Published online Aug 27, 2024. doi: 10.4240/wjgs.v16.i8.2409
Revised: May 27, 2024
Accepted: June 24, 2024
Published online: August 27, 2024
Processing time: 161 Days and 6.3 Hours
Liver cancer represents a grave hepatic condition and constitutes a significant global health concern. Surgical resection remains the principal therapeutic modality for liver cancer. Nevertheless, perioperative malnutrition exerts a notable impact on patients with liver cancer, emerging as an independent risk factor for disease mortality and adverse outcomes. Hence, precise nutritional diagnosis and timely nutritional support hold the potential to enhance therapeutic efficacy and quality of life for liver cancer patients. This study represents a meticulous foray into the literature, extracting data from PubMed, Web of Science, and EMBASE databases, with a focus on the past 5 years. It scrutinizes the impact of malnutrition on patients undergoing liver cancer surgery, the etiological underpinnings of malnutrition within this patient cohort, the critical assessment of perioperative nutritional status, and the strategic approaches to nutritional support. Utilizing rigorous inclusion and exclusion criteria, the amassed scholarly works are meticulously synthesized, methodically organized, and categorically elaborated upon. Ultimately, the authors propose the incorporation of a multidisciplinary nutrition management team during the perioperative period, comprising nutritionists, pharmacists, physicians, nurses, psychologists, and rehabilitation therapists, among other specialized professionals. Together, they collaborate to devise and implement personalized nutritional support plans, monitor patients’ nutritional status, and make necessary adjustments as required. Through comprehensive management and intervention, improvements in the nutritional status of liver cancer patients can be achieved, thereby enhancing surgical success rates and facilitating postoperative recovery. It is believed that this manuscript will offer valuable insights to advance the nutritional management during the perioperative phase of liver cancer, aiding in ameliorating patients' nutritional status and treatment outcomes.
Core Tip: Liver cancer is a severe hepatic ailment, constituting a paramount global health concern. Nonetheless, the impact of perioperative malnutrition on patients with liver cancer is notably profound, emerging as an independent risk factor for disease mortality and adverse outcomes. This manuscript provides a comprehensive review focusing on the influence of malnutrition on liver cancer surgical patients, the etiology of malnutrition in liver cancer patients, perioperative nutritional status assessment, and nutritional support strategies. It is believed that this paper will offer reference opinions and theoretical groundwork to aid in the formulation of standardized perioperative nutritional support protocols.
- Citation: Li XQ, Liang Y, Huang CF, Li SN, Cheng L, You C, Liu YX, Wang T. Advancements in nutritional diagnosis and support strategies during the perioperative period for patients with liver cancer. World J Gastrointest Surg 2024; 16(8): 2409-2425
- URL: https://www.wjgnet.com/1948-9366/full/v16/i8/2409.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i8.2409
Liver cancer is a major global health concern due to its high prevalence in many countries[1]. Primary liver cancer is the sixth most commonly diagnosed cancer and third leading cause of cancer-related deaths worldwide in 2020, with approximately 906000 new cases and 830000 deaths[2]. Liver cancer, a severe liver disease, entails the destruction of hepatic cells, resulting in abnormal liver function and metabolic disturbances. This leads to malnutrition symptoms such as hypoglycemia, hypoalbuminemia, and electrolyte imbalances. The heightened metabolic demands and compromised liver function in liver cancer patients contribute to their susceptibility to malnutrition, which subsequently weakens their immune function. Liver cancer treatment options are diverse and include surgical resection, liver transplantation, transarterial chemoembolization, ablation, neoadjuvant therapy[3-6]. However, surgical resection remains the primary treatment modality for liver cancer. Additionally, liver resection surgery, as a complex procedure, necessitates elevated nutritional requirements. Preexisting malnutrition impairs postoperative wound healing and increases the risk of infection[7]. Insufficient postoperative nutritional support further augments the likelihood of complications, prolongs patient recovery time, and exacerbates the financial burden. Malnutrition exerts a significant impact on liver cancer patients, emerging as an independent risk factor for disease mortality and adverse outcomes[8]. Accurate nutritional diagnosis and timely nutritional support can improve treatment efficacy and quality of life for liver cancer patients. This text aims to comprehensively explore the impact of malnutrition on patients undergoing liver cancer surgery, the causes of malnutrition in liver cancer patients, perioperative nutritional status assessment, and nutritional support strategies, providing reference opinions and theoretical basis for establishing standardized perioperative nutritional support protocols.
Firstly, a search strategy was employed: TS = ("liver cancer" OR "hepatic carcinoma" OR "hepatocellular carcinoma" OR "hepatoma" OR "liver neoplasm" OR "hepatic neoplasm" OR "hepatocellular cancer" OR "hepatic cancer") AND TS = (nutrition OR nutritional). The search was conducted in the electronic databases PubMed (
Selection was carried out by screening the titles and abstracts of all publications. The retrieved articles were manually categorized into four aspects: "impact", "causes", "assessment", and "treatment". They were then arranged in descending order based on their publication dates. If an article covered multiple aspects mentioned above, it was included multiple times.
The articles were independently reviewed by two authors who checked the inclusion and exclusion criteria. The inclusion criteria were as follows: (1) Articles with open access to abstracts and full texts; and (2) Articles written in English. The exclusion criteria were as follows: (1) Protocols and articles unrelated to the topic; (2) Conference pro
The factors contributing to early-stage malnutrition in liver cancer patients primarily depend on the extent of liver dysfunction, while late-stage malnutrition factors need to be considered in conjunction with tumor-related factors[9-13]. The main type of malnutrition observed in liver cancer patients is energy-protein deficiency. The primary reasons for this are as follows: (1) Reduced food intake: Symptoms such as early satiety, taste disturbances, abdominal distension, and diarrhea can significantly decrease the normal food intake; (2) Decreased nutrient absorption: Portal hypertension leads to congestion and edema of the gastric and intestinal mucosa, resulting in decreased autonomous neural regulation and weakened peristalsis, leading to reduced absorption capacity; (3) Excessive loss of nutrients: Ascites, gastrointestinal bleeding, infections, and other factors can cause significant protein loss; (4) Impaired liver function leading to decreased protein synthesis capacity: Increased utilization of branched-chain amino acids (BCAAs) by the body results in decreased uric acid production, increased fatty acid oxidation and ketone body production. Alterations in the growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis: During the progression of liver cancer and cirrhosis, blood levels of GH increase, IGF-1 decreases, and the response of IGF-1 to GH declines, affecting nutritional metabolism[14]; (5) Abnormalities in nutrient metabolism: Liver dysfunction can lead to insulin resistance and glucose intolerance, reducing glycogen storage and oxidation. Impaired production and utilization of fatty acids and ketone bodies occur, along with increased protein breakdown and significant nitrogen consumption in the body[15]; and (6) Metabolic disorders of vitamins and trace elements: Inadequate intake and reduced absorption can result in deficiencies of zinc, selenium, and vitamins. The deficiency of various vitamins and trace elements can further exacerbate liver damage.
Impact of malnutrition on patients undergoing surgery for liver cancer: (1) Decreased immune function: malnutrition will lead to decreased function of the immune system, making patients more prone to infections and other complications. The body needs sufficient nutrition to support the recovery of immune function and wound healing after liver cancer surgery[16,17]; (2) Nutritional metabolic disorders: liver cancer patients are often accompanied by metabolic disorders, including protein metabolism disorders, energy metabolism disorders and microelement metabolism disorders. Malnutrition may aggravate these metabolic disorders and further damage liver function[18,19]; (3) Muscle wasting and weight loss: malnutrition can lead to muscle wasting and weight loss, which can impact patients' surgical tolerance and recovery capacity. After liver cancer surgery, the recovery period is crucial for patients, and maintaining a good nutritional status contributes to improving the speed and quality of postoperative rehabilitation[20,21]; and (4) Hence, maintaining a favorable nutritional status is of paramount importance for liver cancer surgical patients. During the perioperative period, nutritional support should be an integral component of comprehensive treatment.
Accurate nutritional diagnosis is a prerequisite for rational nutritional therapy. The conventional process of nutritional diagnosis, known as the two-tier nutritional diagnosis, comprises nutritional screening and nutritional assessment. In 2015, the Committee of Tumor Nutrition and Supportive Therapy of the Chinese Anti-Cancer Association proposed the concept of a three-tier diagnosis for malnutrition, which was further refined in 2020[22,23]. They recognized that the traditional two-tier diagnosis fails to fully assess the psychological, physiological, neurological, and spiritual changes brought about by malnutrition in patients. Therefore, they recommend conducting a comprehensive evaluation, namely the third-tier nutritional diagnosis, following nutritional assessment. Similarly, ESPEN and ASPEN have also incorporated the additional step of "extended assessment" (ESPEN) or "diagnosis" (ASPEN) after nutritional screening and assessment.
The definition of nutritional screening is the use of simple methods to quickly identify patients in the healthy population who may have underlying diseases without exhibiting symptoms[24]. According to ESPEN, it is the process of rapidly identifying patients in need of nutritional support among all patients[25]. Nutritional screening can swiftly identify liver cancer patients in need of nutritional support. Common methods mainly include the scale method including nutritional risk screening 2002 (NRS 2002), malnutrition universal screening tool (MUST), mini nutritional assessment-short form (MNA-SF), and the Royal Free Hospital Nutritional Prioritizing (RFH-NPT), Liver Disease Undernutrition Screening Tool (LDUST)[26-29].
NRS 2002: NRS 2002 was initially proposed by Kondrup et al[30], was refined by ESPEN in 2002[31]. It encompasses four key dimensions: fluctuations in body weight (weight and weight change), dietary intake (nutritional intake), the impact of the disease on patients (diseases), and age (age). It yields a total score of 7, whereby a score of ≥ 3 signifies the presence of nutritional risk in the patients[32]. A substantial body of research substantiates the heightened sensitivity of NRS 2002 in nutritional screening for individuals with liver cancer. However, it is worth noting the apparent limitations of NRS 2002, specifically its inclusion of body mass index (BMI) calculation, which renders it unsuitable for liver cancer patients who are unable to assume a standing position or have concomitant hepatic encephalopathy or ascites[33].
The MUST: MUST is a nutritional assessment tool proposed by Elia[34] from the United Kingdom in 2003[34]. It includes BMI (body weight), unintentional weight loss in the past 3-6 mo (weight change), presence of acute illness, or inability to eat for more than 5 d (diseases and nutritional intake). The scores are categorized into low risk, medium risk, and high risk. Research by Stratton et al[35] has shown that MUST is more sensitive in predicting the mortality and hospitalization time of elderly patients. Raslan et al[36], in a comparative study with NRS 2002 on hospitalized patients, found that the correlation of MUST results with clinical outcomes is inferior to that of NRS 2002. Currently, the application of MUST in nutritional screening for liver cancer is less common[37].
The MNA-SF: MNA-SF is a simplified version of the original Mini Nutritional Assessment developed by French nutritionist Guigoz et al[38] in 2005. It primarily evaluates weight changes, nutritional intake, the impact of diseases on patients, motility, and other factors such as appetite, psychological stress, mental state, and calf circumference (CC). Its purpose is to provide a rapid and effective method for assessing the nutritional status of elderly individuals. It has been widely used in clinical practice and research to help identify and intervene in the nutritional issues of elderly individuals, thus improving their nutritional and overall health status[28,39,40].
The RFH-NPT: The RFH-NPT is specifically developed for patients with chronic liver disease and is considered suitable for nutritional risk screening in patients with liver cirrhosis. The assessment includes weight changes, nutritional intake, considerations for fluid retention and so on. BFH-NPT is more sensitive than NRS 2002 in identifying liver disease patients at risk of malnutrition[41-43]. The 2019 ESPEN guidelines recommend RFH-NPT as the preferred nutritional screening tool for liver disease patients.
The LDUST: LDUST is a tool used to assess the presence of malnutrition in patients with liver disease. It includes aspects of weight change, nutritional intake, mobility, fluid retention, and muscle and fat status in patients with liver disease[44].
The LDUST is a quick, convenient, and easy-to-complete screening tool that is more effective in detecting malnutrition in cirrhotic patients than commonly used nutritional screening tools. The tool has been evaluated and validated by nutritionists. In addition, the study recommended that all hospitalized patients with cirrhosis should be evaluated by a dietitian due to the high prevalence of malnutrition in hospitalized patients with cirrhosis[45]. The American Society for Parenteral and Enteral Nutrition and the Academy of Nutrition and Dietetics have unanimously recognized the strong association between these factors and malnutrition in a consensus statement[46].
The aforementioned nutritional screening tools assess patients’ weight changes and nutritional intake. NRS 2002, MNA-SF, and RFH-NPT evaluate the impact of disease on patients, while MNA-SF and LDUST assess patients' functional capacity. RFH-NPT and LDUST also consider the influence of fluid retention. Additionally, NRS 2002 includes age as a screening factor, MNA-SF includes appetite, psychological stress, mental state, and CC, and LDUST includes fats and muscle. RFH-NPT and LDUST are modified scales designed specifically for nutritional screening in patients with liver diseases, taking into account the characteristics of the disease and catering to specialized needs compared to other scales (Table 1).
| Feature | NRS 2002 | MUST | MNA-SF | RFH-NPT | LDUST |
| Age | √ | ||||
| Diseases | √ | √ | √ | ||
| Weight | √ | √ | √ | √ | |
| Weight change | √ | √ | √ | √ | √ |
| Nutritional intake | √ | √ | √ | √ | √ |
| Appetite | √ | ||||
| Motility | √ | √ | |||
| Psychological stress | √ | ||||
| Mental stat | √ | ||||
| Fluid retention | √ | √ | |||
| Fats | √ | ||||
| Muscle | √ | ||||
| Calf circumference | √ |
Nutritional status is fundamental to a patient's overall well-being[47]. Liver cancer patients should undergo routine nutritional screening within 24 h of admission, performed by the nurse handling the admission procedures. For patients with negative nutritional screening results, screening should be repeated after one treatment course. For patients with positive nutritional screening results, nutritional assessment should be conducted, and a nutritional treatment plan or education should be developed. Currently, China has made positive nutritional screening a prerequisite for the use of enteral and parenteral nutrition preparations and medical insurance coverage[48].
However, nutritional screening has a higher false-negative rate in special patient populations such as cancer patients, critically ill patients, and elderly patients (age ≥ 65)[23]. Regardless of the initial diagnostic result from nutritional screening, routine nutritional assessment should be conducted. Therefore, for patients with liver cancer, direct nutritional assessment is recommended to reduce the rate of underdiagnosed malnutrition in this population.
For patients who have tested positive on nutritional screening, a secondary level diagnosis, known as nutritional assessment, should be conducted. Special populations such as cancer patients, critically ill individuals, and the elderly (≥ 65-years-old) should undergo routine nutritional assessment, regardless of the results of the primary level diagnosis (nutritional screening), as nutritional screening tends to have a higher false-negative rate in these populations[49,50]. Nutritional assessment should be conducted by a qualified nutrition professional (such as a nutrition nurse, dietitian, or oncologists) within 48 h of the patient’s admission[51]. Common methods used include nutritional assessment scales, dietary surveys, anthropometric measurements, and estimations of energy requirements.
Nutritional assessment scales: The commonly used nutritional assessment scales include subjective global assessment (SGA), patient-generated SGA (PG-SGA), Royal Free Hospital Global Assessment (RFH-GA) and the Global Leadership Initiative on Malnutrition (GLIM).
SGA: SGA was initially designed in 1984 to evaluate the nutritional status of patients receiving parenteral nutrition. The assessment includes weight changes, dietary changes, gastrointestinal symptoms, functional capacity, metabolic stress, physical examination findings such as triceps skinfold thickness (TSF) and ankle edema. It has been widely used in clinical practice and is a simple yet effective nutritional assessment tool applicable to outpatient and inpatient populations of different diseases and age groups[52].
SGA combines subjective feedback from patients and objective evaluations from healthcare professionals. Its reliability and validity have been extensively tested, making it the gold standard for nutritional assessment. SGA is suitable for various patient populations, including those with hepatic cirrhosis, liver cancer, liver transplantation, and long-term dialysis, as it is simple to administer, repeatable, applicable to a wide range of patients, easy to learn, and not influenced by sodium and water retention[53] (Table 2).
| Indicator | A | B | C |
| Optimal nutritional status | Mild to moderate malnutrition | Severe malnutrition | |
| Recent weight change | None/increase | Decrease of less than 5% | Decrease of more than 5% |
| Modification in dietary habits | None | Reduction | Abstaining from food/low-calorie liquid diet |
| Gastrointestinal symptoms | None/decreased appetite | Mild nausea, vomiting | Severe nausea, vomiting |
| Change in mobility | None/decreased | Able to walk out of bed | Bedridden |
| Stress response | None/low degree | Moderate degree | High degree |
| Muscle depletion | None | Mild | Severe |
| Triceps skinfold thickness in mm | Normal | Slight decrease | Significant decrease |
| Ankle swelling | None | Mild | Severe |
Regarding weight changes, if there has been a significant change over the past 6 mo or recent 2 wk, but no loss or gain in the past month, or if weight has stabilized after treatment in the past 2 wk, weight loss should not be considered.
Gastrointestinal symptoms should persist for at least 2 wk; occasional occurrences once or twice should not be considered.
Stress reference: Extensive burns, high fever, or significant bleeding are considered high stress; prolonged fever or chronic diarrhea are considered moderate stress; long-term low-grade fever or malignant tumors are considered low stress.
If five or more items in the evaluation results are classified as grade C or grade B, it can be diagnosed as severe or moderate malnutrition.
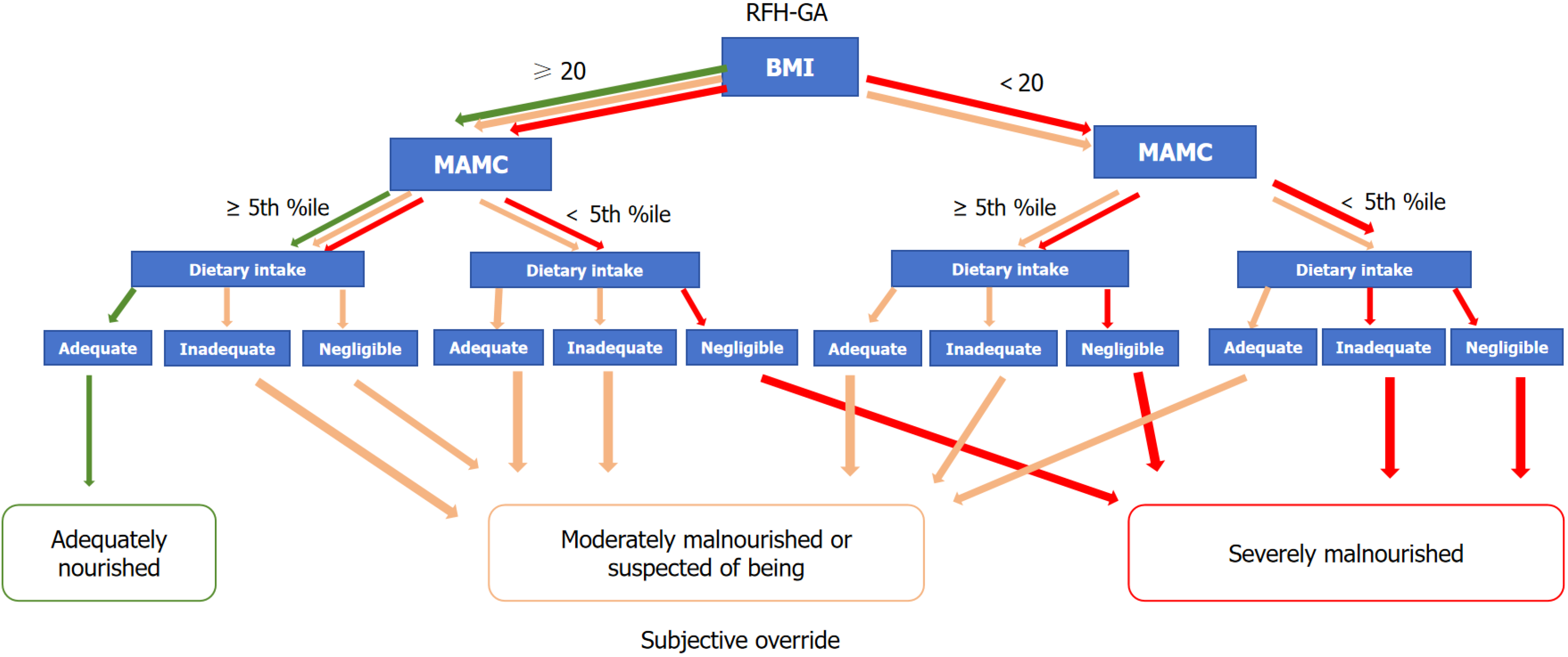
RFH-GA: In 2006, the Royal Free Hospital in London expanded upon SGA by adding BMI, mid-arm muscle circumference (MAMC), and dietary components, creating the modified SGA (RFH-GA). This modified version is specifically used to assess the nutritional status of end-stage liver disease patients and has improved its accuracy to some extent[54] (Figure 1).
The RFH-GA incorporates both subjective and objective variables to assess nutrition, including BMI, MAMC and dietary intake. In the case of fluid retention, BMI needs to be corrected for the patient’s dry weight, commonly estimated by the postparacentesis body weight or the weight recorded before fluid retention if available, or by subtracting a percentage of the weight based upon the severity of the ascites (mild 5%, moderate 10%, and severe 15%), with an additional 5% subtracted if bilateral pedal oedema is present, as described in several studies. Then the dry-weight BMI is calculated by dividing the patient’s estimated dry weight (kg) by the square of the patient’s height (m). The TSF (mm) and mid-arm circumference (MAC, cm) were measured using Holtain/Tanner-Whitehouse skinfold calipers. These two measurements are used to calculate the MAMC: MAMC (cm) = MAC (cm) - [3.14 × TSF (cm)]. Measures of the MAMC below the 5th percentile are indicative of a risk of malnutrition.
PG-SGA: PG-SGA is an improved nutritional assessment method based on SGA, specifically designed for cancer patients[55]. It has received strong recommendations from organizations such as the American Dietetic Association[56]. PG-SGA includes assessments from both the patient and healthcare professionals, with a total of seven sections. The patient assessment consists of four parts: body weight, dietary intake, subjective symptoms, and activity and physical function. The healthcare professional assessment includes three parts: disease and nutritional requirements, metabolic needs, and physical examination. The assessment results of PG-SGA can be categorized into qualitative and quantitative evaluations, with the quantitative assessment being the major highlight of PG-SGA[57]. Studies conducted by Gabrielson et al[58] have shown that when PG-SGA was applied to cancer patients in outpatient settings, it achieved a sensitivity of 97.0% and specificity of 86.0%. When using PG-SGA for nutritional evaluation of liver cancer patients, it is recommended to provide training in order to identify patients' nutritional deficiencies at an earlier stage and ensure timely nutritional therapy[59] (Table 3).
| Feature | Patient self-assessment | Medical staff assessment | |||||
| Items | Weight change | Food intake | Gastrointestinal symptoms | Activities and functions | Diseases and Age | Metabolic emergency | Physical fitness (muscle, fat, body fluids) |
| Score | 0-5 | 0-5 | 0-23 | 0-3 | 0-6 | 0-9 | 0-3 |
Nutritional grading recommendations: the total score is used to determine specific nutritional interventions, including patient and family education, symptomatic treatment including medication interventions, and appropriate nutritional interventions (dietary modifications, nutritional supplements, enteral nutrition, parenteral nutrition). First-line nutritional intervention involves optimal symptom management.
0-1: No intervention required at present, reassessment and standard treatment.
2-3: Nutritional analysis by a dietitian, nurse, or other healthcare professional, followed by appropriate education for the patient and their family.
4-8: Requires intervention by a dietitian, with assistance from nurses and doctors.
≥ 9: Urgent need for symptom management and/or nutritional intervention.
GLIM: The GLIM is a multidisciplinary working group composed of experts from the international nutrition community. It was established in 2016 with the aim of improving global understanding and management of malnutrition. GLIM focuses on developing a consensus approach to the identification, assessment, and management of malnutrition[60].
By bringing together experts from different disciplines and leveraging their collective knowledge and expertise, GLIM aims to advance the field of malnutrition management and contribute to improved outcomes for individuals affected by malnutrition[61].
GLIM is a relatively concise set of assessment criteria, making it more convenient to use compared to other assessment tools[62]. However, there is currently no unified standard for assessing muscle mass, and the reliability and validity of GLIM are being extensively validated from various perspectives. The reliability and validity of GLIM are currently undergoing extensive validation from various perspectives. The goal is to ensure that GLIM provides accurate and reliable assessments of malnutrition[63-65] (Table 4).
| Phenotypic indicators | Etiological indicators | |||
| Involuntary decline in body mass | Reduction in BMI | Decrease in muscle mass | Decreased food intake or impaired digestion and absorption | Inflammation or disease burden |
| Body mass reduction exceeding 5% within a span of 6 mo | Below 20 kg/m2 for individuals under 70 yr of age; below 22 kg/m2 for individuals aged 70 and above (according to European and American standards) | None | Food intake below 50% of energy requirements for more than 1 wk | Acute illness or injury |
| Body mass reduction exceeding 10% over a period of more than 6 mo | Below 18.5 kg/m2 for individuals under 70 yr of age; below 20 kg/m2 for individuals aged 70 and above (according to Asian standards) | Food intake reduction for more than 2 wk | Chronic disease | |
| — | — | Any gastrointestinal symptoms that affect digestion and absorption | ||
The aforementioned nutritional assessment tools are commonly employed in practical applications. However, it is important to note that the GLIM, despite its recent introduction, still requires extensive validation from multiple sources. The SGA is applicable for nutritional assessment in almost all hospitalized patients. Nonetheless, due to its limited consideration for specific diseases and circumstances, derived tools such as the PG-SGA and RFH-GA have been developed based on the SGA. The PG-SGA is better suited for assessing patients with tumors, while the RFH-GA primarily. Focuses on liver disease patients.
Dietary assessment: Dietary assessment is commonly conducted using dietary assessment software and the 24-h dietary recall method. Through dietary assessment, healthcare professionals can gain insights into a patient's nutrient intake and calculate their daily energy intake. This information can help determine the type of malnutrition a patient may be experiencing, such as energy deficiency, protein deficiency, or a mixed type[66].
Dietary assessment software provides a systematic approach to collecting and analyzing dietary data, allowing for a comprehensive evaluation of nutrient intake[67-69]. The software often includes a food database that contains nutritional information for various foods and allows for the calculation of nutrient intake based on the reported food consumption.
The 24-h dietary recall method involves asking the patient to recall all the foods and beverages consumed in the past 24 h[70,71]. This method provides a snapshot of the patient's recent dietary habits and allows for a detailed assessment of nutrient intake.
By conducting dietary assessments, healthcare professionals can identify deficiencies or excesses in specific nutrients and develop tailored nutritional interventions to address the patient's specific needs. Additionally, dietary assessments can monitor changes in dietary patterns over time, evaluate the effectiveness of dietary interventions, and guide nutritional counseling and education.
Anthropometric measurements: In addition to height, weight, and BMI, there are several other anthropometric mea
TSF, MAC, and MAMC are anthropometric measurements used to assess muscle and fat mass in the human body. These measurements are less susceptible to the influence of sodium and fluid retention and are positively correlated with the severity of liver cirrhosis and liver cancer. Therefore, they have become relatively accurate indicators for evaluating malnutrition in end-stage liver disease patients[72]. HGS is a simple and sensitive method for evaluating muscle function. It is not influenced by sodium and fluid retention, and the test results are reliable. Handgrip strength is well-correlated with upper arm circumference in assessing muscle reserve, making it an effective method for independently predicting malnutrition in end-stage liver disease patients. It is also a good indicator for predicting complications in liver cirrhosis and liver cancer[73]. Some researchers suggest performing dynamic handgrip strength tests using the non-dominant hand, as this method is more reliable in clinical practice[59]. However, this testing method is not suitable for patients with impaired consciousness or hepatic encephalopathy. Recent studies have shown that handgrip strength testing is more sensitive than upper arm circumference and TSF in detecting malnutrition in liver disease patients[74].
Anthropometric measurements provide valuable information about body composition and muscle function, allowing healthcare professionals to assess nutritional status and monitor changes over time. These measurements, along with other assessment tools, contribute to a comprehensive evaluation of a patient's nutritional health.
Energy requirements: The energy requirements of patients encompass resting energy expenditure (REE), basal energy expenditure, and total energy expenditure. There are various methods for measuring energy requirements, including direct calorimetry, indirect calorimetry (IC), and estimation equations. Direct calorimetry is primarily used in laboratory settings, while IC and energy estimation equations are commonly employed in clinical practice[75,76].
IC is a method used to measure the energy expenditure in the human body. It estimates the metabolic rate by measuring the oxygen consumption and carbon dioxide production in exhaled gases. IC is considered the gold standard for accurately assessing the REE of each patient. Ideally, any patient with uncertain energy requirements should undergo this measurement. By doing so, it can prevent underfeeding, overfeeding, and their associated complications[77].
Estimation equations, such as the thumb rule or formula methods, are commonly used to estimate energy requirements. The Harris-Benedict equation is a classic example, although the Mifflin-St Jeor equation is currently recommended. Estimation equations are simple, convenient, and easy to use. However, it is important to note their limitations, as they are less accurate than IC and may not be suitable for patients requiring precise measurement of energy expenditure[78,79].
Using nutritional assessment, patients can be classified into two categories: those with and without malnutrition. Patients without malnutrition do not require nutritional intervention. However, for patients with malnutrition, it is important to assess the severity and implement further comprehensive evaluations or initiate nutritional therapy accordingly. Regardless of the presence of malnutrition, a follow-up nutritional assessment should be conducted after completing one course of treatment for the underlying condition.
During this stage, the underlying causes of malnutrition in liver cancer patients can be identified through the analysis of patient medical history, physical examination, laboratory tests, and instrumental examinations. Factors such as reduced intake, absorption disorders, increased demand, and enhanced energy expenditure can be identified. Based on the energy expenditure level, stress, inflammatory response, and metabolic status, appropriate nutritional support strategies can be formulated.
Medical history inquiry: Emphasis should be placed on the nutrition-related medical history of liver cancer patients such as changes in food intake, gastrointestinal symptoms, and weight fluctuations. The Karnofsky Performance Status score is commonly used to assess the physical activity capacity of liver cancer patients[80]. Quality of life surveys can be con
Physical examination and fitness assessment: During nutritional physical examination of liver cancer patients, special attention should be given to assessing muscle mass, fat deposition, edema, and ascites. Physical performance measurements may include the 6-min walk test (cardiorespiratory endurance)[83], 30-s chair stand test (muscular endurance), isometric knee extension (lower limb strength)[84], single-leg standing time (static balance), and maximum stride length (dynamic balance/coordination)[85], among others.
Laboratory indicators: (1) Blood glucose: elevated blood glucose levels in liver cancer patients (excluding those with diabetes) often indicate a stress response; (2) Lymphocyte count: it can indirectly reflect the nutritional and immune status of liver cancer patients. However, it is influenced by factors such as infection, sodium and fluid retention, tumor cell growth, splenic hyperfunction and liver cirrhosis, which limits its evaluation value; (3) Inflammation levels: elevated levels of tumor necrosis factor alpha, interleukin 1, interleukin 6, C-reactive protein (CRP), thiobarbituric acid reactive substances, and superoxide dismutase indicate the presence of an inflammatory response, which can contribute to the complexity of malnutrition in liver cancer patients. Studies have found that an increase in CRP levels has a greater predictive value for the prognosis of cancer patients compared to decreased albumin levels[86]. The relationship between Glasgow Prognostic Score (GPS) results and the survival of hepatocellular carcinoma patients is closely linked[87]. Elevated GPS (1-2) serves as an independent prognostic indicator for overall survival in liver cancer patients after surgical resection. Patients with higher GPS experience poorer overall survival[88]; (4) Albumin: Albumin serves as a commonly employed nutritional indicator; however, its evaluation in liver cancer patients is impeded by the prevalent occurrence of hypoalbuminemia and ascites, which compromise the accuracy of measurements. Furthermore, exogenous blood products can also impact albumin levels. Consequently, the utility of albumin in assessing the nutritional status of liver cancer patients is relatively diminished. A study conducted by Ferreira et al[89] encompassed statistical analyses of patients with liver cirrhosis and liver cancer awaiting liver transplantation, affirming the limited correlation between albumin levels and nutritional status. This observation underscores the inadequacy of relying solely on albumin levels to evaluate the nutritional status of liver cancer patients. Hence, a comprehensive assessment incorporating other clinical and laboratory indicators is imperative to obtain a more comprehensive and precise evaluation[89]; (5) Prealbumin: It represents the precursor form of albumin, with a half-life of 1.9 d. In comparison to albumin, prealbumin exhibits heightened sensitivity to changes and remains unaffected by exogenous blood products. Consequently, it can serve as an evaluative marker for malnutrition in liver cancer patients. However, the levels of prealbumin can be influenced by hepatic reserve function, feeding status, and sodium and water retention, thereby diminishing its value in nutritional assessment[90]; (6) Creatinine height index: It is the ratio of 24-h urinary creatinine to height and accurately reflects the state of protein synthesis and breakdown in the body. A value of 60%-80% or less than 60% can be used as an evaluation criterion for moderate and severe protein deficiency. It is not affected by sodium and water retention and serves as a sensitive indicator for nutritional evaluation in liver cancer patients with normal renal function and no infection[91]; (7) Prognostic nutritional index (PNI): It is calculated based on the levels of albumin and total lymphocyte count. It is categorized into low, moderate, and severe risk groups. Research has shown that PNI is significantly correlated with overall survival and recurrence-free survival in liver cancer resection patients. A low PNI can serve as an effective marker for predicting the prognosis of such patients[92]; and (8) Monitoring of liver and kidney function in liver cancer patients, as well as the assessment of intestinal barrier function (diamine oxidase, D-lactate, and bacterial endotoxins), helps understand the metabolism of factors and their products (protein catabolism inducers, fat mobilization factors, free fatty acids, glucose, and lactate), which aids in determining the absorption of nutrients and guides the development of appropriate nutritional plans for patients.
Instrument inspection: Dual-energy X-ray, magnetic resonance imaging, computed tomography, and B-mode ultrasound examinations can offer insights into the composition of lean body tissue and adipose tissue in patients. However, these diagnostic methods are constrained in their broader application to liver cancer patients due to their elevated cost and the expertise required for their administration and interpretation.
Bioelectrical impedance analysis: On the other hand, is a widely used, simple, non-invasive, and convenient method in clinical practice[93]. Bioelectrical impedance analysis (BIA) is a technique that measures body composition by utilizing the electrical properties and variations of biological tissues and organs. It can provide information about fat mass, body fat percentage, non-fat mass, skeletal muscle mass, estimated bone mass, protein mass, water content, water percentage, extracellular fluid volume, intracellular fluid volume, basal metabolic rate, phase angle, visceral fat level, body shape, and more. Phase angle, in particular, has been identified as an important predictive factor for nutritional status. Since the phase angle of the trunk tends to be larger, it is recommended to measure the phase angle of the arms and thighs[94].
Research by Cotogni et al[95] has shown that BIA is a useful tool for analyzing the nutritional status and survival rate of cancer patients. Both are related to changes in cell membrane integrity and fluid balance, and the phase angle reflects changes in soft tissue quality and quantity (i.e. cell membrane permeability and soft tissue hydration). The phase angle can also be used for preoperative volume assessment to reduce blood loss during liver resection. Dzierżek et al[96] have also demonstrated that in advanced pancreatic cancer patients, the phase angle is a more effective survival indicator compared to traditional nutritional assessment markers such as albumin, prealbumin, and transferrin. Patients with low preoperative phase angles had significantly higher rates of severe postoperative infections and mortality compared to those with normal or high phase angles[97].
Genetic testing: In recent years, genetic testing has been widely used in clinical medicine. With the continuous development of genetics and genomics research, genetic testing is guiding the progress of predictive medicine and being applied in various aspects such as disease prevention, genetic diagnosis, and personalized medicine.
Through genetic metabolic capacity testing, it is possible to discover an individual's ability to absorb, metabolize, and store nutrients in the body. This information can guide the selection of nutritional support substances, quantities, and routes. By analyzing specific genes related to nutrient metabolism, such as those involved in the metabolism of vitamins, minerals, carbohydrates, fats, and proteins, genetic testing can provide insights into an individual's unique nutritional requirements and responses[98-101].
The perioperative nutritional support for malnourished patients with liver cancer encompasses both preoperative and postoperative phases. Precise and well-designed nutritional support plays a crucial role in promoting patient recovery and improving clinical outcomes.
In 2017, the European Society for Clinical Nutrition and Metabolism published guidelines that emphasized the importance of preoperative nutritional support for patients with severe malnutrition or significant nutritional risk, even if it necessitates delaying the surgery[102]. Administering preoperative nutritional support to liver cancer patients at risk of malnutrition has shown various benefits, including improved nutritional status, enhanced liver function, faster gastrointestinal recovery, and reduced complications.
Nutritional support time: The duration of preoperative nutritional support for liver cancer patients should be determined based on their nutritional status and the timing of the surgery. Short durations may not effectively achieve the desired nutritional support. Current guidelines recommend short-term nutritional support of 7-10 d for patients with mild malnutrition, while patients with moderate to severe malnutrition may require longer support of 10-14 d, combined with resistance exercise. Studies have shown that providing preoperative nutritional support for liver cancer patients at nutritional risk for 7-10 d improves their nutritional status and reduces the incidence of postoperative intra-abdominal fluid accumulation[103,104]. In a study by Fukuda et al[105], preoperative nutritional support for malnourished cancer patients for more than 10 d resulted in a significantly lower occurrence of postoperative infections compared to support durations less than 10 d or no nutritional support at all.
Modes of nutritional support: The preoperative nutritional support for liver cancer patients should be tailored to their specific conditions, such as disease status, appetite, nutritional status, and gastrointestinal tolerance. The preferred approach is nutritional health education, which is conducted by specialized nurses or dietitians and is integrated throughout the various stages of nutritional support[106]. When nutritional health education is insufficient to meet the patient's nutritional needs or in cases of moderate malnutrition, oral nutritional supplementation (ONS) can be considered[107]. Compared to enteral and parenteral nutrition, oral supplementation has higher compliance[108]. It is recommended to use high-protein oral nutritional supplements or immune nutrition, with a suggestion to ensure three oral supplementations per day, providing a minimum of 400-600 kcal of energy[109]. When patients are unable to meet their nutritional needs through ONS, enteral nutrition should be administered via a feeding tube for a minimum of 7 d. If intolerance symptoms occur during the nutritional support process (such as abdominal distension, abdominal pain, significant reduction in anal gas and bowel movements, gastric residual volume > 500 mL, abnormal abdominal imaging, etc), reducing or temporarily suspending oral nutrition and diet should be considered[110]. If ONS and enteral nutrition fail to meet protein and/or calorie requirements (less than 50% of recommended intake), preoperative parenteral nutrition support is advised to improve nutritional status. Enteral nutrition supplementation provides anti-inflammatory stimulation to the intestinal mucosa-associated lymphoid tissue, promotes the secretion of immunoglobulin A, helps maintain the mucosal barrier, and prevents the movement of bacterial microorganisms into the portal venous system, thus reducing the risk of infection and complications[111]. Total parenteral nutrition is suitable for patients with severe malnutrition and gastrointestinal dysfunction[112]. The choice of preoperative nutritional support mode should adhere to the five-step principle of nutritional support, tailored to the patient's specific needs[106]. However, in clinical practice, due to the invasive nature of enteral nutrition through tube feeding, it may cause patient discomfort and alter their perception of well-being. Patients and their families may have low acceptance of preoperative invasive nutrition support. Therefore, in practical terms, preoperative nutritional support for patients with moderate to severe malnutrition often involves a combination of partial enteral and partial parenteral nutrition support.
Selection of nutritional support substances and quantities: (1) Energy: The daily energy intake for liver cancer patients should aim to alleviate catabolic metabolism and endogenous protein hydrolysis. During the stable phase, the recommended energy intake for non-obese liver cancer patients (BMI < 18.5 kg/m2) can be based on the guidelines provided by the European Association for the Study of the Liver, suggesting an energy intake of ≥ 35 kcal/kg/d (based on corrected actual body weight after fluid retention) or 1.3 times the REE. The European Society for Clinical Nutrition and Metabolism (ESPEN) recommends 30-35 kcal/kg/d[113], while the Chinese Medical Association suggests an intake of 30-35 kcal/kg/d or 1.3-1.4 times the REE for patients with liver cirrhosis in China[114]. To prevent refeeding syndrome, initial energy intake should be reduced for liver cancer patients with moderate to severe malnutrition, generally following a standard of 20-25 kcal/kg/d[115]. If the patient's malnutrition is severe and of long duration, the initial energy intake should be further reduced to 10-15 kcal/kg/d[116]; (2) Protein: Excessive protein consumption is one of the characteristics of liver cancer patients. The daily protein requirement depends on the metabolic capacity and protein consumption levels in the patient's body. Liver damage leads to increased aromatic amino acids (AAAs) and decreased BCAAs, which may cause hepatic encephalopathy or other neurological complications[107]. Therefore, guidelines recommend a protein intake of 1.2-1.5 g/kg/d for liver cancer patients, along with an increased intake of BCAA to adjust the BCAA to AAA ratio. Studies have found that increasing dietary BCAA intake can improve liver function reserve and albumin levels in patients[117]; (3) Lipids: Lipids are an important source of calories for malnourished patients, accounting for 40%-50% of the total energy intake[118]. Due to impaired lipid metabolism in liver cancer patients, incomplete metabolism of long-chain triglycerides often occurs. It is recommended to use medium-chain triglycerides as an alternative form of lipid, which does not require bile salt absorption and can provide calories for patients with poor lipid absorption[53]; (4) Carbohydrates: Carbohydrates should account for 50%-60% of the total energy intake[103]. Liver cancer patients have a reduced glycogen storage and impaired gluconeogenesis, increasing the risk of hypoglycemia. A 12-h fasting period for these patients is equivalent to 2-3 d of fasting for healthy individuals[119]. Liver cancer patients can follow the principle of "small and frequent meals" and have a supplementary meal before bedtime to alleviate impaired glucose metabolism[120]; and (5) Vitamins: Due to reduced oral intake and impaired liver function, vitamin deficiencies are common in these patients. For example, patients with bleeding tendencies should be supplemented not only with water-soluble and fat-soluble vitamins but also with vitamin K. Patients with liver cirrhosis who use diuretics experience increased loss of water-soluble vitamins with increased urine output, necessitating enhanced supplementation[121].
Immunonutrition: Immunonutrition primarily involves the supplementation of substances that contribute to immune function, such as arginine, glutamine, omega-3 fatty acids, and nucleotides. Arginine is considered an enhancer of T lymphocytes, while glutamine provides oxidative fuel for lymphocytes and macrophages. Nucleotides enhance the host cell's defense capability[122]. These substances help control inflammation and reduce the risk of systemic inflammatory response syndrome during the treatment of malignant tumors. Studies have shown that postoperative inflammation, immune suppression, and liver dysfunction can be improved through preoperative immunonutrition[123]. However, the immunonutrition group and enteral nutrition group showed no statistically significant differences in postoperative complications and length of hospital stay. Russell et al[124] found no advantage in providing 5 d of immunonutritional support compared to the enteral nutrition group in patients undergoing liver resection with relatively good nutritional status. On the other hand, a meta-analysis indicated that immunonutrition is more suitable for malnourished patients, as malnutrition and immune suppression increase the risk of infection and death. Immunonutritional support can minimize early postoperative inflammatory response[122]. Since the immunonutrients take around 5 d to exert their effects on modulating immune and inflammatory responses in the body, it is recommended to use immunonutritional substances preoperatively.
Probiotics and gut microbiota: The gut microbiota is a vast and complex ecosystem consisting of approximately 1013 to 1014 microorganisms, with over 1000 different species, accounting for about 80% of the total microbial population in the body. It plays a significant role in the metabolism and absorption of nutrients such as carbohydrates, lipids, proteins, dietary fibers, and trace elements. The portal vein system, which receives venous blood from the intestines, is closely connected to the liver, forming the "gut-liver axis." When bacterial translocation occurs, the increased levels of endotoxins in the portal vein directly affect liver parenchymal cells, Kupffer cells, and immune cells, leading to the release of a series of inflammatory and cytokine factors by neutrophil aggregation, further aggravating liver damage. Numerous complications associated with gut dysbiosis, decreased colonization ability, bacterial translocation, such as gut-derived endotoxemia, spontaneous bacterial peritonitis, hepatic encephalopathy, are closely related and directly impact the patient's quality of life and prognosis[125,126].
The use of probiotics containing bifidobacteria, lactobacilli, butyric acid bacteria, lactulose, and other probiotic preparations can effectively promote the production of lactic acid in the intestine, inhibit the growth of pathogenic bacteria, maintain an anaerobic environment in the gut, reduce bacterial translocation and endotoxin production, decrease blood ammonia levels, improve liver function, and prognosis[127,128].
Fecal microbiota transplantation involves the transplantation of functional fecal microbiota derived from healthy individuals into the patient's intestinal tract, aiming to reconstruct a new gut microbiota and treat both intestinal and extraintestinal diseases. This revolutionary therapeutic approach has been proven highly effective in the treatment of refractory Clostridium difficile infection. Furthermore, it has demonstrated favorable therapeutic effects in various conditions, including enteritis, obesity, food allergies, multiple sclerosis, and Parkinson's disease[129,130].
It is recommended that liver cancer patients adopt the concept of enhanced recovery after surgery during the perioperative period[131]. Water intake should be restricted for 2 h before anesthesia, and fasting should be observed for 6 h before anesthesia to prevent patients from entering a catabolic state prematurely. Unless there are specific circumstances, patients undergoing liver resection can start drinking water on the day of surgery and progress to a clear liquid diet after 12 h postoperatively, gradually transitioning to a normal diet once anal gas passage and bowel sounds have resumed[132]. Randomized controlled trials have shown that ONS can improve the postoperative nutritional status of liver cancer patients, promote intestinal function recovery (indicated by the return of bowel sounds), shorten hospital stay, and accelerate postoperative recovery[133]. Adequate protein intake should be ensured in the early postoperative period, and it is recommended to increase protein intake through oral nutrition or meals on the day of surgery. Sufficient protein intake is more important than caloric intake in the early postoperative period. The selection of protein sources should be consistent with the preoperative period.
Selection of nutrients: Postoperative nutritional support aims to not only continue the preoperative nutritional support but also prevent the risk of malnutrition resulting from the surgical procedure itself. Randomized controlled clinical trials have found that the addition of total protein-medium chain triglycerides, an enteral nutrition suspension rich in protein and medium-chain triglycerides, can improve postoperative recovery in patients undergoing primary liver cancer resection[134]. It significantly reduces the time to first flatus and first bowel movement. Daily ONS with an energy supply of 400-600 kcal has been shown to significantly improve patient survival and liver function while reducing the incidence of postoperative complications[102]. Currently, postoperative nutrition support often advocates for low-calorie intake to avoid adverse effects caused by excessive caloric supply to the body[104].
Methods of nutritional support: Postoperative nutritional support follows the five-step ladder principle. If neither oral nor enteral nutrition can meet 50% of the protein and caloric requirements for more than 7 d, parenteral nutrition should be considered[135]. Routine gastric tube placement is not common in liver cancer surgery. If there are special circumstances that require gastric tube placement, it is recommended to remove it before the patient regains consciousness from anesthesia[136]. Therefore, postoperative nutritional support for liver cancer patients primarily consists of oral nutrition and daily meals.
The incidence and mortality rates of liver cancer are indeed considerably high, and currently, surgical resection is the primary modality of treatment. However, it is widely observed that liver cancer patients commonly experience malnutrition, leading to compromised immune function and an increased incidence of postoperative complications. Detecting early signs of nutritional deficiencies or potential issues in liver cancer patients and analyzing the underlying causes of malnutrition can be guided by the recommended tri-level nutritional diagnosis model proposed by the Chinese Society of Oncology Nutrition. Malnutrition in liver cancer primarily manifests as inadequate energy and protein intake. Consequently, nutritional support should primarily focus on energy and protein, while also ensuring the supplementation of other essential nutrients. Since the majority of liver cancer patients possess intact gastrointestinal tracts and unimpaired functionality, the primary mode of nutritional support is oral nutrient supplementation. In cases where some liver cancer patients or those in advanced stages experience digestive symptoms such as abdominal distension, which hinder oral nutrient intake, a combination of partial oral and enteral nutrition support can be considered. To evaluate the effectiveness of nutritional support, regular weekly nutritional reassessment should be conducted to gain insights into the patient's nutritional status. Therefore, it is recommended to involve a multidisciplinary team of nutrition management throughout the perioperative period, comprising professionals such as dietitians, pharmacists, physicians, nurses, psychotherapists, and rehabilitation therapists. Such a team can collaboratively develop and implement personalized nutritional support plans, monitor patients' nutritional status, and make necessary adjustments as required. Through comprehensive management and intervention, it is possible to improve the nutritional status of liver cancer patients, enhance the success rate of surgical procedures, and facilitate postoperative recovery. Certainly, this study still has its limitations. The viewpoints presented herein are derived from the synthesis and summarization of literature search findings. Incorporating specific case studies from the authors' institution could further substantiate the arguments presented in this paper. Regrettably, due to the nature of this manuscript being a review article, such content was not included. Nevertheless, we believe that this article will serve as a valuable reference for advancing the nutritional management during the perioperative phase of liver cancer, contributing to the amelioration of patients' nutritional status and treatment outcomes.
| 1. | Rich NE. Changing Epidemiology of Hepatocellular Carcinoma Within the United States and Worldwide. Surg Oncol Clin N Am. 2024;33:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64636] [Article Influence: 16159.0] [Reference Citation Analysis (176)] |
| 3. | Rizzo A, Ricci AD, Brandi G. Systemic adjuvant treatment in hepatocellular carcinoma: tempted to do something rather than nothing. Future Oncol. 2020;16:2587-2589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Rizzo A, Ricci AD, Brandi G. Immune-based combinations for advanced hepatocellular carcinoma: shaping the direction of first-line therapy. Future Oncol. 2021;17:755-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 5. | Mollica V, Rizzo A, Marchetti A, Tateo V, Tassinari E, Rosellini M, Massafra R, Santoni M, Massari F. The impact of ECOG performance status on efficacy of immunotherapy and immune-based combinations in cancer patients: the MOUSEION-06 study. Clin Exp Med. 2023;23:5039-5049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 108] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 6. | Dall'Olio FG, Rizzo A, Mollica V, Massucci M, Maggio I, Massari F. Immortal time bias in the association between toxicity and response for immune checkpoint inhibitors: a meta-analysis. Immunotherapy. 2021;13:257-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 7. | Takagi K, Domagala P, Polak WG, Buettner S, Ijzermans JNM. Prognostic significance of the controlling nutritional status (CONUT) score in patients undergoing hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, Esfandiari N, Lieffers JR, Sawyer MB. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 9. | Palmer LB, Kuftinec G, Pearlman M, Green CH. Nutrition in Cirrhosis. Curr Gastroenterol Rep. 2019;21:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 10. | Schütte K, Schulz C, Malfertheiner P. Nutrition and Hepatocellular Cancer. Gastrointest Tumors. 2016;2:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Traub J, Reiss L, Aliwa B, Stadlbauer V. Malnutrition in Patients with Liver Cirrhosis. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 12. | Ruiz-Margáin A, Román-Calleja BM, Moreno-Guillén P, González-Regueiro JA, Kúsulas-Delint D, Campos-Murguía A, Flores-García NC, Macías-Rodríguez RU. Nutritional therapy for hepatocellular carcinoma. World J Gastrointest Oncol. 2021;13:1440-1452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Yang C, Huang X, Liu Z, Qin W, Wang C. Metabolism-associated molecular classification of hepatocellular carcinoma. Mol Oncol. 2020;14:896-913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 14. | Puri P, Dhiman RK, Taneja S, Tandon P, Merli M, Anand AC, Arora A, Acharya SK, Benjamin J, Chawla YK, Dadhich S, Duseja A, Eapan CE, Goel A, Kalra N, Kapoor D, Kumar A, Madan K, Nagral A, Pandey G, Rao PN, Saigal S, Saraf N, Saraswat VA, Saraya A, Sarin SK, Sharma P, Shalimar, Shukla A, Sidhu SS, Singh N, Singh SP, Srivastava A, Wadhawan M. Nutrition in Chronic Liver Disease: Consensus Statement of the Indian National Association for Study of the Liver. J Clin Exp Hepatol. 2021;11:97-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Plauth M, Schütz T, Buckendahl DP, Kreymann G, Pirlich M, Grüngreiff S, Romaniuk P, Ertl S, Weiss ML, Lochs H. Weight gain after transjugular intrahepatic portosystemic shunt is associated with improvement in body composition in malnourished patients with cirrhosis and hypermetabolism. J Hepatol. 2004;40:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Fan Y, Yao Q, Liu Y, Jia T, Zhang J, Jiang E. Underlying Causes and Co-existence of Malnutrition and Infections: An Exceedingly Common Death Risk in Cancer. Front Nutr. 2022;9:814095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Stephensen CB. Primer on Immune Response and Interface with Malnutrition. In: Humphries DL, Scott ME, Vermund SH, editors. Nutrition and Infectious Diseases: Shifting the Clinical Paradigm. Cham: Springer International Publishing, 2021: 83–110. |
| 18. | Zhu M, Liu C. [Medical nutrition therapy for patients of liver cancer]. Zhongliu Daixie Yu Yingyang Dianzi Zazhi. 2020;7:5. [DOI] [Full Text] |
| 19. | Nilsson A, Haanstra JR, Engqvist M, Gerding A, Bakker BM, Klingmüller U, Teusink B, Nielsen J. Quantitative analysis of amino acid metabolism in liver cancer links glutamate excretion to nucleotide synthesis. Proc Natl Acad Sci U S A. 2020;117:10294-10304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Fischer M, JeVenn A, Hipskind P. Evaluation of muscle and fat loss as diagnostic criteria for malnutrition. Nutr Clin Pract. 2015;30:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Landi F, Camprubi-Robles M, Bear DE, Cederholm T, Malafarina V, Welch AA, Cruz-Jentoft AJ. Muscle loss: The new malnutrition challenge in clinical practice. Clin Nutr. 2019;38:2113-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 22. | Shi HP, Zhao QC, Wang KH, Xu HX, Li W, Fang Y, Yu SC, Qi YM, Zhu GY, Lu Q, Luo Q, Zhang XW, Tan RS, Jiao GY, Jia RP, Zhou L, Ge S, Cong MH, Li ZN, Wang K, Zhang XT, Yu Kg, Liu YH, Zhou Y, Xie CH, Cui JW, Guan WX, Shi WY, Hu W, Zhao CH, Chen W, Zhu CF, Li SY, Gao FL, Ba Y, Chen ZH, Lin Y, Cao WX, Chen GY. [Tertiary three-level diagnosis of malnutrition]. Zhongliu Daixie Yu Yingyang Dianzi Zazhi. 2015;2:31-36. [DOI] [Full Text] |
| 23. | Wang H, Ye YF, Lin LH, Wang ZF, Chen ZZ, Li L. [Study on perioperative tertiary nutrition diagnosis in patients with gastric and colorectal cancer]. Shequ Yixue Zazhi. 2023;21:1047-1051 + 1057. [DOI] [Full Text] |
| 24. | Loud JT, Murphy J. Cancer Screening and Early Detection in the 21(st) Century. Semin Oncol Nurs. 2017;33:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 25. | Soriano-Moreno DR, Dolores-Maldonado G, Benites-Bullón A, Ccami-Bernal F, Fernandez-Guzman D, Esparza-Varas AL, Caira-Chuquineyra B, Taype-Rondan A. Recommendations for nutritional assessment across clinical practice guidelines: A scoping review. Clin Nutr ESPEN. 2022;49:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | de van der Schueren MAE, Jager-Wittenaar H. Malnutrition risk screening: New insights in a new era. Clin Nutr. 2022;41:2163-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 27. | Du H, Liu B, Xie Y, Liu J, Wei Y, Hu H, Luo B, Li Z. Comparison of different methods for nutrition assessment in patients with tumors. Oncol Lett. 2017;14:165-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, Jensen GL, Malone A, Muscaritoli M, Nyulasi I, Pirlich M, Rothenberg E, Schindler K, Schneider SM, de van der Schueren MA, Sieber C, Valentini L, Yu JC, Van Gossum A, Singer P. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36:49-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 908] [Cited by in RCA: 1481] [Article Influence: 164.6] [Reference Citation Analysis (0)] |
| 29. | van Bokhorst-de van der Schueren MA, Guaitoli PR, Jansma EP, de Vet HC. Nutrition screening tools: does one size fit all? A systematic review of screening tools for the hospital setting. Clin Nutr. 2014;33:39-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 30. | Kondrup J, Rasmussen HH, Hamberg O, Stanga Z; Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1485] [Cited by in RCA: 1756] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 31. | Kondrup J, Allison SP, Elia M, Vellas B, Plauth M; Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 1901] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 32. | Sahli L, Hagenbuch N, Ballmer PE, Rühlin M, Imoberdorf R. NRS-2002 components, nutritional score and severity of disease score, and their association with hospital length of stay and mortality. Swiss Med Wkly. 2021;151:w20517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Hersberger L, Bargetzi L, Bargetzi A, Tribolet P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, Kutz A, Kägi-Braun N, Hoess C, Pavlicek V, Schmid S, Bilz S, Sigrist S, Brändle M, Benz C, Henzen C, Nigg M, Thomann R, Brand C, Rutishauser J, Aujesky D, Rodondi N, Donzé J, Stanga Z, Mueller B, Schuetz P. Nutritional risk screening (NRS 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: secondary analysis of a prospective randomised trial. Clin Nutr. 2020;39:2720-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 34. | Elia M. Screening for malnutrition: a multidisciplinary responsibility. Development and use of the Malnutrition Universal Screening Tool (‘MUST’) for adults. Redditch: BAPEN, 2003. [cited 3 June 2024]. Available from: https://www.bapen.org.uk/pdfs/must/must-report.pdf. |
| 35. | Stratton RJ, King CL, Stroud MA, Jackson AA, Elia M. 'Malnutrition Universal Screening Tool' predicts mortality and length of hospital stay in acutely ill elderly. Br J Nutr. 2006;95:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 36. | Raslan M, Gonzalez MC, Dias MC, Nascimento M, Castro M, Marques P, Segatto S, Torrinhas RS, Cecconello I, Waitzberg DL. Comparison of nutritional risk screening tools for predicting clinical outcomes in hospitalized patients. Nutrition. 2010;26:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Wang Z, Xu J, Song G, Pang M, Guo B, Xu X, Wang H, Zhou Y, Ren L, Zhou H, Ma J, Fan H. Nutritional status and screening tools to detect nutritional risk in hospitalized patients with hepatic echinococcosis. Parasite. 2020;27:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev. 1996;54:S59-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 834] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 39. | Kokkinakis S, Venianaki M, Petra G, Chrysos A, Chrysos E, Lasithiotakis K. A Comparison of the Malnutrition Universal Screening Tool (MUST) and the Mini Nutritional Assessment-Short Form (MNA-SF) Tool for Older Patients Undergoing General Surgery. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Ozturk Y, Sarikaya D, Emin Kuyumcu M, Yesil Y, Koca M, Guner Oytun M, Unsal P, Balci C, Balam Dogu B, Cankurtaran M, Halil M. Comparison of Mini Nutritional Assessment-Short and Long Form to predict all-cause mortality up to 7 years in geriatric outpatients. Nutr Clin Pract. 2022;37:1418-1428. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Rajab N, Abdul Hamid SB, Mohd Said AH, Md Isa KA. Validation Of Nutrition Screening Tool: Royal Free Hospital Nutritional Prioritizing Tool (RFH-NPT) For Chronic Liver Disease Patients. MJMHS. 2023;19:130-137. [DOI] [Full Text] |
| 42. | Tan JYT, Cheah CCM, Wang YT, Chang PEJ, Krishnamoorthy TL, Tan HK, Salazar E. Outpatient screening with the Royal Free Hospital-Nutrition Prioritizing Tool for patients with cirrhosis at risk of malnutrition. Nutrition. 2023;114:112139. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 43. | Wu Y, Zhu Y, Feng Y, Wang R, Yao N, Zhang M, Liu X, Liu H, Shi L, Zhu L, Yang N, Chen H, Liu J, Zhao Y, Yang Y. Royal Free Hospital-Nutritional Prioritizing Tool improves the prediction of malnutrition risk outcomes in liver cirrhosis patients compared with Nutritional Risk Screening 2002. Br J Nutr. 2020;124:1293-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | Casas-Deza D, Bernal-Monterde V, Betoré-Glaria E, Julián-Gomara AB, Yagüe-Caballero C, Sanz-París A, Fernández-Bonilla EM, Fuentes-Olmo J, Arbones-Mainar JM. Liver Disease Undernutrition Screening Tool Questionnaire Predicts Decompensation and Mortality in Cirrhotic Outpatients with Portal Hypertension. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | McFarlane M, Hammond C, Roper T, Mukarati J, Ford R, Burrell J, Gordon V, Burch N. Comparing assessment tools for detecting undernutrition in patients with liver cirrhosis. Clin Nutr ESPEN. 2018;23:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy Malnutrition Work Group; A. S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr. 2012;36:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 842] [Article Influence: 64.8] [Reference Citation Analysis (1)] |
| 47. | Yu K, Liu L, Shi H. [Nutritional status should be a basic vital sign]. Zhongliu Daixie Yu Yingyang Dianzi Zazhi. 2019;6:391-395. [DOI] [Full Text] |
| 48. | XU J, Jiang Z. [Learning of 2017 edition Chinese national reimbursement list of clinical nutrition drugs about the limitation based on the nutritional risk and patient]. Zhongguo Linchuang Yingyang Zazhi. 2017;25:4. [DOI] [Full Text] |
| 49. | Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C, Preiser JC, van Zanten ARH, Oczkowski S, Szczeklik W, Bischoff SC. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1528] [Article Influence: 218.3] [Reference Citation Analysis (0)] |
| 50. | Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Goisser S, Hooper L, Kiesswetter E, Maggio M, Raynaud-Simon A, Sieber CC, Sobotka L, van Asselt D, Wirth R, Bischoff SC. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38:10-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 746] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 51. | Compher C, Bingham AL, McCall M, Patel J, Rice TW, Braunschweig C, McKeever L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. 2022;46:12-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 315] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 52. | Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1939] [Cited by in RCA: 1955] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 53. | Johnson TM, Overgard EB, Cohen AE, DiBaise JK. Nutrition assessment and management in advanced liver disease. Nutr Clin Pract. 2013;28:15-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 54. | Morgan MY, Madden AM, Soulsby CT, Morris RW. Derivation and validation of a new global method for assessing nutritional status in patients with cirrhosis. Hepatology. 2006;44:823-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 55. | Ottery FD. Rethinking nutritional support of the cancer patient: the new field of nutritional oncology. Semin Oncol. 1994;21:770-778. [PubMed] |
| 56. | Mulasi U, Vock DM, Kuchnia AJ, Jha G, Fujioka N, Rudrapatna V, Patel MR, Teigen L, Earthman CP. Malnutrition Identified by the Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition Consensus Criteria and Other Bedside Tools Is Highly Prevalent in a Sample of Individuals Undergoing Treatment for Head and Neck Cancer. JPEN J Parenter Enteral Nutr. 2018;42:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 57. | Beirer A. Malnutrition and cancer, diagnosis and treatment. memo. 2021;14:168-173. [DOI] [Full Text] |
| 58. | Gabrielson DK, Scaffidi D, Leung E, Stoyanoff L, Robinson J, Nisenbaum R, Brezden-Masley C, Darling PB. Use of an abridged scored Patient-Generated Subjective Global Assessment (abPG-SGA) as a nutritional screening tool for cancer patients in an outpatient setting. Nutr Cancer. 2013;65:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 59. | Medhat AS, Ahmed AO, Ahmed FT, Amal MAA. Creatinine height index as a predictor of nutritional status among patients with liver cirrhosis. J Public Health Epidemiol. 2016;8:220-228. [DOI] [Full Text] |
| 60. | Cederholm T, Jensen GL. To Create a Consensus on Malnutrition Diagnostic Criteria. JPEN J Parenter Enteral Nutr. 2017;41:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Correia MITD, Tappenden KA, Malone A, Prado CM, Evans DC, Sauer AC, Hegazi R, Gramlich L. Utilization and validation of the Global Leadership Initiative on Malnutrition (GLIM): A scoping review. Clin Nutr. 2022;41:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 62. | Jensen GL, Cederholm T, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, de Baptista GA, Barazzoni R, Blaauw R, Coats AJS, Crivelli A, Evans DC, Gramlich L, Fuchs-Tarlovsky V, Keller H, Llido L, Malone A, Mogensen KM, Morley JE, Muscaritoli M, Nyulasi I, Pirlich M, Pisprasert V, de van der Schueren M, Siltharm S, Singer P, Tappenden KA, Velasco N, Waitzberg DL, Yamwong P, Yu J, Compher C, Van Gossum A. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report From the Global Clinical Nutrition Community. JPEN J Parenter Enteral Nutr. 2019;43:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 424] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 63. | Fontane L, Reig MH, Garcia-Ribera S, Herranz M, Miracle M, Chillaron JJ, Estepa A, Toro S, Ballesta S, Navarro H, Llaurado G, Pedro-Botet J, Benaiges D. Validity and Applicability of the Global Leadership Initiative on Malnutrition (GLIM) Criteria in Patients Hospitalized for Acute Medical Conditions. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 64. | Crestani MS, Stefani GP, Scott LM, Steemburgo T. Accuracy of the GLIM Criteria and SGA Compared to PG-SGA for the Diagnosis of Malnutrition and Its Impact on Prolonged Hospitalization: A Prospective Study in Patients with Cancer. Nutr Cancer. 2023;75:1177-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 65. | Zhou L, Fu J, Ding Z, Jin K, Wu R, Ye LX. Comparison of GLIM, SGA, PG-SGA, and PNI in diagnosing malnutrition among hepatobiliary-pancreatic surgery patients. Front Nutr. 2023;10:1116243. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 66. | de van der Schueren MAE, Keller H; GLIM Consortium, Cederholm T, Barazzoni R, Compher C, Correia MITD, Gonzalez MC, Jager-Wittenaar H, Pirlich M, Steiber A, Waitzberg D, Jensen GL. Global Leadership Initiative on Malnutrition (GLIM): Guidance on validation of the operational criteria for the diagnosis of protein-energy malnutrition in adults. Clin Nutr. 2020;39:2872-2880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 67. | Marchetti BS, Steluti J, Fisberg RM, Marchioni DML. Performance of the Brazilian version of GloboDiet software for dietary intake assessment. Nutrire. 2018;43:13. [DOI] [Full Text] |
| 68. | Wittig F, Krems C, Engelbert AK, Strassburg A. Validation of the Updated GloboDiet Version by Protein and Potassium Intake for the German National Nutrition Monitoring. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 69. | Aglago EK, Landais E, Nicolas G, Margetts B, Leclercq C, Allemand P, Aderibigbe O, Agueh VD, Amuna P, Annor GA, El Ati J, Coates J, Colaiezzi B, Compaore E, Delisle H, Faber M, Fungo R, Gouado I, El Hamdouchi A, Hounkpatin WA, Konan AG, Labzizi S, Ledo J, Mahachi C, Maruapula SD, Mathe N, Mbabazi M, Mirembe MW, Mizéhoun-Adissoda C, Nzi CD, Pisa PT, El Rhazi K, Zotor F, Slimani N. Evaluation of the international standardized 24-h dietary recall methodology (GloboDiet) for potential application in research and surveillance within African settings. Global Health. 2017;13:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | St George SM, Van Horn ML, Lawman HG, Wilson DK. Reliability of 24-Hour Dietary Recalls as a Measure of Diet in African-American Youth. J Acad Nutr Diet. 2016;116:1551-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 71. | Pannucci TE, Thompson FE, Bailey RL, Dodd KW, Potischman N, Kirkpatrick SI, Alexander GL, Coleman LA, Kushi LH, Groesbeck M, Sundaram M, Clancy H, George SM, Kahle L, Subar AF. Comparing Reported Dietary Supplement Intakes between Two 24-Hour Recall Methods: The Automated Self-Administered 24-Hour Dietary Assessment Tool and the Interview-Administered Automated Multiple Pass Method. J Acad Nutr Diet. 2018;118:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 72. | Campillo B, Richardet JP, Bories PN. Validation of body mass index for the diagnosis of malnutrition in patients with liver cirrhosis. Gastroenterol Clin Biol. 2006;30:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Nascimento SC, Pinto IC, Silva CP. [Comparison of strength of the handshake with anthropometric and subjective parameters at the patients' nutritional assessment with liver diseas]. Acta Gastroenterol Latinoam. 2013;43:218-226. [PubMed] |
| 74. | Hiraoka A, Michitaka K, Ueki H, Kaneto M, Aibiki T, Okudaira T, Kawakami T, Yamago H, Suga Y, Tomida H, Miyamoto Y, Azemoto N, Mori K, Miyata H, Tsubouchi E, Ninomiya T, Hirooka M, Abe M, Matsuura B, Hiasa Y. Sarcopenia and two types of presarcopenia in Japanese patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2016;28:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 75. | Sabatino A, Theilla M, Hellerman M, Singer P, Maggiore U, Barbagallo M, Regolisti G, Fiaccadori E. Energy and Protein in Critically Ill Patients with AKI: A Prospective, Multicenter Observational Study Using Indirect Calorimetry and Protein Catabolic Rate. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Fraipont V, Preiser JC. Energy estimation and measurement in critically ill patients. JPEN J Parenter Enteral Nutr. 2013;37:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 77. | Delsoglio M, Achamrah N, Berger MM, Pichard C. Indirect Calorimetry in Clinical Practice. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 78. | Quiroz-Olguín G, Medina-Vera I, Serralde-Zúñiga AE, Gulias-Herrero A, Sánchez-Rosales AI, Guevara-Cruz M. Accurate determination of energy requirements in hospitalised patients with parenteral nutrition. J Hum Nutr Diet. 2018;31:810-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 79. | Eleutério M, Vale B, Mendes I, Carvalho R, Leite M, Sousa I. Energy Requirements Determined By Indirect Calorimetry And Estimated By Predictive Formulas In The Surgical Patient. Clin Nutr ESPEN. 2023;54:693. [DOI] [Full Text] |
| 80. | Shamseddeen H, Pike F, Ghabril M, Patidar KR, Desai AP, Nephew L, Anderson M, Kubal C, Chalasani N, Orman ES. Karnofsky performance status predicts outcomes in candidates for simultaneous liver-kidney transplant. Clin Transplant. 2021;35:e14190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 81. | Schwenkglenks M, Matter-Walstra K. Is the EQ-5D suitable for use in oncology? An overview of the literature and recent developments. Expert Rev Pharmacoecon Outcomes Res. 2016;16:207-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 82. | Annunziata MA, Muzzatti B, Bidoli E, Flaiban C, Bomben F, Piccinin M, Gipponi KM, Mariutti G, Busato S, Mella S. Hospital Anxiety and Depression Scale (HADS) accuracy in cancer patients. Support Care Cancer. 2020;28:3921-3926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 83. | ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6981] [Cited by in RCA: 8295] [Article Influence: 360.7] [Reference Citation Analysis (0)] |
| 84. | Carty CP, Barrett RS, Cronin NJ, Lichtwark GA, Mills PM. Lower limb muscle weakness predicts use of a multiple- versus single-step strategy to recover from forward loss of balance in older adults. J Gerontol A Biol Sci Med Sci. 2012;67:1246-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 85. | Medell JL, Alexander NB. A clinical measure of maximal and rapid stepping in older women. J Gerontol A Biol Sci Med Sci. 2000;55:M429-M433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 619] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 87. | Lu L, Lin K, Zheng J, Wu H, Li D. Glasgow Prognostic Score and modified Glasgow Prognostic Score and survival in patients with hepatocellular carcinoma: a meta-analysis. BMJ Open. 2021;11:e053061. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 88. | Abe T, Tashiro H, Kobayashi T, Hattori M, Kuroda S, Ohdan H. Glasgow Prognostic Score and Prognosis After Hepatectomy for Hepatocellular Carcinoma. World J Surg. 2017;41:1860-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 89. | Ferreira LG, Anastácio LR, Lima AS, Correia MI. Assessment of nutritional status of patients waiting for liver transplantation. Clin Transplant. 2011;25:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 90. | Ranasinghe RN, Biswas M, Vincent RP. Prealbumin: The clinical utility and analytical methodologies. Ann Clin Biochem. 2022;59:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 91. | Sharma D, Kannan R, Tapkire R, Nath S. Evaluation of Nutritional Status of Cancer Patients during Treatment by Patient-Generated Subjective Global Assessment: a Hospital-Based Study. Asian Pac J Cancer Prev. 2015;16:8173-8176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Nogueiro J, Santos-Sousa H, Pereira A, Devezas V, Fernandes C, Sousa F, Fonseca T, Barbosa E, Barbosa JA. The impact of the prognostic nutritional index (PNI) in gastric cancer. Langenbecks Arch Surg. 2022;407:2703-2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 93. | Zhou S, Yu Z, Shi X, Zhao H, Dai M, Chen W. The Relationship between Phase Angle, Nutrition Status, and Complications in Patients with Pancreatic Head Cancer. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 94. | Silva AM, Campa F, Stagi S, Gobbo LA, Buffa R, Toselli S, Silva DAS, Gonçalves EM, Langer RD, Guerra-Júnior G, Machado DRL, Kondo E, Sagayama H, Omi N, Yamada Y, Yoshida T, Fukuda W, Gonzalez MC, Orlandi SP, Koury JC, Moro T, Paoli A, Kruger S, Schutte AE, Andreolli A, Earthman CP, Fuchs-Tarlovsky V, Irurtia A, Castizo-Olier J, Mascherini G, Petri C, Busert LK, Cortina-Borja M, Bailey J, Tausanovitch Z, Lelijveld N, Ghazzawi HA, Amawi AT, Tinsley G, Kangas ST, Salpéteur C, Vázquez-Vázquez A, Fewtrell M, Ceolin C, Sergi G, Ward LC, Heitmann BL, da Costa RF, Vicente-Rodriguez G, Cremasco MM, Moroni A, Shepherd J, Moon J, Knaan T, Müller MJ, Braun W, García-Almeida JM, Palmeira AL, Santos I, Larsen SC, Zhang X, Speakman JR, Plank LD, Swinburn BA, Ssensamba JT, Shiose K, Cyrino ES, Bosy-Westphal A, Heymsfield SB, Lukaski H, Sardinha LB, Wells JC, Marini E. The bioelectrical impedance analysis (BIA) international database: aims, scope, and call for data. Eur J Clin Nutr. 2023;77:1143-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 95. | Cotogni P, Monge T, Fadda M, De Francesco A. Bioelectrical impedance analysis for monitoring cancer patients receiving chemotherapy and home parenteral nutrition. BMC Cancer. 2018;18:990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 96. | Dzierżek P, Kurnol K, Hap W, Frejlich E, Diakun A, Karwowski A, Kotulski K, Rudno-Rudzińska J, Kielan W. Assessment of changes in body composition measured with bioelectrical impedance in patients operated for pancreatic, gastric and colorectal cancer. Pol Przegl Chir. 2020;92:8-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 97. | Di Vincenzo O, Marra M, Di Gregorio A, Pasanisi F, Scalfi L. Bioelectrical impedance analysis (BIA) -derived phase angle in sarcopenia: A systematic review. Clin Nutr. 2021;40:3052-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 98. | Guasch-Ferré M, Dashti HS, Merino J. Nutritional Genomics and Direct-to-Consumer Genetic Testing: An Overview. Adv Nutr. 2018;9:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 99. | Braakhuis A, Monnard CR, Ellis A, Rozga M. Consensus Report of the Academy of Nutrition and Dietetics: Incorporating Genetic Testing into Nutrition Care. J Acad Nutr Diet. 2021;121:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 100. | Garcia-Bailo B, El-Sohemy A. Recent advances and current controversies in genetic testing for personalized nutrition. Curr Opin Clin Nutr Metab Care. 2021;24:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Martin DS. Nutritional Genomics: Emerging Science with Implications for Us All. J Acad Nutr Diet. 2018;118:545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 102. | Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, Laviano A, Ljungqvist O, Lobo DN, Martindale R, Waitzberg DL, Bischoff SC, Singer P. ESPEN guideline: Clinical nutrition in surgery. Clin Nutr. 2017;36:623-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 1062] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 103. | Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Mühlebach S, Muscaritoli M, Oldervoll L, Ravasco P, Solheim T, Strasser F, de van der Schueren M, Preiser JC. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1318] [Cited by in RCA: 1780] [Article Influence: 197.8] [Reference Citation Analysis (1)] |
| 104. | Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, Laviano A, Ljungqvist O, Lobo DN, Martindale RG, Waitzberg D, Bischoff SC, Singer P. ESPEN practical guideline: Clinical nutrition in surgery. Clin Nutr. 2021;40:4745-4761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 352] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 105. | Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Maeda S, Haraguchi N, Miyake M, Hama N, Miyamoto A, Ikeda M, Nakamori S, Sekimoto M, Fujitani K, Tsujinaka T. Prevalence of Malnutrition Among Gastric Cancer Patients Undergoing Gastrectomy and Optimal Preoperative Nutritional Support for Preventing Surgical Site Infections. Ann Surg Oncol. 2015;22 Suppl 3:S778-S785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 106. | Shi HP, Xu HX, Li SY, Cao WX, Li W, Ba Y, Chen GY, Wang KH, Qi YM, Chen ZH, Yu K, Jiang H, Hu W, Cong MH, Chen JQ, Li ZN, Fang Y, Ge S, Wang XY, Lin Y, Guan WX, Luo Q. [Five-step treatment of malnutrition]. Zhongliu Daixie Yu Yingyang Dianzi Zazhi. 2015;2:29-33. |
| 107. | Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 403] [Article Influence: 67.2] [Reference Citation Analysis (3)] |
| 108. | Hubbard GP, Elia M, Holdoway A, Stratton RJ. A systematic review of compliance to oral nutritional supplements. Clin Nutr. 2012;31:293-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 109. | Wischmeyer PE, Carli F, Evans DC, Guilbert S, Kozar R, Pryor A, Thiele RH, Everett S, Grocott M, Gan TJ, Shaw AD, Thacker JKM, Miller TE, Hedrick TL, McEvoy MD, Mythen MG, Bergamaschi R, Gupta R, Holubar SD, Senagore AJ, Abola RE, Bennett-Guerrero E, Kent ML, Feldman LS, Fiore JF Jr; Perioperative Quality Initiative (POQI) 2 Workgroup. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Nutrition Screening and Therapy Within a Surgical Enhanced Recovery Pathway. Anesth Analg. 2018;126:1883-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 110. | Yasuda H, Kondo N, Yamamoto R, Asami S, Abe T, Tsujimoto H, Tsujimoto Y, Kataoka Y. Monitoring of gastric residual volume during enteral nutrition. Cochrane Database Syst Rev. 2021;9:CD013335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Chen Y, Zhuang Y, Cai Z, Zhang Y, Guo S. Effects of enteral nutrition on pro-inflammatory factors and intestinal barrier function in patients with acute severe pancreatitis. Eur J Inflamm. 2019;17:205873921982721. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 112. | Xu Y, Wei FX. A retrospective study of enteral nutrition on immune and inflammatory factors after liver cancer surgery. Medicine (Baltimore). 2021;100:e27718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 113. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 672] [Article Influence: 112.0] [Reference Citation Analysis (2)] |
| 114. | Beijing Medical Association Committee of Parenteral and Enteral Nutrition; Expert Panel on Consensus on the parenteral and Enteral Nutritional and the Dietary Intervention for Patients with Chronic Liver Diseases. [Consensus on the clinical nutritional intervention for patients with chronic liver diseases]. Linchuang Gandanbing Zazhi. 2017;33:1236-1245. [DOI] [Full Text] |
| 115. | Aubry E, Aeberhard C, Leuenberger M, Stirnimann J, Friedli N, Schütz P, Stanga Z. Refeeding-Syndrom: Ein konsensusbasierter Algorithmus für stationäre Patienten. Aktuel Ernahrungsmed. 2019;44:33-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 116. | Reber E, Friedli N, Vasiloglou MF, Schuetz P, Stanga Z. Management of Refeeding Syndrome in Medical Inpatients. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 117. | Chen L, Chen Y, Wang X, Li H, Zhang H, Gong J, Shen S, Yin W, Hu H. Efficacy and safety of oral branched-chain amino acid supplementation in patients undergoing interventions for hepatocellular carcinoma: a meta-analysis. Nutr J. 2015;14:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 118. | Shuja A, Malespin M, Scolapio J. Nutritional Considerations in Liver Disease. Gastroenterol Clin North Am. 2018;47:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 119. | Chong CCN, Chung WY, Cheung YS, Fung AKY, Fong AKW, Lok HT, Wong J, Lee KF, Chan SKC, Lai PBS. Enhanced recovery after surgery for liver resection. Hong Kong Med J. 2019;25:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 120. | Plauth M, Cabré E, Campillo B, Kondrup J, Marchesini G, Schütz T, Shenkin A, Wendon J; ESPEN. ESPEN Guidelines on Parenteral Nutrition: hepatology. Clin Nutr. 2009;28:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 121. | Blumberg JB, Cena H, Barr SI, Biesalski HK, Dagach RU, Delaney B, Frei B, Moreno González MI, Hwalla N, Lategan-Potgieter R, McNulty H, van der Pols JC, Winichagoon P, Li D. The Use of Multivitamin/Multimineral Supplements: A Modified Delphi Consensus Panel Report. Clin Ther. 2018;40:640-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 122. | Yu K, Zheng X, Wang G, Liu M, Li Y, Yu P, Yang M, Guo N, Ma X, Bu Y, Peng Y, Han C, Yu K, Wang C. Immunonutrition vs Standard Nutrition for Cancer Patients: A Systematic Review and Meta-Analysis (Part 1). JPEN J Parenter Enteral Nutr. 2020;44:742-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 123. | Mikagi K, Kawahara R, Kinoshita H, Aoyagi S. Effect of preoperative immunonutrition in patients undergoing hepatectomy; a randomized controlled trial. Kurume Med J. 2011;58:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 124. | Russell K, Zhang HG, Gillanders LK, Bartlett AS, Fisk HL, Calder PC, Swan PJ, Plank LD. Preoperative immunonutrition in patients undergoing liver resection: A prospective randomized trial. World J Hepatol. 2019;11:305-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 125. | Wang Z, Li L, Wang S, Wei J, Qu L, Pan L, Xu K. The role of the gut microbiota and probiotics associated with microbial metabolisms in cancer prevention and therapy. Front Pharmacol. 2022;13:1025860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 126. | Tan J, Fu B, Zhao X, Ye L. Novel Techniques and Models for Studying the Role of the Gut Microbiota in Drug Metabolism. Eur J Drug Metab Pharmacokinet. 2024;49:131-147. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 127. | Shimizu K, Ojima M, Ogura H. Gut Microbiota and Probiotics/Synbiotics for Modulation of Immunity in Critically Ill Patients. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 128. | Vitetta L, Saltzman ET, Thomsen M, Nikov T, Hall S. Adjuvant Probiotics and the Intestinal Microbiome: Enhancing Vaccines and Immunotherapy Outcomes. Vaccines (Basel). 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 129. | Meighani A, Alimirah M, Ramesh M, Salgia R. Fecal Microbiota Transplantation for Clostridioides Difficile Infection in Patients with Chronic Liver Disease. Int J Hepatol. 2020;2020:1874570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 130. | Konturek PC, Koziel J, Dieterich W, Haziri D, Wirtz S, Glowczyk I, Konturek K, Neurath MF, Zopf Y. Successful therapy of Clostridium difficile infection with fecal microbiota transplantation. J Physiol Pharmacol. 2016;67:859-866. [PubMed] |
| 131. | Esakov YS, Raevskaya MB, Sizov VA, Pechetov AA, Ruchkin DV, Gorin DS, Kazennov VV, Khlan TN. [The philosophy of rapid rehabilitation in thoracoabdominal surgery]. Khirurgiia (Mosk). 2016;88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 132. | Brustia R, Monsel A, Skurzak S, Schiffer E, Carrier FM, Patrono D, Kaba A, Detry O, Malbouisson L, Andraus W, Vandenbroucke-Menu F, Biancofiore G, Kaido T, Compagnon P, Uemoto S, Rodriguez Laiz G, De Boer M, Orloff S, Melgar P, Buis C, Zeillemaker-Hoekstra M, Usher H, Reyntjens K, Baird E, Demartines N, Wigmore S, Scatton O. Guidelines for Perioperative Care for Liver Transplantation: Enhanced Recovery After Surgery (ERAS) Recommendations. Transplantation. 2022;106:552-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 133. | Williams DGA, Ohnuma T, Krishnamoorthy V, Raghunathan K, Sulo S, Cassady BA, Hegazi R, Wischmeyer PE. Impact of early postoperative oral nutritional supplement utilization on clinical outcomes in colorectal surgery. Perioper Med (Lond). 2020;9:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 134. | Yan X, Liu L, Zhang Y, Song T, Liang Y, Liu Z, Bao X, Mao L, Qiu Y. Perioperative Enteral Nutrition Improves Postoperative Recovery for Patients with Primary Liver Cancer: A Randomized Controlled Clinical Trial. Nutr Cancer. 2021;73:1924-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 135. | McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, Gervasio JM, Sacks GS, Roberts PR, Compher C; Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JPEN J Parenter Enteral Nutr. 2016;40:159-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1695] [Cited by in RCA: 1866] [Article Influence: 207.3] [Reference Citation Analysis (3)] |