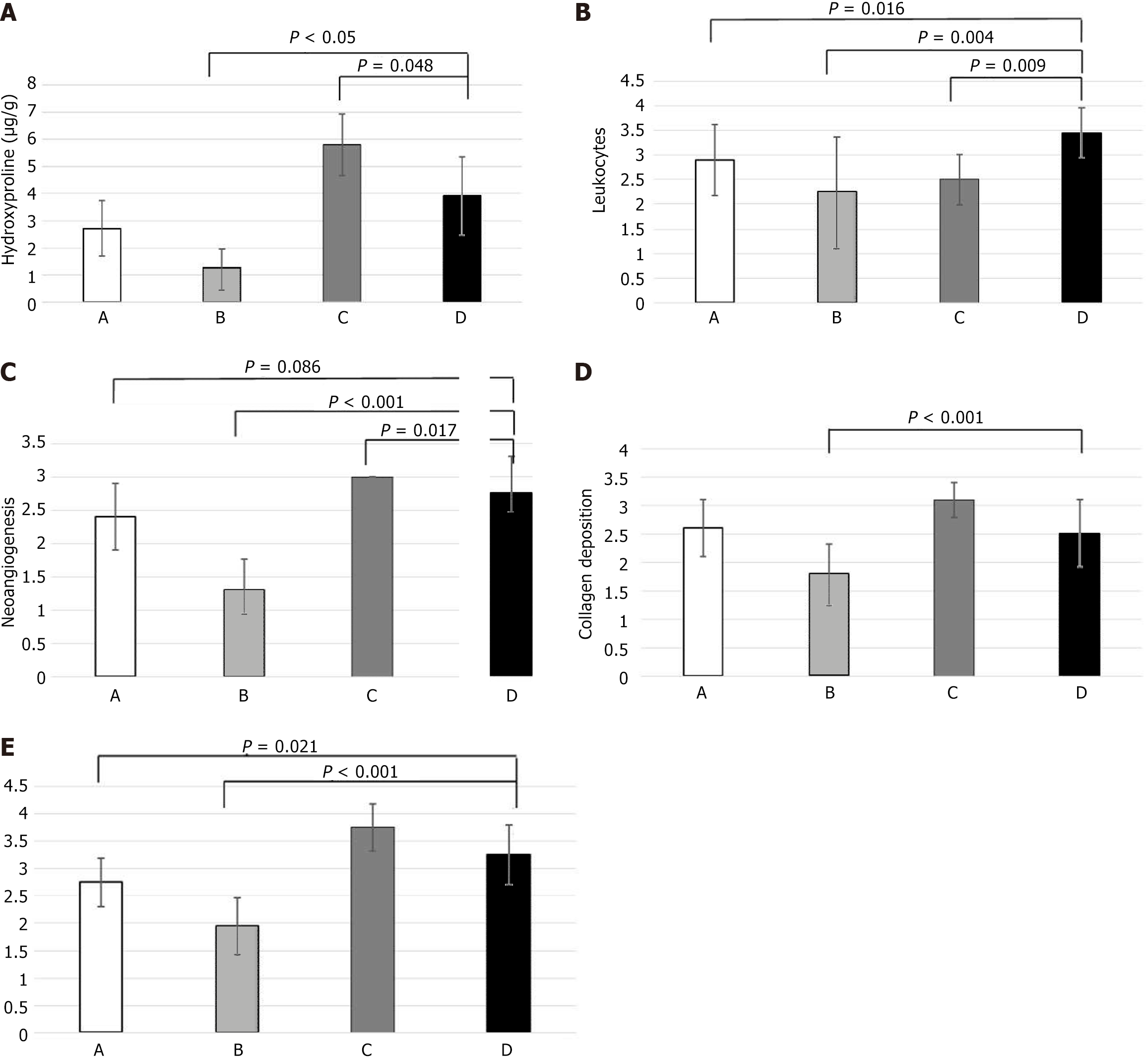
A segment of the anastomosis was sent for measurement of hydroxyproline levels for quantification of the amount of collagen. Group D showed a significantly higher mean level of hydroxyproline than group B (P = 0.05) and a significantly lower mean level than group C (P = 0.048). Compared to the control group, group D showed higher values of hydroxyproline, however the difference was not statistically significant. Figure 3A summarizes the values of hydroxyproline among the groups.
Anastomotic healing was assessed histologically by measuring inflammatory cell infiltration, neoangiogenesis, fibroblast activity levels and collagen deposition. Statistical data showed significant differences in group D compared to the control group in two histological parameters: inflammation (3.450 vs 2.900, P = 0.016) and fibroblast activity (2.75 vs 3.25, P = 0.021). Neoangiogenesis and collagen deposition were not significantly different between the control group and Group D.
The average inflammatory cell infiltration was significantly higher in group D than in the control group (P < 0.001), group B (P = 0.004) and group C (P = 0.009). With regard to neoangiogenesis, group D demonstrated a statistically significant increase compared to group B (P < 0.001). Group C and D were found to have a significant increase in neoangiogenesis (P = 0.017). Collagen deposition was significantly increased in group D compared to B (P < 0.001). A statistically significant increase in fibroblast activity was found in group D compared to group A (P = 0.021) and group B (P < 0.001). Figure 3B-E summarize inflammatory cell infiltration, neoangiogenesis, collagen deposition and fibroblast activity, respectively.
Tissue healing is a complex pathophysiological process which is divided into 4 phases: Hemostasis, the inflammatory phase, the proliferative phase, and the remodeling phase[33]. These phases are distinct and present in a consecutive order. Extrinsic factors can inhibit one or more of the healing phases resulting in delayed healing or an insufficient tissue defect[34]. Similar to any other tissue, the colon follows the same consecutive phases of healing after trauma, surgery etc. A healing delay or defect will have significant repercussions, such as dehiscence or rupture of an anastomosis[7]. Chemotherapy is one systemic factor that can cause healing delay in patients with colon cancer that require excision and colon reconstruction via an anastomosis[35]. It has been established by numerous studies that a surgical wound, such a colonic anastomosis, can be ameliorated using GH, a strong healing promoter[26,36-40].
Factors that have been used to restrict the negative effects of chemotherapy on the healing process of colonic anastomosis are synthetic adhesives (fibrin glue) and the administration of Milepost, interferon or GM-CSF factors[41-44]. A combination of fibrin glue and GH has also been experimented, showing an improvement in the physical and histological characteristics of the anastomosis[45]. While these studies have shown positive results, they do not fully reverse the adverse effects of chemotherapy. In our study, GH managed to overcome the negative effects that chemotherapeutics induced on body weight changes during the experiment. This was shown in group D, as the average body weight loss in rats was statistically significantly smaller than the loss in group B. The body weight loss in group D did not differ significantly from that of the control group (9.00 g vs 13.00 g, P = 1.0).
The administration of GH in group D resulted in a decreased rate of anastomotic rupture and dehiscence compared to group B which is an important indicator of the positive effect of GH. The high rate of anastomotic rupture (40%) observed in group B was improved by GH, which was reduced to a lower level (10%).
The anastomoses in group D maintained their mechanical strength. Although the mechanical strength was lower in group D than in group C, it was proportional to that of the control group. The rupture level during the bursting pressure test in the anastomoses of group D was 50%, compared to 36% in group B. This result confirms the increased anastomoses strength. Adhesion formation around the anastomosis site further enhanced the mechanical anastomosis strength. GH led to a statistically significant larger number of adhesions at the anastomosis site in group D compared to those observed in the control group. These adhesions were fewer compared to group C with no statistically significant difference.
Hydroxyproline level assessment is a reliable method of measuring the total collagen formed during wound healing. Thus, collagen formation was significantly higher in group D compared to group B, while not different to that in group A.
GH had an evident beneficial effect as it was shown to overcome the negative effect of chemotherapeutics in collagen synthesis. A contiguous study in 2016 researched the positive effect of GH on colonic anastomoses in rats receiving the immunosuppressive drug everolimus[46]. The experiment showed that GH improved the negative effects of everolimus on colonic anastomosis, taking bursting pressure and hydroxyproline concentration into account. During the histological assessment of tissue healing in this experiment, inflammation, defined by inflammatory cell infiltration (white blood cell count), remained unchanged in group D compared to group B. Neoangiogenesis on the other hand was significantly increased in group D in the presence of GH compared to group B. The increase in neovascularization secondary to GH administration is a potential compensatory factor to the negative effects of chemotherapeutics.
Finally, GH administration resulted in a higher rate of fibroblast activation and collagen deposition. Thus, group D showed a significant increase in fibroblasts and neocollagen compared to group B. It could therefore be deduced that the increase in mechanical strength of anastomoses due to GH action is due to fibroblast activation and increased collagen deposition in the anastomosis.
The immediate postoperative intraperitoneal administration of 5-FU, bleomycin and cisplatin in rats has a negative effect in the healing process of colonic anastomoses. Specifically, it impairs fibroblast proliferation and reduces collagen formation and deposition at the anastomosis site. Thus, an increase in the frequency of anastomotic rupture and reduction of mechanical strength are observed, as expressed by the decrease in the values of corresponding bursting pressure. In addition, inflammation is increased while neoangiogenesis is reduced at the anastomosis site.
The action of GH on colon anastomoses in rats resulted in a qualitative and quantitative increase in collagen levels, as shown by hydroxyproline levels. The result was an increase in the mechanical strength of the anastomoses, as expressed by the increase in bursting pressure values.
Administration of GH to rats undergoing intraperitoneal administration of 5-FU, bleomycin and cisplatin resulted in an increase of their metabolic profile as directly expressed by a mean increase in body weight. Simultaneously, GH caused an increase in the mechanical strength of anastomoses at levels not statistically different from those of the control group. As a result, the rate of anastomotic rupture and dehiscence in rats following intraperitoneal administration of 5-FU, bleomycin and cisplatin was reduced when GH was simultaneously administered, mimicking the characteristics of the control group anastomoses. In conclusion, GH administration to rats undergoing intraperitoneal administration of 5-FU, bleomycin and cisplatin results in attenuation of the negative effects of these chemotherapeutic agents on the healing of colonic anastomoses.
| 1. | Goulder F. Bowel anastomoses: The theory, the practice and the evidence base. World J Gastrointest Surg. 2012;4:208-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 2. | Karanjia ND, Corder AP, Holdsworth PJ, Heald RJ. Risk of peritonitis and fatal septicaemia and the need to defunction the low anastomosis. Br J Surg. 1991;78:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 160] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Kanellos I, Blouhos K, Demetriades H, Pramateftakis MG, Mantzoros I, Zacharakis E, Betsis D. The failed intraperitoneal colon anastomosis after colon resection. Tech Coloproctol. 2004;8 Suppl 1:s53-s55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Verhofstad MH, Lange WP, van der Laak JA, Verhofstad AA, Hendriks T. Microscopic analysis of anastomotic healing in the intestine of normal and diabetic rats. Dis Colon Rectum. 2001;44:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Tritt L. Nutritional assessment and support of kidney transplant recipients. J Infus Nurs. 2004;27:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Shogan BD, Carlisle EM, Alverdy JC, Umanskiy K. Do we really know why colorectal anastomoses leak? J Gastrointest Surg. 2013;17:1698-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 7. | Kanellos I, Vasiliadis K, Angelopoulos S, Tsachalis T, Pramateftakis MG, Mantzoros I, Betsis D. Anastomotic leakage following anterior resection for rectal cancer. Tech Coloproctol. 2004;8 Suppl 1:s79-s81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Durães Lde C, Durães EF, Lobato LF, Oliveira PG, Sousa JB. Correlation between bursting pressure and breaking strength in colonic anastomosis. Acta Cir Bras. 2013;28:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, Ungerleider JS, Emerson WA, Tormey DC, Glick JH, Veeder MH, Mailliard JA. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 657] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 10. | Macdonald JS, Astrow AB. Adjuvant therapy of colon cancer. Semin Oncol. 2001;28:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Sugarbaker PH, Gianola FJ, Barofsky I, Hancock SL, Wesley R. 5-Fluorouracil chemotherapy and pelvic radiation in the treatment of large bowel cancer. Decreased toxicity in combined treatment with 5-fluorouracil administration through the intraperitoneal route. Cancer. 1986;58:826-831. [PubMed] [DOI] [Full Text] |
| 12. | Sugarbaker PH. Intraperitoneal chemotherapy for treatment and prevention of peritoneal carcinomatosis and sarcomatosis. Dis Colon Rectum. 1994;37:S115-S122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Goel R, Cleary SM, Horton C, Kirmani S, Abramson I, Kelly C, Howell SB. Effect of sodium thiosulfate on the pharmacokinetics and toxicity of cisplatin. J Natl Cancer Inst. 1989;81:1552-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Piccart MJ, Abrams J, Dodion PF, Crespeigne N, Sculier JP, Pector JC, Finet C, Nouwijnck C, Bondue H, Atassi G. Intraperitoneal chemotherapy with cisplatin and melphalan. J Natl Cancer Inst. 1988;80:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Los G, Mutsaers PH, Ruevekamp M, McVie JG. The use of oxaliplatin versus cisplatin in intraperitoneal chemotherapy in cancers restricted to the peritoneal cavity in the rat. Cancer Lett. 1990;51:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Markman M, Hakes T, Reichman B, Hoskins W, Rubin S, Jones W, Almadrones L, Yordan EL Jr, Eriksson J, Lewis JL Jr. Intraperitoneal cisplatin and cytarabine in the treatment of refractory or recurrent ovarian carcinoma. J Clin Oncol. 1991;9:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Martens MF, Hendriks T, Wobbes T, De Pont JJ. Intraperitoneal cytostatics impair early post-operative collagen synthesis in experimental intestinal anastomosesP6. Br J Cancer. 1992;65:649-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | de Roy van Zuidewijn DB, Hendriks T, Wobbes T, de Boer HH. Intraperitoneal cytostatics impair healing of experimental intestinal anastomoses. Br J Cancer. 1991;63:937-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Graf W, Westlin JE, Påhlman L, Glimelius B. Adjuvant intraperitoneal 5-fluorouracil and intravenous leucovorin after colorectal cancer surgery: a randomized phase II placebo-controlled study. Int J Colorectal Dis. 1994;9:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Kanellos I, Mantzoros I, Goulimaris I, Zacharakis E, Zavitsanakis A, Betsis D. Effects of the use of fibrin glue around the colonic anastomosis of the rat. Tech Coloproctol. 2003;7:82-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Arikan AY, Senel FM, Akman RY, Can C. Comparison of the effects of various anticancer agents on intestinal anastomosis after intraperitoneal administration. Surg Today. 1999;29:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Hu Q, Xu J, Ke J, Zhang Z, Chu T. S-1 and 5-Fluorouracil-related adverse events in patients with advanced gastric cancer: A meta-analysis. PLoS One. 2023;18:e0290003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Negarandeh R, Salehifar E, Saghafi F, Jalali H, Janbabaei G, Abdhaghighi MJ, Nosrati A. Evaluation of adverse effects of chemotherapy regimens of 5-fluoropyrimidines derivatives and their association with DPYD polymorphisms in colorectal cancer patients. BMC Cancer. 2020;20:560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Romani AMP. Cisplatin in cancer treatment. Biochem Pharmacol. 2022;206:115323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 154] [Reference Citation Analysis (0)] |
| 25. | Christensen H, Jørgensen PH, Oxlund H, Laurberg S. Growth hormone increases the mass, the collagenous proteins, and the strength of rat colon. Scand J Gastroenterol. 1990;25:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Lyra Junior HF, de Lucca Schiavon L, Rodrigues IK, Couto Vieira DS, de Paula Martins R, Turnes BL, Latini AS, D'Acâmpora AJ. Effects of Ghrelin on the Oxidative Stress and Healing of the Colonic Anastomosis in Rats. J Surg Res. 2019;234:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Oines MN, Krarup PM, Jorgensen LN, Agren MS. Pharmacological interventions for improved colonic anastomotic healing: a meta-analysis. World J Gastroenterol. 2014;20:12637-12648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li P, Li X, McKnight JR, Satterfield MC, Spencer TE. Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids. 2011;40:1053-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 451] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 29. | Rygl M, Novotna J, Herget J, Skaba R, Snajdauf J. Parameters of healing in approximative intestinal anastomosis. Eur J Pediatr Surg. 2009;19:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Phillips JD, Kim CS, Fonkalsrud EW, Zeng H, Dindar H. Effects of chronic corticosteroids and vitamin A on the healing of intestinal anastomoses. Am J Surg. 1992;163:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 146] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Lambrou I, Mantzoros I, Ioannidis O, Tatsis D, Chasampalioti M, Bitsianis S, Pramateftakis MG, Kotidis E, Ouzounidis N, Angelopoulos S, Dimitriadis C, Tsalis K. The effect of chemotherapy with 5-fluorouracil, bleomycin and cisplatin in the healing of colonic anastomoses in rats. Ann Ital Chir. 2020;91:552-562. [PubMed] |
| 32. | Lambrou I, Ioannidis O, Manztoros I, Tatsis D, Chasampalioti M, Varnalidis I, Pramateftakis MG, Kotidis E, Ouzounidis N, Angelopoulos S, Zaramboukas T, Demetriades H, Tsalis K. The effects of growth hormone on the healing of colonic anastomoses in rats. Ann Ital Chir. 2021;92:424-434. [PubMed] |
| 33. | Larouche J, Sheoran S, Maruyama K, Martino MM. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv Wound Care (New Rochelle). 2018;7:209-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 381] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 34. | Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of Acute and Chronic Wound Healing. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 463] [Article Influence: 115.8] [Reference Citation Analysis (0)] |
| 35. | Raptis D, Pramateftakis MG, Kanellos I. Our 20-year experience with experimental colonic anastomotic healing. J Med Life. 2018;11:5-14. [PubMed] |
| 36. | Wheeless CR Jr, Zanagnolo V, Bowers D, Brenner MJ, Lilley R. The effect of growth hormone on the bursting strength of ileal anastomotic segments in radiation-injured rat bowel. Gynecol Oncol. 1998;70:121-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Ward HC, Halliday D, Sim AJ. Protein and energy metabolism with biosynthetic human growth hormone after gastrointestinal surgery. Ann Surg. 1987;206:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 151] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Hammarqvist F, Strömberg C, von der Decken A, Vinnars E, Wernerman J. Biosynthetic human growth hormone preserves both muscle protein synthesis and the decrease in muscle-free glutamine, and improves whole-body nitrogen economy after operation. Ann Surg. 1992;216:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Yarimkaya A, Apaydin B, Unal E, Karabicak I, Aydogan F, Uslu E, Erginoz E, Artis T, Eyuboglu E. Effects of recombinant human growth hormone and nandrolone phenylpropionate on the healing of ischemic colon anastomosis in rats. Dis Colon Rectum. 2003;46:1690-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Donmez R, Oren D, Ozturk G, Kisaoglu A, Ozogul B, Atamanalp SS. The combined effects of glutamine and growth hormone on intestinal anastomosis in the rat intra-abdominal sepsis model. J Surg Res. 2013;182:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Kanellos I, Mantzoros I, Demetriades H, Kalfadis S, Kelpis T, Sakkas L, Betsis D. Healing of colon anastomoses covered with fibrin glue after immediate postoperative intraperitoneal administration of 5-fluorouracil. Dis Colon Rectum. 2004;47:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Kanellos I, Christoforidis E, Kanellos D, Pramateftakis MG, Sakkas L, Betsis D. The healing of colon anastomosis covered with fibrin glue after early postoperative intraperitoneal chemotherapy. Tech Coloproctol. 2006;10:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Bostanoğlu S, Dinçer S, Keskin A, Bostanoğlu A, Dursun A, Serim C. Beneficial effect of Iloprost on impaired colonic anastomotic healing induced by intraperitoneal 5-fluorouracil infusion. Dis Colon Rectum. 1998;41:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Erdem E, Dinç S, Erdem D, Ustün H, Caydere M, Alagöl H. Effects of intraperitoneal chemotherapy and GM-CSF on anastomotic healing: an experimental study in rats. J Surg Res. 2002;108:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Zhang SY, Shi L, Chi Q. [Effects of fibrin glue and growth hormone on the healing of colon anastomoses in the condition of immediate postoperative intraperitoneal chemotherapy]. Zhonghua Wei Chang Wai Ke Za Zhi. 2006;9:452-454. [PubMed] |
| 46. | Küper MA, Trütschel S, Weinreich J, Königsrainer A, Beckert S. Growth hormone abolishes the negative effects of everolimus on intestinal wound healing. World J Gastroenterol. 2016;22:4321-4329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |