Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2183
Revised: May 13, 2024
Accepted: May 28, 2024
Published online: July 27, 2024
Processing time: 104 Days and 22 Hours
According to the theory of traditional Chinese medicine (TCM), the spleen and stomach are the basis of acquired nature and the source of qi and blood bio
To investigate the effect of Buzhong Yiqi decoction on spleen and stomach qi deficiency in patients with colorectal cancer.
One hundred patients with colorectal cancer who underwent preoperative chemotherapy and laparoscopy at The First TCM Hospital of Changde from January 2022 to October 2023 were retrospectively analyzed. The patients were divided equally into control and observation groups. Both groups underwent conventional rehabilitation surgery, and the observation group was supplemented with Buzhong Yiqi decoction. SPSS 26.0 was used for statistical analyses. The χ2 test was used for univariate analysis; independent sample t-tests were used in all cases.
No significant differences were observed preoperatively in the general characteristics of the two groups. Fourteen days post-surgery, the abdominal distension, emaciation, loose stool, loss of appetite, and vomiting scores were significantly lower in the observation group than in the control group (P < 0.05). Immune function and interleukin (IL)-10 levels in the observation group were significantly higher than those of the control group, whereas IL-6, tumor necrosis factor-α, and C-reactive protein levels, tumor biological indexes, and adverse reactions in the observation group were significantly lower than those of the control group (P < 0.05). One month after surgery, the patients’ quality of life in the observation group was significantly higher than that of the patients in the control group (P < 0.05).
Buzhong Yiqi decoction can regulate inflammatory responses and metabolic processes by enhancing immune function, thereby promoting overall immune nutrition and restoring the body’s balance.
Core Tip: Through a retrospective analysis, this study investigated the therapeutic effects of Buzhong Yiqi decoction in patients with spleen and stomach qi deficiency after neoadjuvant chemotherapy and laparoscopic radical surgery for colorectal cancer. The results showed that Buzhong Yiqi decoction significantly improved symptoms, enhanced immune function, reduced the levels of inflammatory mediators, and improved the quality of life of the patients.
- Citation: Hu Q, Chen XP, Tang ZJ, Zhu XY, Liu C. Therapeutic effects of Buzhong Yiqi decoction in patients with spleen and stomach qi deficiency after routine surgery and chemotherapy for colorectal cancer. World J Gastrointest Surg 2024; 16(7): 2183-2193
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2183.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2183
Colorectal cancer, a malignancy affecting the gastrointestinal system globally, is witnessing an alarming increase, profoundly affecting both the well-being and survival of affected individuals[1]. The traditional Chinese medicine (TCM) names of colorectal cancer are “conglomeration”, “abdominal mass”, “anal cryptitis”, “intestinal qin”, “anorectal cancer”, and “intestinal rock”. Owing to the invasion of dampness and heat, patients with colorectal cancer suffer from healthy qi deficiency, middle-jiao damage, long-term retention of toxic pathogens in the intestine, and, eventually, vein damage[2]. Currently, surgical intervention is the cornerstone for managing colorectal cancer, with laparoscopic radical surgery emerging as a leading modality owing to its minimally invasive nature and accelerated recovery. However, the physiological trauma inflicted by such procedures may disrupt normal body functions, precipitating an imbalance in spleen and stomach functions, subsequently leading to the spleen and stomach qi deficiency syndrome[3]. Consequently, a compromised immune function increases the vulnerability to postoperative infections and tumor recurrence. Preoperative neoadjuvant chemotherapy, which is chemotherapy implemented after a clear diagnosis of the tumor, aims to induce tumor cell apoptosis, reduce tissue edema, delay the clinical stage, and reduce the lesion size, thus creating more favorable conditions for surgical treatment and enhancing therapeutic effects. Buzhong Yiqi decoction, a revered formula known for its qi-invigorating and spleen-fortifying ability, is widely used for the management of spleen and stomach qi deficiency syndrome. It replenishes Zhong Yiqi, invigorates Yang, assuages despondency, and ameliorates symptoms such as physical disability and diminished appetite[4]. The key ingredients of Buzhong Yiqi decoction include astragalus, codonopsis, white atractylodes, and chenpi. This ancient remedy has garnered recognition in modern medical studies for its remarkable anti-fatigue, immune-boosting, and anti-inflammatory properties among patients with spleen deficiency[5]. This study aimed to elucidate the impact of Buzhong Yiqi decoction on immune function and inflammatory markers in patients with spleen and stomach qi deficiency following laparoscopic radical surgery for colorectal cancer. Its primary aim was to unravel the clinical efficacy of Buzhong Yiqi decoction post-surgery, thereby offering novel perspectives and methodologies that seamlessly integrate TCM into contemporary Western medical practices in the multifaceted management of colorectal cancer.
One hundred patients with colorectal cancer who underwent preoperative neoadjuvant chemotherapy and laparoscopic radical surgery at The First Traditional Chinese Medicine Hospital of Changde from January 2022 to October 2023 were retrospectively analyzed and divided into control (n = 50) and observation groups (n = 50) on the basis of the patients’ perioperative treatment regimen. The inclusion criteria were as follows: (1) Pathologically confirmed diagnosis of colorectal cancer; (2) meeting the diagnostic criteria for spleen and stomach qi deficiency according to TCM; (3) undergoing radical laparoscopic surgery; and (4) availability of complete clinical data. The exclusion criteria were as follows: (1) Hepatic or renal insufficiency; (2) poor perioperative compliance; (3) co-occurrence of systemic immune disorders; and (4) the presence of inflammatory gastrointestinal diseases.
All patients exhibited symptoms consistent with spleen deficiency syndrome according to the TCM diagnosis and treatment guide for cancer. These symptoms include fatigue, loose stools, and diminished appetite, whereas secondary symptoms include weak pulse; slow, fine, and fat tongue or teeth marks; thin, white tongue coating; weak tongue; nausea and vomiting; abdominal distension after eating; body fatigue; and laundry speech. The presence of two or more main syndromes and one or more secondary syndromes can yield a diagnosis. Western medicine criteria for colorectal cancer are similar to the Chinese criteria for colorectal cancer diagnosis and treatment, that is, typical symptoms (such as abdominal pain, abdominal distension, changes in stool, anal or abdominal digital mass, and dyspepsia), imaging and colonoscopy features, pathological examination of surgical specimens, and indications for mucosal biopsy are common.
Control group: Preoperative neoadjuvant chemotherapy with FOLFOX4 chemotherapy regimen [oxaliplatin (85 mg/m2) was administered by intravenous drip for 2 h on day 1; calcium folinate (200 mg/m2) was administered by intravenous drip for 2 h on days 1-2; and fluorouracil (400 mg/m2)] was administered intravenously. The dosage of fluorouracil was then increased to 600 mg/m2 and intravenously pumped at a rate of 5 mL /h for 22 h, and a total of four cycles (14 d as a cycle) were given. On the basis of the patient’s vital signs, laparoscopic radical rectal cancer surgery was performed 4 wk after chemotherapy. The following conventional rehabilitation surgical measures were used: (1) Preoperatively: Gastrointestinal decompression was performed before radical surgery, and the average postoperative extraction was 24-48 h. The patient fasted for 24 h, and drinking of water was forbidden for 8 h. The patient took the Chinese patent medicine orally in the evening 1 d before the operation, and intestinal cleaning was performed by professional nursing staff. Early in the morning, routine gastric and urinary catheters were inserted, and the patient underwent routine general anesthesia; (2) intraoperatively: Patients were given appropriate nutritional fluids, maintained at a normal body temperature, and closely monitored for changes in body functions; and (3) postoperatively: Self-controlled analgesic pumps (opioids) were used on the basis of patients’ pain level. The patient was routinely administered rehydration fluid at 3000 mL/d while monitoring the electrolytes and internal environment by adding electrolytes at the right time, correcting the acid-base imbalance in time, and monitoring the water intake and release from the body to prevent excessive or insufficient fluid rehydration, for 5 d. The patient was administered anti-infective treatment after the surgery, began to eat after the emergence of anal exhaustion, and gradually switched from bed activities to passive activities and out-of-bed activities after being bedridden for 3 d. The patient was also administered antibiotics for postoperative treatment.
Observation group: In addition to the treatment received by the control group, the observation group was orally administered Buzhong Yiqi decoction. The composition of the formula included Angelica sinensis and yam (15 g each); orange peel, bupleurum, and licorice (6 g each); ginseng, cohosh, white atractylodes, amomum kernel, and poria (9 g each); and astragalus (18 g). The two medications were administered daily, one in the morning and one in the evening, for 14 d.
The following indicators were collected from both groups of patients after laparoscopic radical surgery, 1 d preoperatively and 14 d postoperatively: (1) TCM syndrome evaluation: The TCM syndrome score was determined by observing the symptoms and the severity of symptoms, including abdominal distension, fatigue, lethargy, loose stool, loss of appetite, and vomiting. On the basis of the frequency of attacks, the four grades were no, occasional, frequent, and daily attacks, and the score for each symptom ranged from 0-3 points; (2) Karnofsky performance status (KPS) score: The KPS score is used to determine the functional status (0-100 points) of patients. The higher the score, the better the general condition and self-care ability. A score of 80 or more is considered non-dependent, 50-70 is semi-dependent, and 50 or less is dependent; and (3) immune function and inflammatory mediator levels: Serum tumor necrosis factor (TNF-α), C-reactive protein (CRP), interleukin (IL)-6, and IL-10 levels in fasting peripheral venous blood samples of patients in both groups were measured using an enzyme-linked immunosorbent assay (Specification: Yue Xie Zhu Zhun 20192220393, Model: MR-96A). Serum immunoglobulin (Ig) G, IgA, and IgM levels were detected by immunoturbidimetric assay using an immunoglobulin M assay kit (Specification: Zhe Xie Zhu Zhun 20162400137, Model: reagent 70 mL × 3). Peripheral blood CD3+ T lymphocyte percentage, CD4+ T lymphocyte percentage, CD8+ T lymphocyte percentage, CD4+/CD8+ T lymphocyte ratio, and natural killer (NK) cell percentage were detected by flow cytometry (Specification: Yue Xie Zhu Zhun 20202221588, Model: EasyCell 103A0).
One month after completing the treatment regimen, patients from both groups were asked to complete the SF-36 questionnaire to assess their health status and functionality. The SF-36 questionnaire, developed in 1988 at the Boston Health Research Institute, is a widely used tool comprising 36 items. It comprehensively evaluates health-related life qualities across age groups, diverse populations, and health conditions. The questionnaire covers eight dimensions: Role limitations owing to physical health, physical functioning, general health perceptions, bodily pain, vitality, role limitations due to emotional problems, mental health, and social functioning. Each response option was assigned a value, and the cumulative score across all dimensions was used to provide an overall measure of health and well-being. Additionally, the SF-36 questionnaire enables comparison with normative data and assessment of treatment outcomes over time.
Common complications, including nausea, vomiting, abdominal distension, and postoperative incision infections within 7 d post-treatment, were compared between the two groups.
Statistical analyses were performed using SPSS, version 26.0. For data with a normal distribution, the mean and standard deviation (mean ± SD) are used to represent measurement data, and an independent sample t-test was performed for comparison. Count data are expressed as sample size in percentages [n (%)], and univariate analysis was conducted using the chi-square test. Differences were considered statistically significant at P < 0.05.
No significant differences were observed in age, body-mass index, sex distribution, smoking status, or alcohol consumption between the two groups (P > 0.05), as presented in Table 1.
| Group | Control group (n = 50) | Observation group (n = 50) | t/χ2 | P value | |
| Age (years, mean ± SD) | 47.12 ± 13.37 | 46.97 ± 14.21 | 0.246 | 0.806 | |
| Sex, n (%) | Male | 25 (50.00) | 27 (54.00) | 0.160 | 0.689 |
| Female | 25 (50.00) | 23 (46.00) | |||
| BMI (kg/m2) | 21.62 ± 4.03 | 21.36 ± 3.84 | 0.453 | 0.652 | |
| History of smoking, n (%) | 7 (14.00) | 6 (12.00) | 0.088 | 0.766 | |
| History of alcohol use, n (%) | 10 (20.00) | 12 (24.00) | 0.629 | 0.629 | |
One day before the surgery, no significant differences were observed in the TCM syndrome scores between the two groups (P > 0.05). After the treatment for 14 d, both groups of participants experienced reductions in scores for symptoms such as abdominal distension, fatigue, lethargy, loose stool, loss of appetite, and vomiting, as well as enhancements in the KPS scores compared with the baseline levels (P < 0.05). Moreover, the observation group showed significantly reduced scores for symptoms such as abdominal bloating, weight loss, diarrhea, appetite loss, and vomiting compared with the control group after treatment. Additionally, their KPS scores were significantly higher (P < 0.05), as presented in Table 2.
| Group | Abdominal bloating | Anesthesia | Emaciation | Loose stool | Loss of appetite | Vomit | KPS score | |||||||
| 1 d preoperati | 14 d postoperati | 1 d preoperati | 14 d postoperati | 1 d preoperati | 14 d postoperati | 1 d preoperati | 14 d postoperati | 1 d preoperati | 14 d postoperati | 1 d preoperati | 14 d postoperati | 1 d preoperati | 14 d postoperati | |
| Control group (n = 50) | 1.98 ± 0.40 | 1.33 ± 0.37a | 1.87 ± 0.32 | 1.46 ± 0.22a | 1.96 ± 0.51 | 1.02 ± 0.46a | 1.81 ± 0.47 | 1.00 ± 0.50a | 1.94 ± 0.34 | 1.52 ± 0.28a | 1.92 ± 0.45 | 1.21 ± 0.40a | 69.78 ± 6.98 | 78.13 ± 7.21a |
| Observation group (n = 50) | 1.93 ± 0.42 | 0.74 ± 0.21a | 1.84 ± 0.30 | 1.10 ± 0.17a | 2.00 ± 0.50 | 0.67 ± 0.38a | 1.77 ± 0.45 | 0.61 ± 0.36a | 1.90 ± 0.31 | 1.18 ± 0.20a | 1.94 ± 0.42 | 0.79 ± 0.31a | 68.12 ± 6.77 | 83.32 ± 6.54a |
| t value | 0.969 | 9.717 | 0.289 | 0.770 | 0.555 | 4.319 | 0.393 | 4.516 | 0.923 | 7.201 | 0.251 | 5.633 | 1.533 | 4.307 |
| P value | 0.335 | < 0.001 | 0.773 | 0.443 | 0.580 | < 0.001 | 0.695 | < 0.001 | 0.358 | < 0.001 | 0.803 | < 0.001 | 0.129 | < 0.001 |
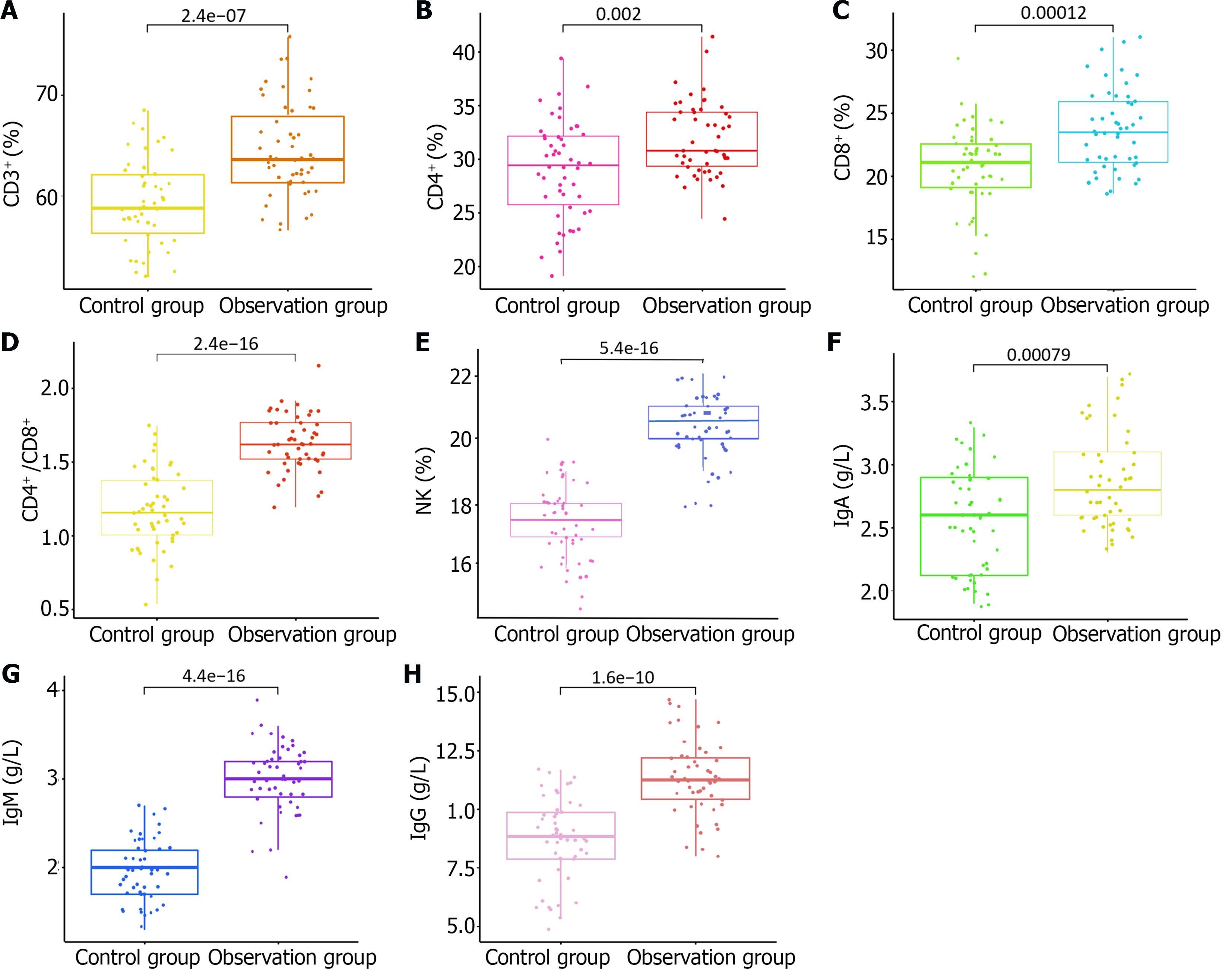
Preoperatively, no significant differences were observed in the immune function between the two groups (P > 0.05). However, after 14 d of treatment, both groups experienced an enhancement in various immune parameters. The proportions of CD3+ and CD8+ T lymphocytes, as well as the ratios of CD4+/CD8+ T lymphocytes and NK cells, increased with elevated levels of IgG, IgA, and IgM in the peripheral blood compared with the baseline levels. Notably, the observation group demonstrated superior immune function compared with the control group, with significantly higher levels of these immune markers (P < 0.05). These findings are presented in Table 3 and depicted in Figure 1.
| Index | Control group (n = 50) | Observation group (n = 50) | t value | P value | |
| CD3+ (%) | 1 d preoperatively | 55.31 ± 3.72 | 55.86 ± 3.88 | 1.448 | 0.151 |
| 14 d postoperatively | 59.03 ± 4.26a | 64.32 ± 4.67a | 6.025 | < 0.001 | |
| CD4+ (%) | 1 d preoperatively | 28.26 ± 4.46 | 28.84 ± 4.62 | 0.132 | 0.895 |
| 14 d postoperatively | 29.16 ± 4.53 | 31.78 ± 3.46a | 3.619 | < 0.001 | |
| CD8+ (%) | 1 d preoperatively | 26.89 ± 4.33 | 26.77 ± 4.67 | 0.077 | 0.939 |
| 14 d postoperatively | 20.67 ± 3.35a | 23.77 ± 3.24a | 4.576 | < 0.001 | |
| CD4+/CD8+ (%) | 1 d preoperatively | 1.17 ± 0.26 | 1.11 ± 0.24 | 1.088 | 0.279 |
| 14 d postoperatively | 1.32 ± 0.18a | 1.63 ± 0.19a | 10.051 | < 0.001 | |
| NK cell (%) | 1 d preoperatively | 16.23 ± 1.46 | 16.75 ± 1.49 | 1.558 | 0.122 |
| 14 d postoperatively | 17.31 ± 1.17 | 20.45 ± 1.01a | 13.240 | < 0.001 | |
| IgG (g/L) | 1 d preoperatively | 6.21 ± 1.53 | 6.07 ± 1.47 | 0.405 | 0.686 |
| 14 d postoperatively | 8.77 ± 1.78a | 11.24 ± 1.58a | 7.882 | < 0.001 | |
| IgA (g/L) | 1 d preoperatively | 2.12 ± 0.32 | 2.04 ± 0.36 | 1.110 | 0.270 |
| 14 d postoperatively | 2.57 ± 0.40a | 2.89 ± 0.38a | 4.144 | < 0.001 | |
| IgM (g/L) | 1 d preoperatively | 1.89 ± 0.33 | 1.85 ± 0.29 | 0.578 | 0.565 |
| 14 d postoperatively | 1.97 ± 0.34a | 2.29 ± 0.38a | 14.364 | < 0.001 | |
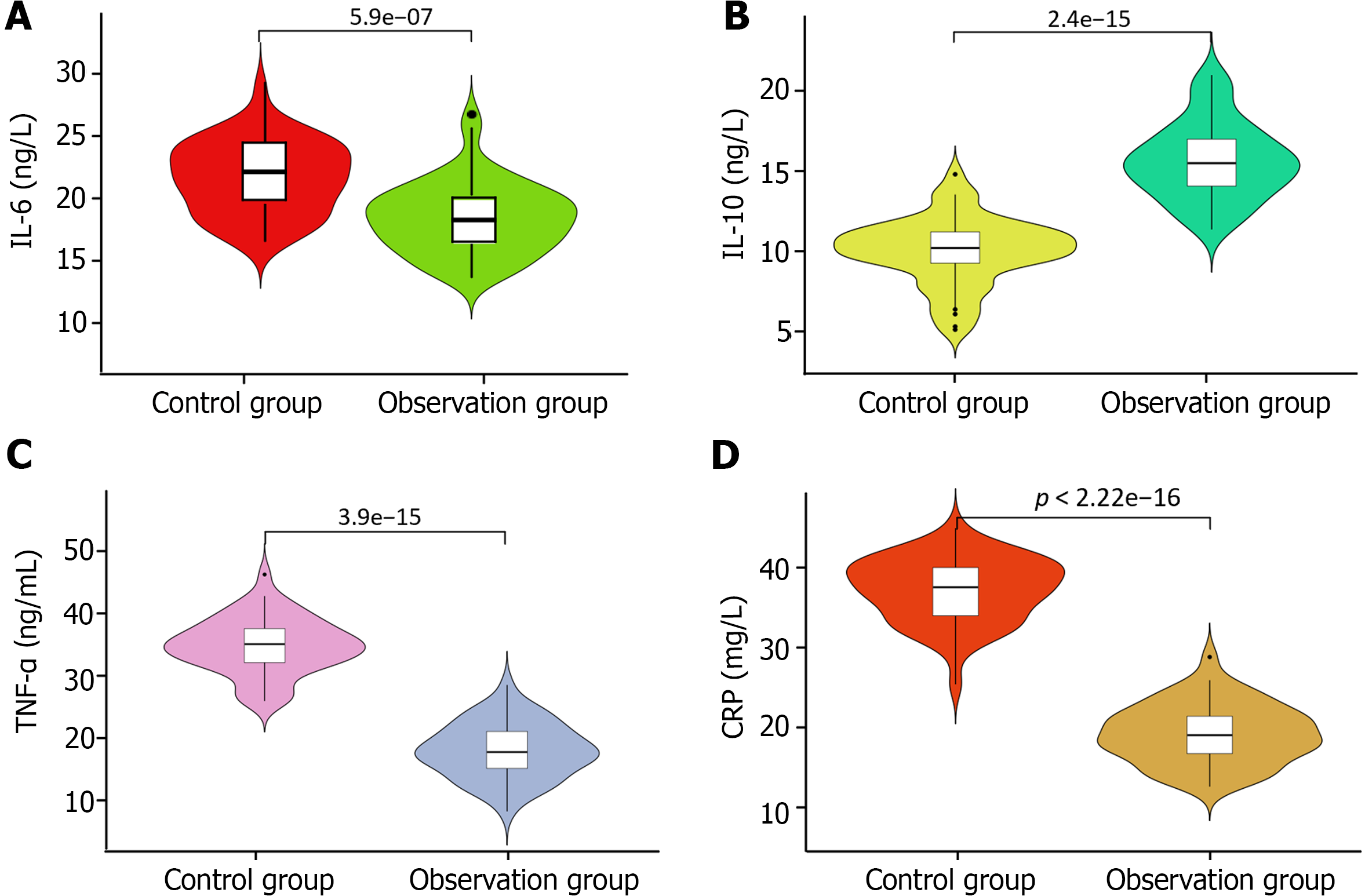
Preoperatively, no significant differences were observed in the levels of inflammatory markers including IL-10, IL-6, TNF-α, and CRP between the two groups (P > 0.05). After treatment for 14 d, a significant increase in the levels of these markers was observed in both groups compared with their baseline levels (P < 0.05). Notably, the observation group exhibited significantly higher levels of IL-10 post-treatment than the control group. Conversely, the levels of IL-6, TNF-α, and CRP were significantly lower in the observation group than in the control group (P < 0.05). Further details regarding these changes are presented in Table 4 and depicted in Figure 2.
| Index | Control group (n = 50) | Observation group (n = 50) | t value | P value | |
| IL-10 (ng/L) | 1 d preoperatively | 8.73 ± 1.97 | 8.62 ± 2.01 | 1.012 | 0.849 |
| 14 d postoperatively | 10.21 ± 2.14a | 15.32 ± 2.43a | 11.311 | < 0.001 | |
| IL-6 (ng/L) | 1 d preoperatively | 7.41 ± 1.16 | 7.29 ± 1.07 | 0.190 | 0.314 |
| 14 d postoperatively | 20.78 ± 3.46a | 16.72 ± 3.28a | 5.733 | < 0.001 | |
| TNF-α (ng/mL) | 1 d preoperatively | 18.21 ± 4.21 | 18.73 ± 4.56 | 0.701 | 0.485 |
| 14 d postoperatively | 34.32 ± 5.02a | 22.31 ± 4.94a | 12.060 | < 0.001 | |
| CRP (mg/L) | 1 d preoperatively | 6.45 ± 0.37 | 6.53 ± 0.34 | 1.802 | 0.075 |
| 14 d postoperatively | 31.68 ± 3.72a | 19.27 ± 3.45a | 17.097 | < 0.001 | |
Before the surgery, no significant differences were observed in carcinoembryonic antigen, cancer antigen 19-9, vascular endothelial growth factor, or tumor specific growth factor levels between the two groups (P > 0.05). However, after the treatment period, both groups showed a noticeable decrease in tumor biological indices compared with their baseline levels. As presented in Table 5, it is noteworthy that the observation group exhibited a significant reduction in these tumor markers compared with the control group (P < 0.05).
| Group | CEA (ng/mL) | CA19-9 (U/mL) | VEGF (ng/mL) | TSGF (U/mL) | ||||
| 1 d preoperatively | 14 d postoperatively | 1 d preoperatively | 14 d postoperatively | 1 d preoperatively | 14 d postoperatively | 1 d preoperatively | 14 ds postoperatively | |
| Control group (n = 50) | 27.11 ± 5.72 | 22.12 ± 4.79a | 46.31 ± 5.78 | 40.32 ± 4.78a | 60.28 ± 10.97 | 40.31 ± 8.12a | 74.31 ± 7.26 | 69.28 ± 6.14a |
| Observation group (n = 50) | 26.58 ± 5.87 | 18.33 ± 4.13a | 45.87 ± 5.43 | 36.53 ± 4.01a | 61.09 ± 11.76 | 31.53 ± 9.46a | 73.29 ± 7.53 | 63.28 ± 6.03a |
| t value | 0.671 | 4.428 | 0.148 | 4.420 | 0.811 | 4.905 | 0.160 | 4.777 |
| P value | 0.504 | < 0.001 | 0.883 | < 0.001 | 0.42 | < 0.001 | 0.873 | < 0.001 |
Post-treatment, SF-36 questionnaire scores in the observation group were significantly boosted compared with those in the control group (P < 0.05), indicating an enhancement in the various aspects of health and well-being. This improvement reflects the positive impact of the intervention, suggesting potential benefits for the patients’ quality of life. These findings underscore the importance of a comprehensive assessment of the holistic effects of therapeutic interventions. Further elaboration of the specific domains showing improvement in SF-36 scores could provide valuable insights into treatment outcomes and patient experiences, as shown in Table 6.
| Index | Control group (n = 50) | Observation group (n = 50) | t value | P value |
| SF-36 score | 81.62 ± 7.33 | 92.26 ± 9.33 | 6.982 | < 0.001 |
During treatment, the observation group experienced significantly fewer adverse reactions than the control group (12.00% vs 30.00%, P < 0.05), as presented in Table 7. This finding suggests a favorable safety profile associated with the treatment regimen in the observation group, indicating its potential clinical benefit.
| Group | Nausea and vomiting | Abdominal bloating | Incision infection | Anastomotic fistula | Gastric retention | Anemia | Total occurrence |
| Control group (n = 50) | 4 (8.00) | 5 (10.00) | 3 (6.00) | 2 (4.00) | 0 (0.00) | 1 (2.00) | 15 (30.00) |
| Observation group (n = 50) | 1 (2.00) | 1 (2.00) | 2 (4.00) | 1 (2.00) | 1 (2.00) | 0 (0.00) | 6 (12.00) |
| χ2 | 4.882 | ||||||
| P value | 0.027 |
Colorectal cancer predominantly affects the left half of the colon, including the descending colon, sigmoid colon, and rectum[6]. The disease often manifests with subtle symptoms in the early stages, leading to delayed diagnosis until the intermediate or advanced stages are reached. This delay poses significant hurdles to effective treatment. Furthermore, the long-term survival rate for late-stage colorectal cancer remains low, ranging from 14.4%-39.5%, highlighting a poor prognosis for patients[7]. Recently, colorectal cancer resection surgery has been widely used in clinics. Its success rate has increased significantly with good surgical treatment effects. Preoperative neoadjuvant chemotherapy is an important treatment method. Its unique advantage lies in its ability to significantly shrink tumor tissues and simultaneously kill hard-to-detect metastatic cells as much as possible, thus significantly reducing the risk of patients’ tumors. Hence, preoperative neoadjuvant chemotherapy creates favorable conditions for subsequent surgery and greatly improves the success rate of surgical resection; however, the postoperative efficacy in some patients is still poor due to the heavy surgical stress reaction of the patients, which is detrimental to the treatment[8]. Assessing the immune function and inflammatory mediators is crucial for evaluating patients’ recovery status. Reduced immune function increases the vulnerability to infections and illnesses, whereas abnormal levels of inflammatory mediators can lead to tissue damage and disease advancement[9]. Buzhong Yiqi decoction, a TCM formula rooted in the principle of replenishing depleted qi, has been used by practitioners for generations. This prescription, with astragalus as its principal herb, fortifies qi and strengthens the body’s defenses. Complementary herbs such as ginseng, licorice, and baizhu bolster qi and invigorate the spleen, synergistically enhancing the tonic effects. Additionally, the inclusion of Angelica replenishes the blood, whereas the tangerine peel regulates qi and harmonizes the stomach, ensuring a balanced tonifying effect without inducing stagnation[10]. Hence, the primary aim of this study was to explore the effects of Buzhong Yiqi decoction on the immune function and inflammatory responses of individuals with colorectal cancer and to elucidate its potential therapeutic benefits in enhancing treatment outcomes and improving their life quality.
Fourteen days after the surgery, individuals in the observation group exhibited a significant decrease in TCM syndrome scores and a significantly higher KPS score compared with the control group. Previous research[11] has suggested that surgical procedures can deplete qi and impair blood circulation, resulting in decreased postoperative vital energy. Moreover, according to the modern TCM belief[12], surgery can damage the viscera and veins, leading to abnormal spleen and stomach functions and impaired transport and conduction abilities. This disruption may cause blood stasis and hinder a smooth qi flow, thereby increasing the likelihood of congestion. Studies have implicated neuro-radiation and inflammatory processes induced by surgical interventions as major contributors to postoperative intestinal paralysis[13]. Therefore, the expedited recovery of bodily and gastrointestinal functions after laparoscopic radical surgery is imperative, as the spleen and stomach play crucial roles in maintaining overall health and harmonizing qi and blood levels. Consequently, postoperative care should prioritize qi enhancement and spleen strengthening. Additionally, efforts should be directed toward regulating qi flow, improving blood circulation, and alleviating blood stasis. These interventions restore intestinal function, mitigate blood congestion, and promote smooth blood circulation[14]. Buzhong Yiqi decoction, a classic prescription from the Spleen and Stomach School, is noteworthy in this context. Compared with the sole administration of routine rehabilitation surgical measures, oral supplementation with Buzhong Yiqi decoction can help patients consolidate their foundation, uplift their vitality, nourish qi, and regulate their gastrointestinal functions.
In this study, the observation group exhibited a more pronounced enhancement of inflammatory factor levels. Previous investigations[15] have suggested that surgical procedures in patients with colorectal cancer can trigger the prolonged activation of the sympathetic adrenomedullary system. This leads to increased catecholamine levels, sustained constriction of abdominal visceral blood vessels, and subsequent ischemic and hypoxic conditions in abdominal organs. Following the surgical trauma, the body responds by synthesizing and secreting various inflammatory cytokines, including IL-6, as a part of the inflammatory cascade[16]. This cytokine plays a pivotal role in the body’s immune response to injury and infection and serves as a key mediator of inflammation. Elevated levels following surgery contribute to systemic inflammatory response syndrome, which, if left unchecked, can lead to tissue damage and organ dysfunction. Therefore, monitoring IL-6 levels postoperatively provides valuable insights into the inflammatory status of patients and can guide clinical management strategies aimed at mitigating excessive inflammation and promoting healing. The broad-ranging effects of these inflammatory factors can induce systemic inflammation in patients, activate the coagulation and complement systems, damage vascular endothelial cells, cause tissue and organ injury, and impede postoperative recovery processes[17]. Research[18] suggests that Buzhong Yiqi decoction might exert favorable effects on skin and gastrointestinal tract regulation, immune function, and inflammatory responses. Furthermore, contemporary pharmacological studies[19] have uncovered the potential of codonopsis, a component of Buzhong Yiqi decoction, to enhance immune function, ameliorate intestinal absorption, stimulate intestinal motility, inhibit platelet aggregation, and bolster hematopoietic function. Through anti-platelet aggregation, it can delay thrombosis, reduce blood viscosity, and enhance red blood cell deformability, thereby achieving the pharmacological effects of anti-thrombosis, anti-coagulation, and hemorrhagic improvement. Amomum kernels exhibit biological activities, including the promotion of gastrointestinal motility and anti-inflammatory, analgesic, antibacterial, and antimicrobial properties. Additionally, the saponins and polysaccharides present in ginseng can modulate gastrointestinal function, enhance neuro-humoral regulation, and mitigate postsurgical stress responses[20]. Buzhong Yiqi decoction plays a pivotal role in promoting and modulating the immune function of the body. It enhances cellular metabolic activity, bolsters specific resistance, exerts favorable effects on tumors, and possesses anti-inflammatory properties that aid in suppressing immune-related inflammation.
In this study, the incorporation of Buzhong Yiqi decoction alongside treatment resulted in a significant improvement in immune function compared with routine rehabilitation surgical measures alone. Patients with colorectal cancer frequently experience malnutrition and compromised immune function resulting from tumor-induced nutrient depletion. Moreover, chemotherapy exacerbates stress responses in patients, which can detrimentally affect treatment outcomes and prognosis[21]. A key factor contributing to the observed enhancement in immune function is the diverse effects of astragalus and astragalus polysaccharides found in Buzhong Yiqi decoction, bolstering overall immune function[22]. Furthermore, Angelica sinensis plays a pivotal role in enhancing immune function, whereas orange peels, cohosh, and bupleurum exhibit anti-allergic properties and strengthen cellular immune function, aiding in the recovery from immunosuppression. The immune system is intricately linked to the body’s ability to combat diseases and maintain overall health. Therefore, enhancing immune system function is crucial for improving treatment outcomes and quality of life. Buzhong Yiqi decoction, with its multifaceted effects on the immune system, offers a promising adjunctive therapy for patients with CRC undergoing chemotherapy. By addressing malnutrition and bolstering immune function, Buzhong Yiqi decoction helps mitigate the adverse effects of chemotherapy and improve patients’ resilience to treatment-related stressors. Furthermore, the anti-allergic properties of certain herbs in Buzhong Yiqi decoction contribute to a reduction in allergic reactions and enhancement in cellular immune function. This is particularly beneficial for patients undergoing chemotherapy because chemotherapy drugs often elicit allergic reactions and compromise immune function. Buzhong Yiqi decoction may help patients better tolerate chemotherapy and reduce the risk of treatment-related complications by fortifying the body’s immune defenses, highlighting its potential as a complementary therapy for patients with colorectal cancer undergoing chemotherapy. Its ability to enhance immune function and mitigate the adverse effects of chemotherapy underscores its value for improving treatment outcomes and patient well-being. Further research on the specific mechanisms underlying its immunomodulatory effects is warranted to fully elucidate its therapeutic potential in cancer treatment.
In this study, we observed significant 14-d post-treatment improvements in the observation group, particularly in terms of tumor marker reduction and enhanced quality of life, when compared with their counterparts in the control group. This indicates a potential synergistic effect of the herbal decoction in cancer management. Additionally, the observation group displayed a considerable boost in their overall post-treatment well-being, which highlights the holistic benefits of incorporating TCM into cancer care regimens. The reduced occurrence of adverse reactions among patients receiving the herbal supplementation underscores its favorable safety profile, further supporting its potential as an adjunct therapy in cancer treatment protocols. Astragalus, the principal herb in the Buzhong Yiqi decoction, contains cycloastragaloside alcohol (CAG), a bioactive compound with antitumor properties[23]. The mechanism of action of CAG reveals its ability to inhibit cathepsin B, a key enzyme implicated in MHC-I degradation in tumor cells. By preserving MHC-I molecules, CAG facilitates the improved recognition of tumor cells by immune cells, thereby enhancing antitumor immune responses[24]. This contributes to the growing body of evidence supporting the integration of TCM into modern cancer care approaches. By harnessing the therapeutic potential of herbal remedies such as Buzhong Yiqi decoction, we can explore new avenues for enhancing patient outcomes and minimizing treatment-related complications in the fight against cancer[25]. The sample size of this study was small, which limits the generalizability of the research findings. In the future, efforts should be made to expand the sample size for further investigation.
In conclusion, using Buzhong Yiqi decoction to treat patients with spleen and stomach qi deficiency after laparoscopic radical surgery for colorectal cancer has proven to be effective and safe. This mechanism involves boosting the body’s immune function to regulate inflammation and metabolism, thereby promoting the overall well-being of patients. Future research directions for Buzhong Yiqi decoction in the treatment of colorectal cancer include elucidating its molecular mechanisms, exploring interactions with chemotherapeutic drugs, assessing its efficacy in different patient subpopulations, and conducting long-term follow-up studies. However, these findings highlight its potential for broader clinical applications.
| 1. | Saadh MJ, Allela OQB, Sattay ZJ, Al Zuhairi RAH, Ahmad H, Eldesoky GE, Adil M, Ali MS. Deciphering the functional landscape and therapeutic implications of noncoding RNAs in the TGF-β signaling pathway in colorectal cancer: A comprehensive review. Pathol Res Pract. 2024;255:155158. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Kobayashi T, Sekimoto M, Miki H, Yamamoto N, Harino T, Yagyu T, Hori S, Hatta M, Hashimoto Y, Kotsuka M, Yamasaki M, Inoue K. Laparoscopic polyglycolic acid spacer placement for locally recurrent rectal cancer. Colorectal Dis. 2024;26:760-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Shin JK, Kim HC, Lee WY, Yun SH, Cho YB, Huh JW, Park YA. Is Robotic Surgery Beneficial for Rectal Cancer Patients with Unfavorable Characteristic After Neoadjuvant Chemoradiotherapy? Ann Surg Oncol. 2024;31:3203-3211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Zeng P, Wang F, Zhang J, Ur Rashid H, Li X, Zhang P, Luo Y, Wu X. Integrating network pharmacology and experimental verification to investigate the pharmacological mechanisms of Buzhong Yiqi decoction in the treatment of non-small cell lung cancer. Chem Biol Drug Des. 2024;103:e14414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Yang J, Li Y, Chau CI, Shi J, Chen X, Hu H, Ung COL. Efficacy and safety of traditional Chinese medicine for cancer-related fatigue: a systematic literature review of randomized controlled trials. Chin Med. 2023;18:142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 6. | Dunne PD, Arends MJ. Molecular pathological classification of colorectal cancer-an update. Virchows Arch. 2024;484:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 7. | Xu Y, Lv Y, Zhu Z, Chen Y, Zhou P, Ye L, Tang W, Xu J. Precision medicine in the treatment of colorectal cancer with liver metastases. Cancer Biol Med. 2024;20:942-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Mariusdottir E, Jörgren F, Saeed M, Wikström J, Lydrup ML, Buchwald P. Hartmann’s procedure in rectal cancer surgery is often an intraoperative decision: a retrospective multicenter study. Langenbecks Arch Surg. 2024;409:55. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Yu X, Ou J, Wang L, Li Z, Ren Y, Xie L, Chen Z, Liang J, Shen G, Zou Z, Zhao C, Li G, Hu Y. Gut microbiota modulate CD8(+) T cell immunity in gastric cancer through Butyrate/GPR109A/HOPX. Gut Microbes. 2024;16:2307542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 10. | Xiangru XU, Yi Z, Gang C, Ming L, Wen Z, Xinxin WU, Yuting PU, Caiyu C, Yuting S, Shuang Z, Bangjiang F. Clinical efficacy of Buzhong Yiqi decoction in the treatment of hospital-acquired pneumonia with multi-drug resistant bacteria: a prospective, randomized, multicenter controlled trial. J Tradit Chin Med. 2023;43:1010-1018. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Zhao B, Yu Z, Hu T. Comparative efficacy of uncut Roux-en-Y and Billroth II anastomosis in gastrointestinal reconstruction following laparoscopic radical gastrectomy for distal gastric cancer. Medicine (Baltimore). 2024;103:e37037. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Yoneura N, Shimizu Y, Kondo E, Nishiya K, Ito K, Yokoyama K, Shimizu K, Onai Y, Nakata Y, Nakagawa A, Ishii T. [Perioperative Outcome of Laparoscopic Surgery after Colonic Stent Placement for Obstructive Colorectal Cancer]. Gan To Kagaku Ryoho. 2023;50:1854-1856. [PubMed] |
| 13. | Nguyen TX, Pham NH. Three-dimensional laparoscopic surgery for colorectal cancer: A 2-year follow-up study at Hue Central Hospital. Surg Open Sci. 2024;17:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 14. | Hu B, An HM, Shen KP. [Pharmacological study of Buzhong Yiqi Decoction: a review]. Zhong Xi Yi Jie He Xue Bao. 2008;6:752-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Bekki T, Shimomura M, Hattori M, Sato S, Watanabe A, Ishikawa S, Imaoka K, Ono K, Matsubara K, Mochizuki T, Akabane S, Yano T, Ohdan H. C-Reactive Protein/Albumin Ratio Is an Independent Risk Factor for Recurrence and Survival Following Curative Resection of Stage I-III Colorectal Cancer in Older Patients. Ann Surg Oncol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Zhang T, Miao YC. Prognostic evaluation of preoperative systemic immune inflammatory index in patients with colorectal cancer. Front Oncol. 2023;13:1260796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 17. | Deng D, Chen Z, Jia L, Bu J, Ye M, Sun L, Gen Y, Zhang W, Chen G, Fang B. Treatment of hospital-acquired pneumonia with multi-drug resistant organism by Buzhong Yiqi decoction based on Fuzheng Quxie classical prescription: study protocol for a randomized controlled trial. Trials. 2019;20:817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Yi JL, Wang Y, Jing H, Shi YT, Liu CY. [Buzhong yiqi decoction containing serum reversed resistance of A549/DDP to cisplatin and its effect on the expression of survivin: an experimental research]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34:1250-1255. [PubMed] |
| 19. | Dai HY, Cao KJ, Zhao AB, Chen XY. [Study on the effects of hematopoiesis of anemic mice after marrow-suppressed treated by tonifying kidney, invigorating spleen, and removing blood stasis]. Zhong Yao Cai. 2011;34:250-253. [PubMed] |
| 20. | Wang W, Chen H, Zhang W, Fan D, Deng J, Yang H. Ginsenoside Rk3 Ameliorates Obesity-Induced Colitis by Modulating Lipid Metabolism in C57BL/6 Mice. J Agric Food Chem. 2024;72:2997-3007. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Wei J, Ge X, Qian Y, Jiang K, Chen X, Lu W, Yang H, Fu D, Fang Y, Zhou X, Xiao Q, Tang Y, Ding K. Development and verification of a combined immune- and cancer-associated fibroblast related prognostic signature for colon adenocarcinoma. Front Immunol. 2024;15:1291938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 22. | Tao Y, Yuan J, Zhou H, Li Z, Yao X, Wu H, Shi H, Huang F, Wu X. Antidepressant potential of total flavonoids from Astragalus in a chronic stress mouse model: Implications for myelination and Wnt/β-catenin/Olig2/Sox10 signaling axis modulation. J Ethnopharmacol. 2024;325:117846. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Zhang YM, Miao ZM, Chen YP, Song ZB, Li YY, Liu ZW, Zhou GC, Li J, Shi LL, Chen Y, Zhang SZ, Xu X, He JP, Wang JF, Zhang LY, Liu YQ. Ononin promotes radiosensitivity in lung cancer by inhibiting HIF-1α/VEGF pathway. Phytomedicine. 2024;125:155290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 24. | Li Q, Zhang C, Xu G, Shang X, Nan X, Li Y, Liu J, Hong Y, Wang Q, Peng G. Astragalus polysaccharide ameliorates CD8(+) T cell dysfunction through STAT3/Gal-3/LAG3 pathway in inflammation-induced colorectal cancer. Biomed Pharmacother. 2024;171:116172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 25. | Hu J, Li X, Fang Y, Peng J. Efficacy and safety of Buzhong Yiqi Decoction in improving cancer-related fatigue and immunity of cervical carcinoma patients: A protocol of randomized controlled trial. Medicine (Baltimore). 2021;100:e27938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |