Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2565
Revised: July 2, 2024
Accepted: July 4, 2024
Published online: August 27, 2024
Processing time: 98 Days and 3.1 Hours
Pediatric appendicitis is a common cause of abdominal pain in children and is recognized as a significant surgical emergency. A prompt and accurate diagnosis is essential to prevent complications such as perforation and peritonitis.
To investigate the predictive value of the systemic immune-inflammation index (SII) combined with the pediatric appendicitis score (PAS) for the assessment of disease severity and surgical outcomes in children aged 5 years and older with appendicitis.
Clinical data of 104 children diagnosed with acute appendicitis were analyzed. The participants were categorized into the acute appendicitis group and chronic appendicitis group based on disease presentation and further stratified into the good prognosis group and poor prognosis group based on prognosis. The SII and PAS were measured, and a joint model using the combined SII and PAS was constructed to predict disease severity and surgical outcomes.
Significant differences were observed in the SII and PAS parameters between the acute appendicitis group and chronic appendicitis group. Correlation analysis showed associations among the SII, PAS, and disease severity, with the combined SII and PAS model demonstrating significant predictive value for assessing disease severity [aera under the curve (AUC) = 0.914] and predicting surgical outcomes (AUC = 0.857) in children aged 5 years and older with appendicitis.
The study findings support the potential of integrating the SII with the PAS for assessing disease severity and predicting surgical outcomes in pediatric appendicitis, indicating the clinical utility of the combined SII and PAS model in guiding clinical decision-making and optimizing surgical management strategies for pediatric patients with appendicitis.
Core Tip: This study investigates the combined use of the Systemic Immune-inflammation index (SII) and pediatric appendicitis score (PAS) to predict disease severity and surgical outcomes in children aged 5 years and older with appendicitis. The results demonstrate significant improvements in clinical decision-making and prognosis assessment. Integrating SII and PAS provides a comprehensive assessment of the systemic inflammatory response and clinical symptoms, offering a novel and effective approach to managing pediatric appendicitis.
- Citation: Guo LM, Jiang ZH, Liu HZ. Systemic immune-inflammation index combined with pediatric appendicitis score in assessing the severity and prognosis for paediatric appendicitis. World J Gastrointest Surg 2024; 16(8): 2565-2573
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2565.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2565
Acute appendicitis is one of the most common pediatric surgical emergencies, with a lifetime risk estimated to be around 7%-8%[1]. It is a significant cause of morbidity and requires a prompt diagnosis and timely surgical intervention to prevent potential complications such as perforation and peritonitis[2,3]. Children often present with atypical symptoms, which can pose diagnostic challenges, leading to a high rate of negative appendectomies due to diagnostic uncertainty[4-6]. Therefore, reliable tools must be developed for accurately assessing disease severity and predicting surgical outcomes in pediatric patients with appendicitis.
The systemic immune-inflammation index (SII) is a novel biomarker that reflects the balance between the systemic inflammatory response and the host immune status[7,8]. It is calculated based on the counts of peripheral blood neutrophils, lymphocytes, and platelets, and it has been shown to have prognostic value in various inflammatory and neoplastic conditions[9]. Furthermore, the pediatric appendicitis score (PAS) is a widely used clinical scoring system that incorporates various symptoms, signs, and laboratory findings to aid in the diagnosis of pediatric appendicitis.
In recent years, there has been growing interest in exploring the potential of combining SII with established clinical scoring systems to improve the precision of evaluating disease severity and predicting outcomes in various medical conditions[10]. However, the utility of this combined approach in pediatric appendicitis remains relatively unexplored. Therefore, this study aimed to investigate the predictive value of the SII combined with the PAS for the assessment of disease severity and surgical outcomes in children aged 5 years and older with appendicitis[11,12].
The assessment of disease severity in pediatric appendicitis is crucial for guiding clinical decision-making, including the need for surgical intervention and the appropriate timing of surgical management. Several clinical scoring systems, such as the Alvarado score and the appendicitis inflammatory response (AIR) score, have been developed to assist in the clinical evaluation of pediatric appendicitis[13-15]. However, these scores have limitations, particularly in cases where atypical presentations or diagnostic ambiguity are encountered.
The inclusion of the SII in the assessment of disease severity may offer additional insights into the inflammatory and immune status of pediatric patients with appendicitis[16,17]. Neutrophil, lymphocyte, and platelet counts are routinely assessed in clinical practice, and their integration into a composite index like the SII provides a comprehensive reflection of the systemic immune-inflammatory milieu[18]. Furthermore, the incorporation of SII into the assessment of disease severity may enable a holistic evaluation of the pathophysiological processes underlying pediatric appendicitis, potentially enhancing the accuracy of predicting outcomes and making clinical decisions.
In addition to assessing disease severity, the prediction of surgical outcomes is a critical aspect of managing pediatric patients with appendicitis. The identification of patients at high risk of complicated appendicitis or postsurgical compli
In conclusion, the investigation of novel biomarkers and their integration with established clinical scoring systems is promising for enhancing the precision and accuracy of prognostic assessment in pediatric appendicitis. By exploring the predictive value of the SII combined with the PAS, this study aimed to provide valuable insights into the potential of a comprehensive and integrated approach to assess disease severity and predict the outcome in children aged 5 years and older with appendicitis.
The study retrospectively analyzed the clinical data of children diagnosed with acute appendicitis admitted to our hospital from June 2021 to June 2023, totaling 104 cases. The participants were categorized into the acute appendicitis group (n = 51) and chronic appendicitis group (n = 53) based on the disease presentation. Furthermore, on the basis of the prognosis, the participants were divided into the good prognosis group (n = 90) and the poor prognosis group (n = 14). This study was approved by the Ethics Committee of Qingdao Women and Children’s Hospital (No. QFELL-YJ-2024-24), and the Ethics Committee agreed to waive informed consent.
Inclusion criteria: Patients who met the diagnostic criteria for appendicitis and had a clear history of right lower abdominal pain[21], with symptom onset between 4 and 72 h, were included in the study.
Exclusion criteria: Individuals with coagulation disorders, those with mental or cognitive impairments, individuals with other infectious or immunodeficiency diseases, patients with organ dysfunctions such as cardiac or pulmonary conditions, those with malignant tumors or other acute abdominal conditions, and individuals who had received other medical treatments prior to admission were excluded from the study.
Grouping method: On the basis of the presence of symptoms of acute appendicitis in the children, they were categorized into the acute appendicitis group and chronic appendicitis group.
Symptoms of acute appendicitis: The children exhibited migrating right lower abdominal pain, initially presenting in the upper to mid abdomen or periumbilical region, which later shifted and localized to the right lower abdomen. The pain was characterized by inaccuracy in localization, continuous nature, and progressive aggravation. Additionally, the children experienced symptoms of nausea and vomiting, which were attributed to intestinal inflammation. Low-grade fever, with temperatures between 37.5 °C and 38.5 °C, and high fever for severe cases were also observed.
Measurement of SII: Neutrophil and platelet count: Prior to treatment, fasting venous blood samples of 10 mL were collected from all participants. The samples were centrifuged at 16600 r/min for 10 minutes (with a centrifugal radius of 12.5 cm), and 1.5 mL of the supernatant was extracted. The patients' neutrophil levels were measured by using a fully automatic chemiluminescence immunoassay analyzer (Shanghai De Rui Ke Medical Equipment Co., Ltd.), and serum procalcitonin levels were detected using a double-antibody sandwich chemiluminescent method.
Lymphocyte count: Approximately 5 mL of peripheral venous blood from the patients was collected using a vacuum collection tube, and anticoagulation was performed with EDTA-K2. The samples were transported and stored at room temperature. About 20 μL of antibody was added to a Trucount tube, and 50 μL of anticoagulated whole blood was added using a reverse addition method. The samples were vortex-mixed and then kept in the dark at around 24 °C for 15 minutes. Subsequently, the patients' lymphocytes were analyzed by using a flow cytometer.
SII: The SII was calculated using the following formula: SII = (platelet count × neutrophil count)/lymphocyte count.
The Alvarado scoring criteria included the following: 1 point for migrating right lower abdominal pain, 1 point for anorexia, 1 point for nausea or vomiting, 2 points for tenderness in the right lower abdomen, 1 point for rebound tenderness in the right lower abdomen, 1 point for a temperature > 37.5 °C, and 2 points for a white blood cell count > 10 × 109/L. The total score was calculated by summing the scores of the aforementioned items, with high scores indicating a high likelihood of appendicitis. Patients with a score of 0-3 were considered low risk, suggesting an unlikely possibility of appendicitis. Patients with a score of ≥ 4 were categorized as moderate risk and should undergo further imaging studies to evaluate the likelihood of appendicitis. Patients with a score of ≥ 7 were deemed high risk, indicating a substantial likelihood of acute appendicitis.
The AIR score criteria included the following: 1 point for vomiting; 1 point for tenderness at McBurney's point; rebound tenderness or muscle rigidity (mild: 1 point, moderate: 2 points, and severe: 3 points); 1 point for temperature ≥ 38 °C; 1 point for white blood cell count (109/L), with an additional 2 points if the count is ≥ 15; 1 point for neutrophil ratio (70%-84%, or 2 points if ≥ 85); and 1 point for C-reactive protein (mg/L; 10-49: 1 point, ≥ 50: 2 points). The total score ranged from 0 to 12, with high scores indicating severe clinical symptoms in the child.
Clinical and demographic data were collected from medical records, including age, gender, body mass index (BMI), duration of symptoms, and mode of delivery. Additionally, laboratory values such as neutrophil count, lymphocyte count, and platelet count, which were used to calculate the SII, were recorded. The PAS, Alvarado score, AIR score, and imaging findings score were also evaluated for each patient.
The data were analyzed using SPSS 25.0 statistical software (SPSS Inc., Chicago, IL, United States). For categorical data, n (%) was used for representation. The χ2 test was applied with the basic formula when the sample size was ≥ 40 and the theoretical frequency T was ≥ 5, with the test statistic represented by χ2. When the sample size was ≥ 40 but the theoretical frequency 1 ≤ T < 5, the χ2 test was adjusted using the correction formula. In cases where the sample size was < 40 or the theoretical frequency T < 1, statistical analysis was conducted using Fisher's exact probability method. For normally distributed continuous data, the format (mean ± SD) was employed. Non-normally distributed data were analyzed using Wilcoxon rank-sum test. Statistical significance was set at P < 0.05.
In this study, we evaluated the predictive value of the SII combined with the PAS for the assessment of disease severity and surgical outcomes in children aged 5 years and older with appendicitis (Table 1). A total of 104 children were included, with 51 in the acute appendicitis group and 53 in the chronic appendicitis group. The results of our study revealed no statistically significant differences in demographic characteristics, including age (9.75 ± 2.14 years vs 9.48 ± 2.05 years; t = 0.654, P = 0.515), gender distribution (male/female, 26/25 vs 29/24; t = 0.034, P = 0.853), and BMI (18.52 ± 3.21 kg/m2vs 18.76 ± 3.45 kg/m2; t = 0.367, P = 0.714), between the acute appendicitis and chronic appendicitis groups. Similarly, the mode of delivery showed no significant difference between the groups (vaginal delivery: 56.86% vs 54.72%; cesarean section: 43.14% vs 45.28%; t = 0.001, P = 0.982). However, the duration of symptoms was significantly different, with the acute appendicitis group exhibiting a mean duration of 2.61 ± 1.73 days compared to 30.15 ± 2.66 days in the chronic appendicitis group (t = 62.809, P < 0.001). These findings provide important insights into the demographic characteristics of pediatric patients with appendicitis, laying the groundwork for further investigation into the prognostic value of SII combined with PAS in this population.
| Demographic characteristic | Acute appendicitis group (n = 51) | Chronic appendicitis group (n = 53) | t value | P value |
| Age (years) | 9.75 ± 2.14 | 9.48 ± 2.05 | 0.654 | 0.515 |
| Gender (male/female), n (%) | 26 (50.98)/25 (49.02) | 29 (54.72)/24 (45.28) | 0.034 | 0.853 |
| BMI (kg/m2) | 18.52 ± 3.21 | 18.76 ± 3.45 | 0.367 | 0.714 |
| Duration of symptoms (days) | 2.61 ± 1.73 | 30.15 ± 2.66 | 62.809 | < 0.001 |
| Mode of delivery, n (%) | ||||
| Vaginal delivery, n (%) | 29 (56.86) | 29 (54.72) | 0.001 | 0.982 |
| Cesarean section, n (%) | 22 (43.14) | 24 (45.28) |
The comparison of the SII between the acute appendicitis and chronic appendicitis groups revealed significant differences in neutrophil count, lymphocyte count, platelet count, and the SII itself (Table 2). The acute appendicitis group exhibited a higher mean neutrophil count (7.82 ± 2.03 × 109/L) compared to the chronic appendicitis group (6.76 ± 1.87 × 109/L; t = 2.754, P = 0.007). Conversely, the mean lymphocyte count was significantly lower in the acute appendicitis group (2.54 ± 0.87 × 109/L) than in the chronic appendicitis group (3.11 ± 0.92 × 109/L; t = 3.233, P = 0.002). Additionally, the platelet count was marginally higher in the acute appendicitis group (290.62 ± 45.91 × 109/L) compared to the chronic appendicitis group (271.34 ± 47.68 × 109/L; t = 2.101, P = 0.038). The combined SII value was significantly elevated in the acute appendicitis group (660.41 ± 80.34) relative to the chronic appendicitis group (605.27 ± 78.25; t = 3.544, P < 0.001). These findings indicated the potential of the SII as a valuable biomarker for distinguishing between acute and chronic appendicitis in children aged 5 years and older, highlighting its role in assessing disease severity and guiding clinical decision-making in pediatric appendicitis.
| Parameter | Acute appendicitis group (n = 51) | Chronic appendicitis group (n = 53) | t value | P value |
| Neutrophil count (× 109/L) | 7.82 ± 2.03 | 6.76 ± 1.87 | 2.754 | 0.007 |
| Lymphocyte count (× 109/L) | 2.54 ± 0.87 | 3.11 ± 0.92 | 3.233 | 0.002 |
| Platelet count (× 109/L) | 290.62 ± 45.91 | 271.34 ± 47.68 | 2.101 | 0.038 |
| SII | 660.41 ± 80.34 | 605.27 ± 78.25 | 3.544 | < 0.001 |
The comparison of PAS between the acute appendicitis and chronic appendicitis groups demonstrated significant differences in Alvarado score, AIR score, imaging findings score, and the overall PAS (Table 3). The Alvarado score was lower in the acute appendicitis group (7.13 ± 1.72) compared to the chronic appendicitis group (7.98 ± 1.85; t = 2.427, P = 0.017). Similarly, the AIR score was significantly lower in the acute appendicitis group (5.05 ± 2.01) than in the chronic appendicitis group (5.91 ± 2.15; t = 2.104, P = 0.038). The imaging findings score also showed a significant difference, with the acute appendicitis group scoring 2.07 ± 0.94, whereas the chronic appendicitis group scored 2.65 ± 1.01 (t = 3.032, P = 0.003). Overall, the combined PAS was significantly higher in the chronic appendicitis group (17.71 ± 3.59) compared to the acute appendicitis group (14.32 ± 3.45; t = 4.925, P < 0.001). These results highlight the potential of the PAS as a valuable clinical scoring system for distinguishing between acute and chronic appendicitis in children aged 5 years and older, underscoring its role in assessing disease severity and guiding clinical decision-making in pediatric appendicitis.
| Parameter | Acute appendicitis group (n = 51) | Chronic appendicitis group (n = 53) | t value | P value |
| Alvarado score | 7.13 ± 1.72 | 7.98 ± 1.85 | 2.427 | 0.017 |
| AIR score | 5.05 ± 2.01 | 5.91 ± 2.15 | 2.104 | 0.038 |
| Imaging findings score | 2.07 ± 0.94 | 2.65 ± 1.01 | 3.032 | 0.003 |
| PAS | 14.32 ± 3.45 | 17.71 ± 3.59 | 4.925 | < 0.001 |
The correlation analysis of the SII, PAS, and disease severity in children aged 5 years and older with appendicitis revealed significant associations (Table 4). The neutrophil count showed a negative correlation with disease severity (r = -0.263, P = 0.007), whereas the lymphocyte count exhibited a positive correlation (r = 0.305, P = 0.002). Additionally, the platelet count demonstrated a negative correlation with disease severity (r = -0.204, P = 0.038), and the SII displayed a negative correlation (r = -0.331, P < 0.001). Moreover, the PAS showed a strong positive correlation with disease severity (r = 0.438, P < 0.001). These findings underscore the potential of utilizing the SII and PAS as valuable tools for assessing disease severity in children aged 5 years and older with appendicitis, laying the groundwork for further investigation into their use in evaluating surgical outcomes.
| Parameter | r | R2 | P value |
| Neutrophil count (× 109/L) | -0.263 | 0.069 | 0.007 |
| Lymphocyte count (× 109/L) | 0.305 | 0.093 | 0.002 |
| Platelet count (× 109/L) | -0.204 | 0.041 | 0.038 |
| SII | -0.331 | 0.11 | < 0.001 |
| Alvarado score | 0.233 | 0.054 | 0.017 |
| AIR score | 0.204 | 0.042 | 0.038 |
| Imaging findings score | 0.287 | 0.082 | 0.003 |
| PAS | 0.438 | 0.192 | < 0.001 |
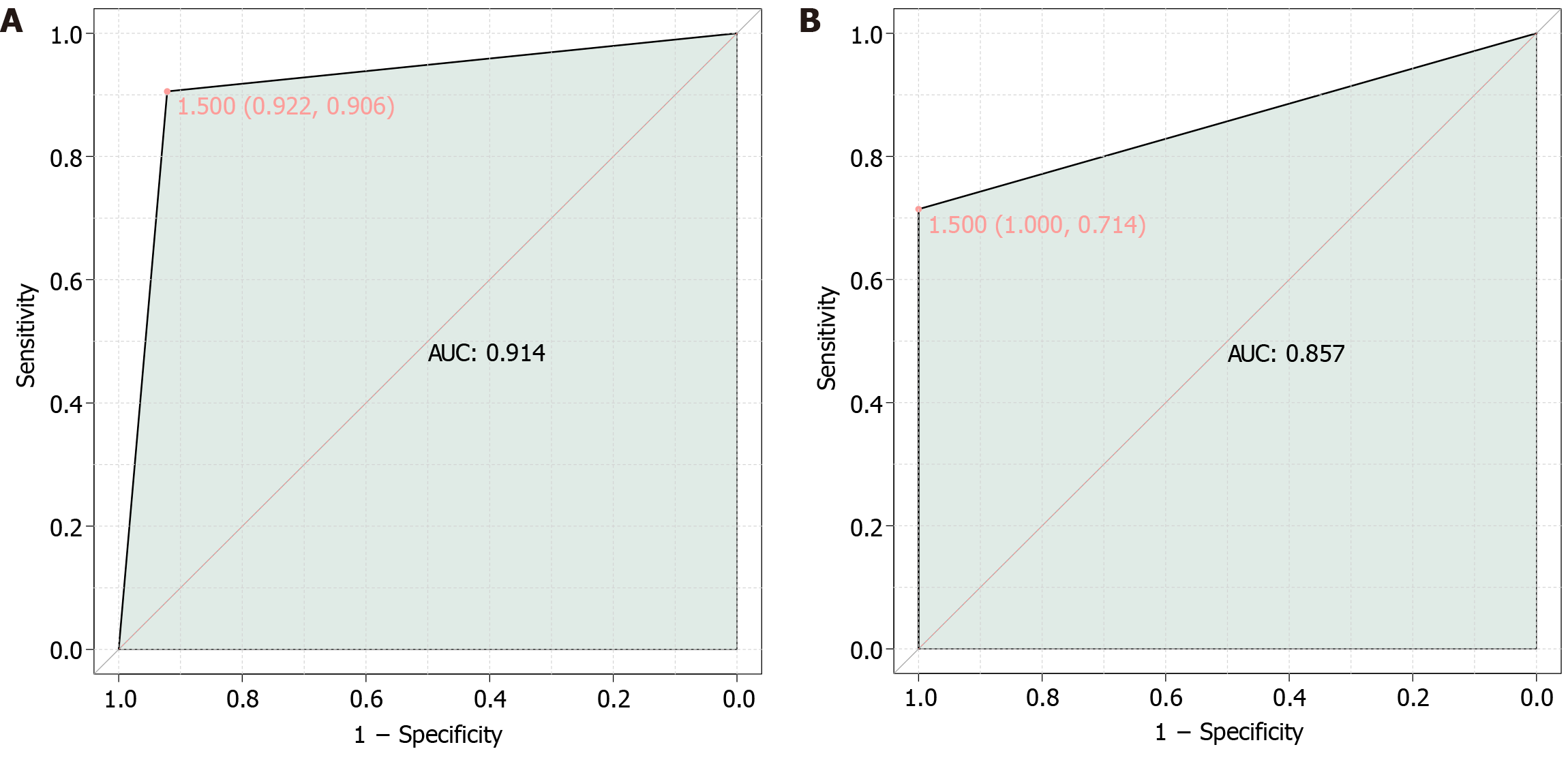
In this study, the SII and PAS were combined to construct a joint model for predicting the condition of children aged 5 years and older with appendicitis (Figure 1A). The results revealed an aera under the curve (AUC) value of 0.914, indicating that the combined SII and PAS model exhibited significant predictive value for assessing the condition of children aged 5 years and older with appendicitis.
Finally, in this study, the SII and PAS were combined to construct a joint model for predicting surgical outcomes in children aged 5 years and older with appendicitis (Figure 1B). The results demonstrated an AUC value of 0.857, indicating that the combined SII and PAS model holds significant predictive value for assessing the surgical outcomes of older children with appendicitis. This result suggested the potential utility of the combined SII and PAS model in effectively predicting surgical outcomes in this patient population.
Acute appendicitis is a common pediatric surgical emergency, necessitating timely diagnosis and intervention to prevent complications such as perforation and peritonitis[22]. However, the atypical presentation of symptoms in children often poses diagnostic challenges, leading to a high rate of negative appendectomies. The development of reliable tools for accurately assessing disease severity and predicting surgical outcomes in pediatric appendicitis is crucial for guiding clinical decision-making[23]. This study's focus on combining the SII with the PAS reflects the growing interest in integrating novel biomarkers with established clinical scoring systems to enhance the accuracy of evaluating disease severity and predicting outcomes in various medical conditions.
The analysis of the SII revealed significant differences in neutrophil count, lymphocyte count, platelet count, and the SII itself between the acute and chronic appendicitis groups. Notably, the SII, which reflects the balance between the systemic inflammatory response and the host immune status, showed a highly significant difference between the two groups. These findings suggested the potential utility of the SII as a valuable biomarker for distinguishing between acute and chronic appendicitis in pediatric patients.
Furthermore, correlation analysis revealed significant associations among the SII, PAS, and disease severity in children aged 5 years and older with appendicitis. The SII exhibited negative correlations with disease severity, highlighting its potential as a marker of disease severity, whereas the PAS showed a strong positive correlation with disease severity, emphasizing its role in assessing the severity of appendicitis in pediatric patients. These findings highlight the value of integrating the SII with the PAS to provide a comprehensive assessment of disease severity and guide clinical decision-making in pediatric appendicitis.
The construction of a joint model using the combined SII and PAS for predicting the condition and surgical outcomes of children aged 5 years and older with appendicitis demonstrated significant predictive value, as evidenced by high AUC values. The AUC values of 0.914 for assessing disease severity and 0.857 for predicting surgical outcomes indicated the robust predictive capability of the combined SII and PAS model in this patient population. These findings suggested the potential clinical utility of this combined approach in enhancing the precision and accuracy of prognostic assessment in pediatric appendicitis, leading to personalized and precise surgical management strategies.
The integration of the SII with the PAS represents a step forward in the search for reliable tools for assessing disease severity and predicting surgical outcomes in pediatric appendicitis[24]. By combining a systemic biomarker reflecting the immune-inflammatory status with an established clinical scoring system, this approach offers a holistic evaluation of the pathophysiological processes underlying pediatric appendicitis[25,26]. The ability to predict disease severity and surgical outcomes with high accuracy has potential for optimizing perioperative care and resource allocation, ultimately leading to improved clinical outcomes in pediatric patients with appendicitis.
By combining the SII, which reflects the systemic immune and inflammatory status, with established clinical scoring systems such as the PAS, healthcare providers can obtain a comprehensive assessment of the patient's condition[27]. This integrated approach allows for a multifaceted evaluation that considers the systemic immune response and the clinical manifestations of the disease, leading to a holistic understanding of disease severity and prognosis. The inclusion of SII in conjunction with clinical scoring systems enhances the prognostic accuracy by providing additional insights into the immune and inflammatory status of pediatric patients with appendicitis. Neutrophil, lymphocyte, and platelet counts, which form the basis of SII calculation, offer valuable information about the systemic inflammatory response and immune status, thereby contributing to a precise prediction of disease severity and surgical outcomes[28].
The strengths of this study lie in its comprehensive analysis of demographic characteristics, biomarker measurements, clinical scoring systems, and correlation analysis to evaluate the combined predictive value of the SII and PAS in children aged 5 years and older with appendicitis. However, certain limitations should be acknowledged. The retrospective nature of the study and the relatively small sample size may have implications for the generalizability of the findings. Therefore, large prospective studies are warranted to validate the findings and further elucidate the clinical utility of the combined SII and PAS approach in pediatric appendicitis.
In conclusion, the findings from this study contribute to the growing body of evidence supporting the potential of integrating the SII with established clinical scoring systems for assessing disease severity and predicting surgical outcomes in pediatric appendicitis. The robust predictive capability of the combined SII and PAS model underscores its clinical utility in guiding clinical decision-making and optimizing surgical management strategies for pediatric patients with appendicitis.
| 1. | Salminen P, Paajanen H, Rautio T, Nordström P, Aarnio M, Rantanen T, Tuominen R, Hurme S, Virtanen J, Mecklin JP, Sand J, Jartti A, Rinta-Kiikka I, Grönroos JM. Antibiotic Therapy vs Appendectomy for Treatment of Uncomplicated Acute Appendicitis: The APPAC Randomized Clinical Trial. JAMA. 2015;313:2340-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 530] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 2. | O'Leary DP, Walsh SM, Bolger J, Baban C, Humphreys H, O'Grady S, Hegarty A, Lee AM, Sheehan M, Alderson J, Dunne R, Morrin MM, Lee MJ, Power C, McNamara D, McCawley N, Robb W, Burke J, Sorensen J, Hill AD. A Randomized Clinical Trial Evaluating the Efficacy and Quality of Life of Antibiotic-only Treatment of Acute Uncomplicated Appendicitis: Results of the COMMA Trial. Ann Surg. 2021;274:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | Salminen P, Sippola S, Haijanen J, Nordström P, Rantanen T, Rautio T, Sallinen V, Löyttyniemi E, Hurme S, Tammilehto V, Laukkarinen J, Savolainen H, Meriläinen S, Leppäniemi A, Grönroos J. Antibiotics versus placebo in adults with CT-confirmed uncomplicated acute appendicitis (APPAC III): randomized double-blind superiority trial. Br J Surg. 2022;109:503-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Minneci PC, Hade EM, Lawrence AE, Sebastião YV, Saito JM, Mak GZ, Fox C, Hirschl RB, Gadepalli S, Helmrath MA, Kohler JE, Leys CM, Sato TT, Lal DR, Landman MP, Kabre R, Fallat ME, Cooper JN, Deans KJ; Midwest Pediatric Surgery Consortium. Association of Nonoperative Management Using Antibiotic Therapy vs Laparoscopic Appendectomy With Treatment Success and Disability Days in Children With Uncomplicated Appendicitis. JAMA. 2020;324:581-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Kang J, Zhang W, Zeng L, Lin Y, Wu J, Zhang N, Xie X, Zhang Y, Liu X, Wang B, Yang R, Jiang X. The modified endoscopic retrograde appendicitis therapy versus antibiotic therapy alone for acute uncomplicated appendicitis in children. Surg Endosc. 2021;35:6291-6299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Patkova B, Svenningsson A, Almström M, Eaton S, Wester T, Svensson JF. Nonoperative Treatment Versus Appendectomy for Acute Nonperforated Appendicitis in Children: Five-year Follow Up of a Randomized Controlled Pilot Trial. Ann Surg. 2020;271:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Mishra S, Johnson L, Gazala MP, Dahiya S, Rahman W, Sreeraj VS. Systemic immune-inflammation index in patients with generalized stage III grade C periodontitis. Oral Dis. 2023;29:3599-3609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Moris D. Comment on "A Randomized Clinical Trial Evaluating the Efficacy and Quality of Life of Antibiotic Only Treatment of Acute Uncomplicated Appendicitis: Results of the COMMA Trial". Ann Surg. 2022;276:e135-e136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Winker M, Stössel S, Neu MA, Lehmann N, El Malki K, Paret C, Joisten N, Bloch W, Zimmer P, Faber J. Exercise reduces systemic immune inflammation index (SII) in childhood cancer patients. Support Care Cancer. 2022;30:2905-2908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Şener K, Çakır A, Kılavuz H, Altuğ E, Güven R. Diagnostic value of systemic immune inflammation index in acute appendicitis. Rev Assoc Med Bras (1992). 2023;69:291-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 11. | Gudjonsdottir J, Marklund E, Hagander L, Salö M. Clinical Prediction Scores for Pediatric Appendicitis. Eur J Pediatr Surg. 2021;31:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Pogorelić Z, Rak S, Mrklić I, Jurić I. Prospective validation of Alvarado score and Pediatric Appendicitis Score for the diagnosis of acute appendicitis in children. Pediatr Emerg Care. 2015;31:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Andersson M, Kolodziej B, Andersson RE; STRAPPSCORE Study Group. Randomized clinical trial of Appendicitis Inflammatory Response score-based management of patients with suspected appendicitis. Br J Surg. 2017;104:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Andersson M, Kolodziej B, Andersson RE. Validation of the Appendicitis Inflammatory Response (AIR) Score. World J Surg. 2021;45:2081-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Deboni VS, Rosa MI, Lima AC, Graciano AJ, Garcia CE. The appendicitis inflammatory response score for acute appendicitis: is it important for early diagnosis? Arq Bras Cir Dig. 2022;35:e1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Bulanova AA, Akhanzaripov ZA. [Immunotherapy for the treatment of acute appendicitis in children]. Khirurgiia (Mosk). 1994;34-36. [PubMed] |
| 17. | Wong DW, Vasinrapee P, Spieth ME, Cook RE, Ansari AN, Jones M Jr, Mandal A. Rapid detection of acute appendicitis with Tc-99m-labeled intact polyvalent human immune globulin. J Am Coll Surg. 1997;185:534-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Markar SR, Karthikesalingam A, Falzon A, Kan Y. The diagnostic value of neutrophil: lymphocyte ratio in adults with suspected acute appendicitis. Acta Chir Belg. 2010;110:543-547. [PubMed] |
| 19. | Di Saverio S, Sibilio A, Giorgini E, Biscardi A, Villani S, Coccolini F, Smerieri N, Pisano M, Ansaloni L, Sartelli M, Catena F, Tugnoli G. The NOTA Study (Non Operative Treatment for Acute Appendicitis): prospective study on the efficacy and safety of antibiotics (amoxicillin and clavulanic acid) for treating patients with right lower quadrant abdominal pain and long-term follow-up of conservatively treated suspected appendicitis. Ann Surg. 2014;260:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 20. | Vons C, Barry C, Maitre S, Pautrat K, Leconte M, Costaglioli B, Karoui M, Alves A, Dousset B, Valleur P, Falissard B, Franco D. Amoxicillin plus clavulanic acid versus appendicectomy for treatment of acute uncomplicated appendicitis: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377:1573-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 440] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 21. | Depinet H, von Allmen D, Towbin A, Hornung R, Ho M, Alessandrini E. Risk Stratification to Decrease Unnecessary Diagnostic Imaging for Acute Appendicitis. Pediatrics. 2016;138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Nana AM, Ouandji CN, Simoens C, Smets D, Mendes da Costa P. Laparoscopic appendectomies: results of a monocentric prospective and non-randomized study. Hepatogastroenterology. 2007;54: 1146-1152. [PubMed] |
| 23. | Ko YS, Lin LH, Chen DF. [Laboratory aid and ultrasonography in the diagnosis of appendicitis in children]. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1995;36:415-419. [PubMed] |
| 24. | Moreno-Pérez D, López-Samanes Á, Larrosa M, Larumbe-Zabala E, Centeno A, Roberts J, Naclerio F. Effects of protein-carbohydrate vs. carbohydrate alone supplementation on immune inflammation markers in endurance athletes: a randomized controlled trial. Eur J Appl Physiol. 2023;123:1495-1505. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Laukhtina E, Schuettfort VM, D'Andrea D, Pradere B, Quhal F, Mori K, Sari Motlagh R, Mostafaei H, Katayama S, Grossmann NC, Rajwa P, Karakiewicz PI, Schmidinger M, Fajkovic H, Enikeev D, Shariat SF. Selection and evaluation of preoperative systemic inflammatory response biomarkers model prior to cytoreductive nephrectomy using a machine-learning approach. World J Urol. 2022;40:747-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 26. | STARSurg Collaborative. Multicentre prospective cohort study of body mass index and postoperative complications following gastrointestinal surgery. Br J Surg. 2016;103: 1157-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Lewis AJ, Zhang X, Griepentrog JE, Yuan D, Collage RD, Waltz PK, Angus DC, Zuckerbraun BS, Rosengart MR. Blue Light Enhances Bacterial Clearance and Reduces Organ Injury During Sepsis. Crit Care Med. 2018;46:e779-e787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Moulis G, Christiansen CF, Darvalics B, Andersen IT, Sørensen HT, Nørgaard M. Platelet counts of adults upon acute hospital admission to internal medicine units are a predictor of mortality. Ann Hematol. 2020;99:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |