Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2145
Revised: May 8, 2024
Accepted: May 27, 2024
Published online: July 27, 2024
Processing time: 109 Days and 21.6 Hours
Patients with different stages of colorectal cancer (CRC) exhibit different abdominal computed tomography (CT) signs. Therefore, the influence of CT signs on CRC prognosis must be determined.
To observe abdominal CT signs in patients with CRC and analyze the correlation between the CT signs and postoperative prognosis.
The clinical history and CT imaging results of 88 patients with CRC who underwent radical surgery at Xingtan Hospital Affiliated to Shunde Hospital of Southern Medical University were retrospectively analyzed. Univariate and multivariate Cox regression analyses were used to explore the independent risk factors for postoperative death in patients with CRC. The three-year survival rate was analyzed using the Kaplan-Meier curve, and the correlation between postoperative survival time and abdominal CT signs in patients with CRC was analyzed using Spearman correlation analysis.
For patients with CRC, the three-year survival rate was 73.86%. The death group exhibited more severe characteristics than the survival group. A multivariate Cox regression model analysis showed that body mass index (BMI), degree of periintestinal infiltration, tumor size, and lymph node CT value were independent factors influencing postoperative death (P < 0.05 for all). Patients with characteristics typical to the death group had a low three-year survival rate (log-rank χ2 = 66.487, 11.346, 12.500, and 27.672, respectively, P < 0.05 for all). The survival time of CRC patients was negatively correlated with BMI, degree of periintestinal infiltration, tumor size, lymph node CT value, mean tumor long-axis diameter, and mean tumor short-axis diameter (r = -0.559, 0.679, -0.430, -0.585, -0.425, and -0.385, respectively, P < 0.05 for all). BMI was positively correlated with the degree of periintestinal invasion, lymph node CT value, and mean tumor short-axis diameter (r = 0.303, 0.431, and 0.437, respectively, P < 0.05 for all).
The degree of periintestinal infiltration, tumor size, and lymph node CT value are crucial for evaluating the prognosis of patients with CRC.
Core Tip: The incidence and mortality rates of colorectal cancer (CRC) are alarming. We analyzed the demographic data, pathological information, and abdominal computed tomography (CT) findings of 88 patients with CRC after radical surgery. This is a retrospective single-center study to investigate the correlation of demographic data, pathological information, and abdominal CT signs with prognosis. We solved the problem of CRC prognosis assessment by observing the changes in the survival rate of patients with CRC under different influencing factors.
- Citation: Yang SM, Liu JM, Wen RP, Qian YD, He JB, Sun JS. Correlation between abdominal computed tomography signs and postoperative prognosis for patients with colorectal cancer. World J Gastrointest Surg 2024; 16(7): 2145-2156
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2145.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2145
Malignant tumors include carcinoma, sarcoma, and carcinosarcoma, which are caused by malignant cell proliferation and are invasive and capable of metastasis. Malignant tumors have become the leading killer of human health[1]. Mutations or abnormal expression of genes cause cells to acquire the ability to maintain proliferation signal transduction, escape growth inhibitors, resist cell death, replicate immortality, induce angiogenesis, and activate invasion and metastasis, leading to tumor occurrence and progression[2].
A common malignant tumor worldwide is colorectal cancer (CRC), and in China, its incidence and mortality rates are increasing[3]. Among the malignant tumors prevalent in China, the mortality rate of CRC ranks second only to stomach, lung, liver, and esophageal cancers. Surgical resection of tumors is currently the primary method used to treat CRC. However, only 20% of patients treated with resection and adjuvant therapy are cured, and 20%-25% of patients newly diagnosed with CRC annually have metastasis or metastatic diseases[4]. In addition, the five-year survival rate of patients with advanced colon cancer in China is only 10.8%, and approximately 50% of the patients die as a result of local recurrence or metastasis after surgery[5]. Therefore, the accurate assessment of CRC prognoses is crucial for guiding clinical interventions and optimizing treatment outcomes. Currently, clinical research is focused on searching for the factors that may affect prognosis after radical CRC surgery. However, the clinicopathological features and prognosis of patients with different stages of CRC exhibit different abdominal computed tomography (CT) signs, which affect the treatment of CRC[6]. Consequently, it is not reasonable to treat CRC patients with different abdominal CT signs using the same regimen. Studies have pointed out that the clinicopathological features and prognosis of CRC patients with different abdominal CT signs differ[7].
Thus, this study aimed to analyze the correlation between abdominal CT signs and postoperative prognosis of patients with CRC and clarify the impact of abdominal CT signs on the short-term prognosis of patients to provide new ideas for early prognosis prediction of CRC and guiding clinicians to identify high-risk patients and take countermeasures to improve the prognosis of CRC.
A retrospective study was conducted on 88 patients with CRC who underwent radical surgery at Xingtan Hospital Affiliated to Shunde Hospital of Southern Medical University from January 2020 to December 2020. The patient selection criteria were as follows. All patients: (1) Were diagnosed with CRC in accordance with the Guidelines for the Diagnosis and Treatment of Colorectal Cancer (2010 Edition), which was confirmed via pathological examination[8]; (2) Underwent radical surgery; and (3) Had no history of radiotherapy or chemotherapy. Patients were excluded if: (1) They had other acute/chronic liver and kidney dysfunction or malignant tumors; (2) They had heart dysfunction or congenital heart disease; or (3) Their follow-up information was incomplete.
The baseline data for all the patients were collected. These data included: (1) Demographic data: Age, sex, body mass index (BMI), smoking history, and drinking history; (2) Pathological information: Pathological stage, vascular thrombus, degree of tumor differentiation, lymph node metastasis, and tumor location; and (3) Abdominal CT signs: The form of intestinal wall thickening, degree of periintestinal infiltration, transformation rate of signal, low-density area after enhancement, lymph node location, tumor size, lymph node CT value, mean tumor long-axis diameter, and mean tumor short-axis diameter.
Intestinal preparation: The patients were required to adhere to a diet with less residue 2 d before the examination, eat liquid food the day before, take 50 g of purgative 50% magnesium sulfate twice the night before the examination, drink more water, and record the timing of their defecations. Ten minutes before the CT scan, 20 mg of the antispasmolytic drug amidoamine hydrochloride was administered intramuscularly to relieve intestinal spasms, reduce colonic tension, and decrease intestinal peristaltic artifacts. The patient was placed in the right lateral decubitus position, and air was injected through a rectal catheter. Next, the patient was placed in a supine or prone position, and additional air was injected. The volume of air injected was 1000-1500 mL, depending on the patient’s tolerance.
CT scanning: A 128-row spiral CT scan was performed. The parameters are as follows: Tube voltage of 120 kVp, tube current of 250 mA, pitch of 0.969, rotation time of 0.75 s, reconstruction interval of 5 mm, layer thickness of 5 mm, and matrix of 512 × 512. During the scan, the patient was placed in a supine position to enable the observation of the inflated status of the colon and rectum. Then, the nonionic contrast agent Ornepiac was quickly injected into the anterior cubital vein with a high-pressure syringe at a dose of 1.5 mL/kg and an injection speed of 2.5-3.0 mL/s. Subsequently, arterial phase, portal vein phase, and delayed phase scans were performed at 23 s, 50 s, and 180 s, respectively. The thickness, reconstruction interval, and pitch were 3.0 mm, 1.5 mm, and 2.0, respectively. The scanning area was the entire colon and liver.
Image processing: Two senior imaging physicians used EBW4.5 to evaluate the imaging features of the lesions without knowing the results of the pathological diagnoses. If the two physicians had different opinions, a consensus was reached through negotiation. The plain scan and enhanced CT values of the solid components of the lesions were measured, and the same region of interest was evaluated at each stage while avoiding areas corresponding to phenomena such as calcification, bleeding, and necrosis fibrosis. The average of the results measured by the two physicians was used as the final result.
The survival rate of the patients at three years after their operations was determined. The end of the follow-up period was December 2023 or the date of death, whichever occurred first for a given patient. Survivors were included in the “survival” group, and the deceased were included in the “death” group.
The data were processed using SPSS version 23.0. Measurement data are expressed as the mean ± SD. Count data are expressed as percentages (%) and were compared using a χ2 test (used when the theoretical frequency is greater than 5) or an exact test (used when the theoretical frequency is less than 5). Multivariate Cox regression was used to analyze the factors affecting the death of patients with CRC three years after their surgeries. The sensitivity and specificity of the independent influencing factors were analyzed using the receiver operating characteristic (ROC) curve. We analyzed the three-year survival of patients according to the degree of periintestinal infiltration, tumor size, lymph node CT value, and BMI using the Kaplan–Meier curve and the log-rank test. The Spearman correlation test was used to evaluate the correlation between non-normal measurement data and rank data. The level of statistical significance was set at P < 0.05.
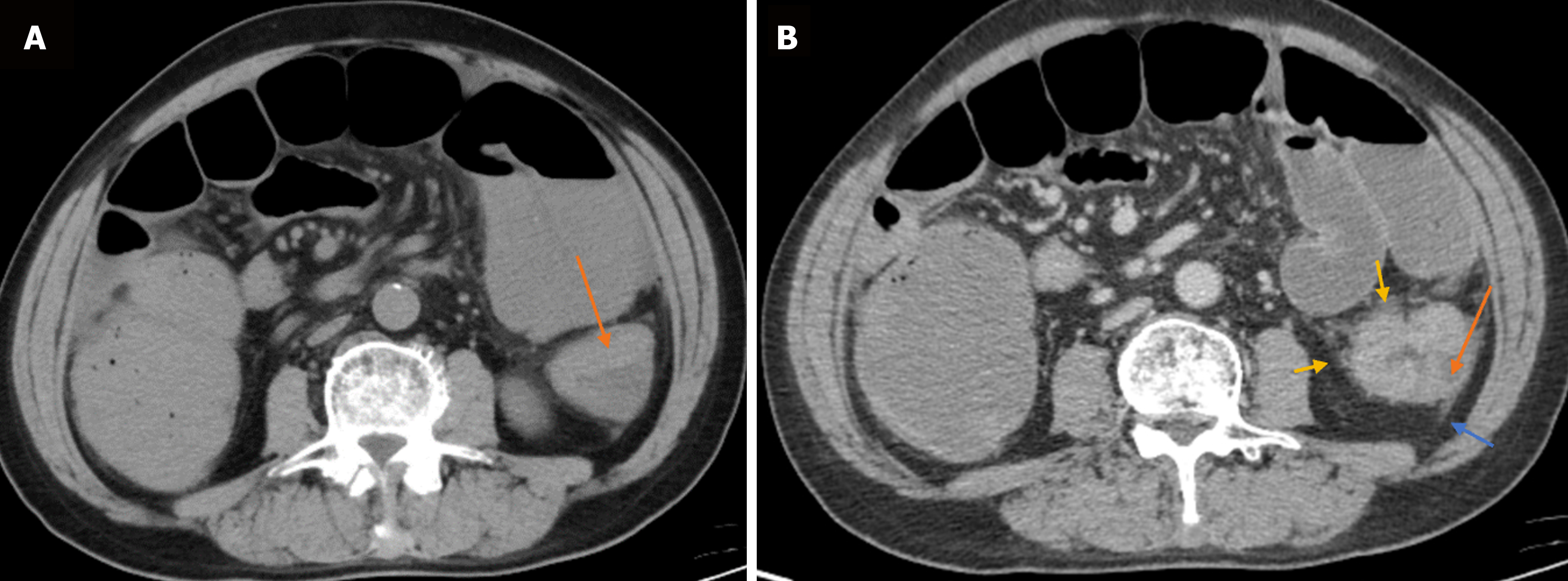
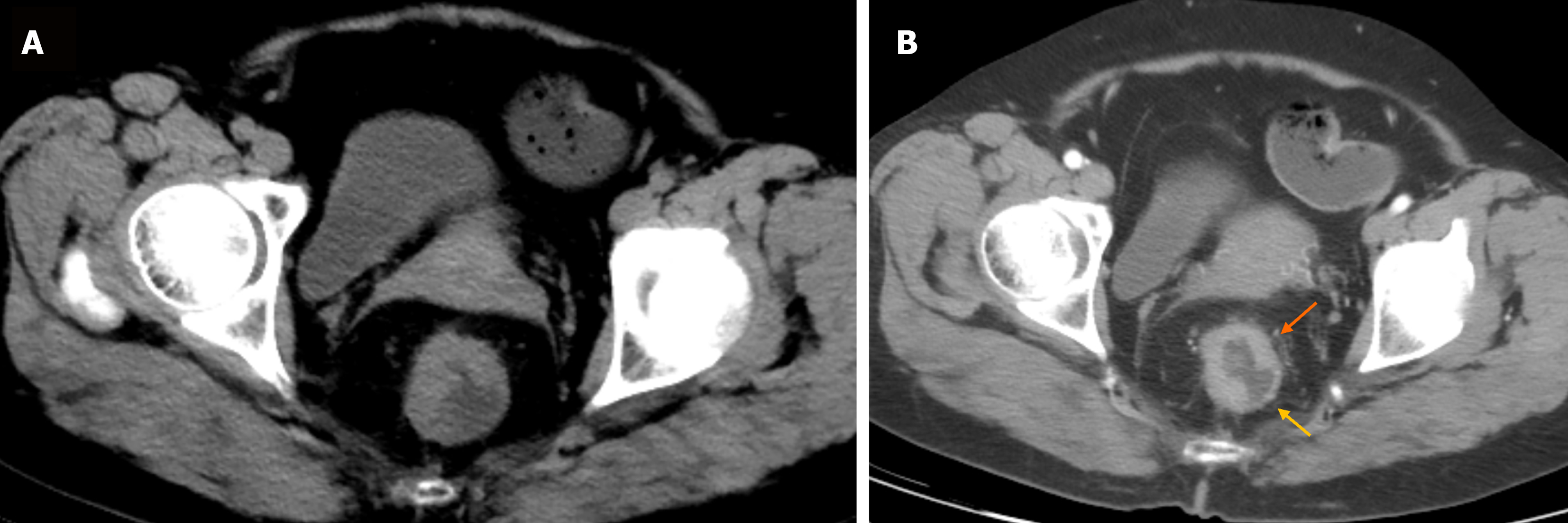
During the three-year follow-up period, 65 of the 88 patients with CRC survived, and 23 died. Hence, the survival rate was 73.86%, and the mortality rate was 26.14%. In 15, 37, 30, and 6 cases, the form of intestinal wall thickening was polyps or cauliflower, eccentric, uneven ring, and uniform ring, respectively. In 33, 31, and 24 cases, the degree of periintestinal infiltration was mild, moderate, and severe, respectively. In 30, 34, and 24 cases, the degree of enhancement was mild, moderate, and severe, respectively. The number of cases with a low-density area after enhancement was 53. In 51, 29, and 8 cases, the lymph node location was the mesenterium, root, and lateral position, respectively. The mean tumor size was 3.95 ± 1.95 cm, the mean lymph node CT value was 27.23 ± 10.11 HU, the mean tumor long-axis diameter was 0.65 ± 0.38 cm, and the mean tumor short-axis diameter was 0.43 ± 0.28 cm. Figures 1 and 2 show the patients’ CT scans.
Compared with the survival group, the death group was more likely to have a BMI > 24 kg/m2, pathological stage IV, highly differentiated tumor, severe periintestinal infiltration, tumor size ≥ 4 cm, lymph node CT value ≥ 30 HU, mean tumor long-axis diameter ≥ 0.6 cm, and mean tumor short-axis diameter ≥ 0.45 cm (P < 0.05 for all), as shown in Table 1.
| Data | Death group (n = 23) | Survival group (n = 65) | χ2 | P value | |
| Gender | Male | 14 (60.87) | 37 (56.92) | 0.109 | 0.742 |
| Female | 9 (39.13) | 28 (43.08) | |||
| Age | ≥ 60 years | 15 (65.22) | 36 (55.38) | 0.674 | 0.412 |
| < 60 years | 8 (34.78) | 29 (44.62) | |||
| BMI | < 18.5 kg/m2 | 0 (0.00) | 4 (6.15) | 16.992 | < 0.001 |
| 18.5-24 kg/m2 | 11 (47.83) | 55 (84.62) | |||
| > 24 kg/m2 | 12 (52.17) | 6 (9.23) | |||
| Smoking history | Yes | 9 (39.13) | 23 (35.38) | 0.103 | 0.748 |
| No | 14 (60.87) | 42 (64.62) | |||
| Drinking history | Yes | 7 (30.43) | 18 (27.69) | 0.063 | 0.802 |
| No | 16 (69.57) | 47 (72.31) | |||
| Pathological stage | I | 0 (0.00) | 10 (15.38) | 15.401 | 0.001 |
| II | 7 (30.43) | 39 (60.00) | |||
| III | 12 (52.17) | 13 (20.00) | |||
| IV | 4 (17.39) | 3 (4.62) | |||
| Blood vessel invasion | Yes | 1 (4.35) | 2 (3.08) | < 0.001 | > 0.999 |
| No | 22 (95.65) | 63 (96.92) | |||
| Degree of tumor differentiation | Poorly differentiated | 6 (26.09) | 5 (7.69) | 8.950 | 0.011 |
| Moderately differentiated | 16 (69.57) | 42 (64.62) | |||
| Highly differentiated | 1 (4.35) | 18 (27.69) | |||
| Lymphatic metastasis | Yes | 10 (43.48) | 14 (21.54) | 4.123 | 0.042 |
| No | 13 (56.52) | 51 (78.46) | |||
| Tumor site | Right semicolon | 8 (34.78) | 12 (18.46) | 2.824 | 0.244 |
| Left semicolon | 5 (21.74) | 14 (21.54) | |||
| Rectum | 10 (43.48) | 39 (60.00) | |||
| Intestinal wall thickening form | Polyps or cauliflower thickening | 6 (26.09) | 9 (13.85) | 2.201 | 0.532 |
| Eccentric thickening | 9 (39.13) | 28 (43.08) | |||
| Uneven ring thickening | 6 (26.09) | 24 (36.92) | |||
| Uniform ring thickening | 2 (8.70) | 4 (6.15) | |||
| Degree of periintestinal infiltration | Mild | 0 (0.00) | 33 (50.77) | 48.385 | < 0.001 |
| Moderate | 4 (17.39) | 27 (41.54) | |||
| Severe | 19 (82.61) | 5 (7.69) | |||
| Transformation rate of signal | Mild | 8 (34.78) | 22 (33.85) | 2.879 | 0.237 |
| Moderate | 6 (26.09) | 28 (43.08) | |||
| Severe | 9 (39.13) | 15 (23.08) | |||
| Low-density area after enhancement | Yes | 13 (56.52) | 40 (61.54) | 0.178 | 0.673 |
| No | 10 (43.48) | 25 (38.46) | |||
| Lymph node location | Mesenterium | 15 (65.22) | 36 (55.38) | 1.234 | 0.540 |
| Root | 7 (30.43) | 22 (33.85) | |||
| Lateral | 1 (4.35) | 7 (10.77) | |||
| Tumor size | < 4 cm | 4 (17.39) | 38 (58.46) | 11.486 | 0.001 |
| ≥ 4 cm | 19 (82.61) | 27 (41.54) | |||
| Lymph node CT value | < 30 HU | 4 (17.39) | 39 (60.00) | 10.697 | 0.001 |
| ≥ 30 HU | 19 (82.61) | 26 (40.00) | |||
| Mean tumor long-axis diameter | < 0.6 cm | 4 (17.39) | 35 (53.85) | 7.731 | 0.005 |
| ≥ 0.6 cm | 19 (82.61) | 30 (46.15) | |||
| Mean tumor short-axis diameter | < 0.45 cm | 5 (21.74) | 36 (55.38) | 7.729 | 0.005 |
| ≥ 0.45 cm | 18 (78.26) | 29 (44.62) |
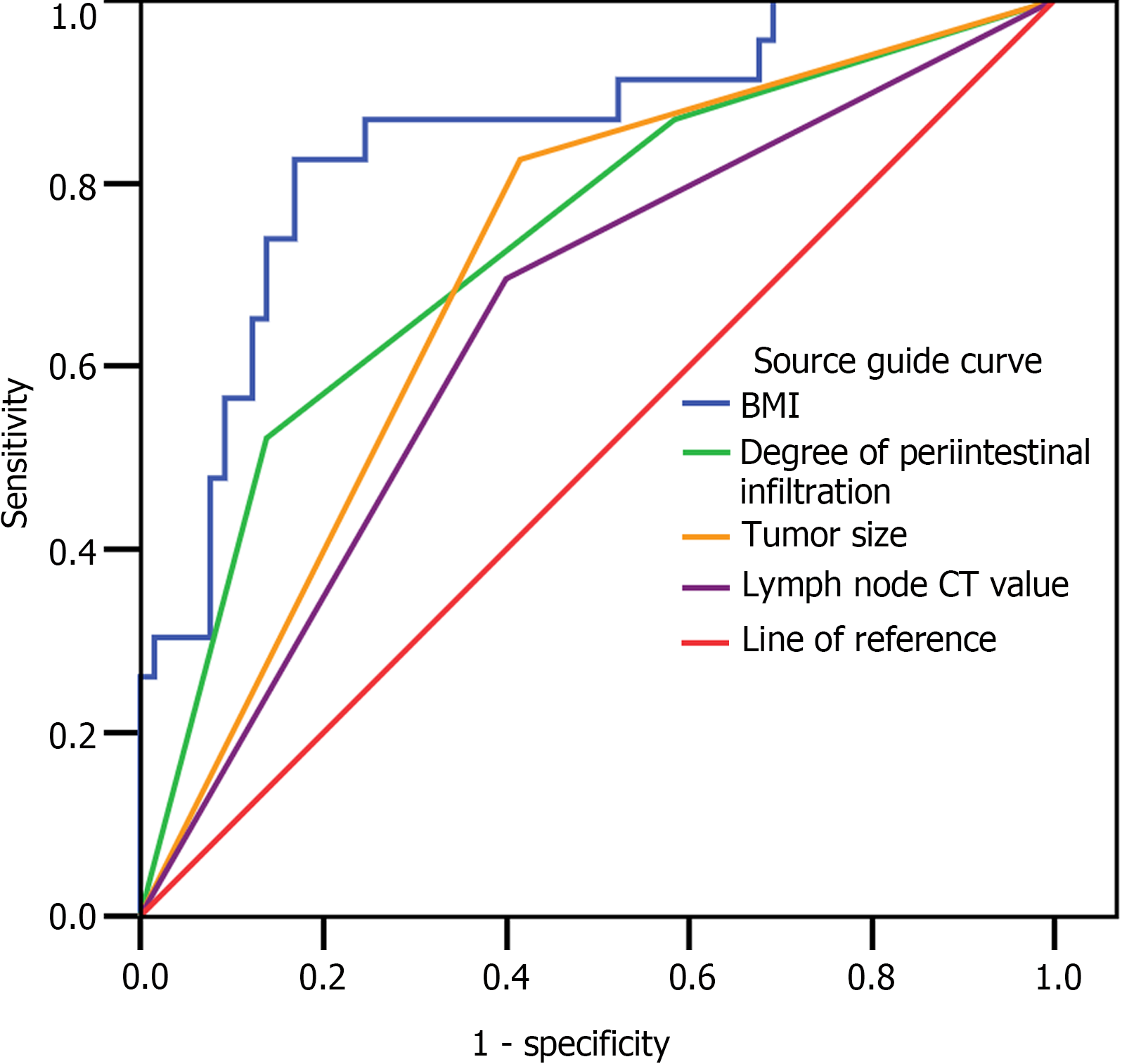
Using the postoperative prognosis of the patients with CRC as the dependent variable (0 = survival, 1 = death), and the statistically significant indicators in the univariate analysis (BMI, pathological stage, degree of intestinal infiltration, tumor size, lymph node CT value, mean tumor long-axis diameter, and mean tumor short-axis diameter) as independent variables (Table 2), multivariate Cox regression analysis demonstrated that BMI, degree of periintestinal infiltration, tumor size, and lymph node CT value were independent factors influencing the likelihood of postoperative death in CRC patients (P < 0.05), as shown in Table 3. ROC curve analysis showed that both BMI and tumor size had the highest sensitivity of 82.6%. The specificity of BMI and degree of periintestinal infiltration was also higher at 83.1% and 86.2%, respectively (Table 4 and Figure 3).
| Independent variable | Assignment |
| BMI | 0: < 18.5 kg/m2; 1: 18.5-24 kg/m2; 2: > 24 kg/m2 |
| Pathological stage | 1: I; 2: II; 3: III; 4: IV |
| Degree of tumor differentiation | 1: Poorly differentiated; 2: Moderately differentiated; 3: Highly differentiated |
| Degree of periintestinal infiltration | 1: Mild; 2: Moderate; 3: Severe |
| Tumor size | 0: < 4 cm; 1: ≥ 4 cm |
| Lymph node CT value | 0: < 30 HU; 1: ≥ 30 HU |
| Mean tumor long-axis diameter | 0: < 0.6 cm; 1: ≥ 0.6 cm |
| Mean tumor short-axis diameter | 0: < 0.45 cm; 1: ≥ 0.45 cm |
| Independent variable | B | SE | Wald | P value | OR | 95%CI |
| BMI | 0.409 | 0.139 | 8.621 | 0.003 | 1.506 | 1.146-1.979 |
| Pathological stage | 2.255 | 0.521 | ||||
| I | -7.741 | 222.369 | 0.001 | 0.972 | 0.000 | 0.000-8.303 |
| II | 0.957 | 0.780 | 1.504 | 0.220 | 2.603 | 0.564-12.003 |
| III | 1.052 | 0.712 | 2.183 | 0.140 | 2.865 | 0.709-11.569 |
| Degree of tumor differentiation | 1.749 | 0.417 | ||||
| Poorly differentiated | 0.686 | 1.290 | 0.283 | 0.595 | 1.986 | 0.158-24.909 |
| Moderately differentiated | -0.302 | 1.114 | 0.074 | 0.786 | 0.739 | 0.083-6.565 |
| Degree of periintestinal infiltration | 7.030 | 0.030 | ||||
| Mild | -12.450 | 167.468 | 0.006 | 0.941 | 0.000 | 0.000-1.390 |
| Moderate | -1.784 | 0.673 | 7.025 | 0.008 | 0.168 | 0.045-0.628 |
| Tumor size | 1.522 | 0.660 | 5.322 | 0.021 | 4.581 | 1.257-16.691 |
| Lymph node CT value | 1.362 | 0.689 | 3.911 | 0.048 | 3.904 | 1.012-15.059 |
| Mean tumor long-axis diameter | 0.918 | 0.809 | 1.287 | 0.257 | 2.505 | 0.513-12.240 |
| Variable | Area (95%CI) | Standard error | P value | Sensitivity | Specificity |
| BMI | 0.847 (0.753-0.942) | 0.048 | < 0.001 | 82.6% | 83.1% |
| Degree of periintestinal infiltration | 0.735 (0.613-0.857) | 0.062 | 0.001 | 52.2% | 86.2% |
| Tumor size | 0.705 (0.587-0.824) | 0.06 | 0.004 | 82.6% | 58.5% |
| Lymph node CT value | 0.648 (0.518-0.778) | 0.066 | 0.036 | 69.6% | 60.0% |
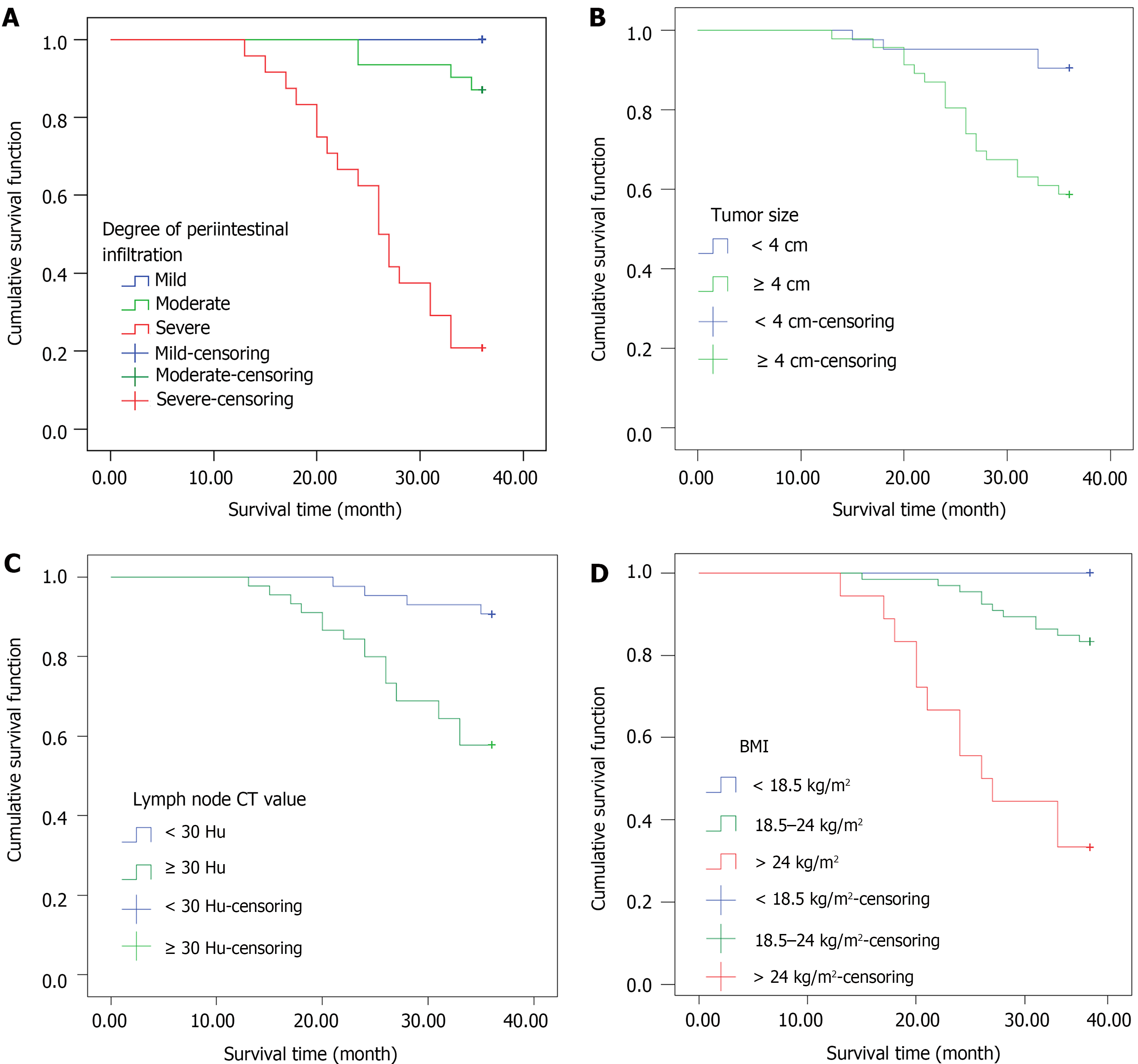
During the three-year follow-up period, it was found that patients with severe periintestinal infiltration (log-rank χ2 = 66.487, P < 0.001), tumor size ≥ 4 cm (log-rank χ2 = 11.346, P = 0.001), lymph node CT value ≥ 30 HU (log-rank χ2 = 12.500, P < 0.001), or BMI > 24 kg/m2 (log-rank χ2 = 27.672, P < 0.001) had a lower survival rate, as shown in Figure 4.
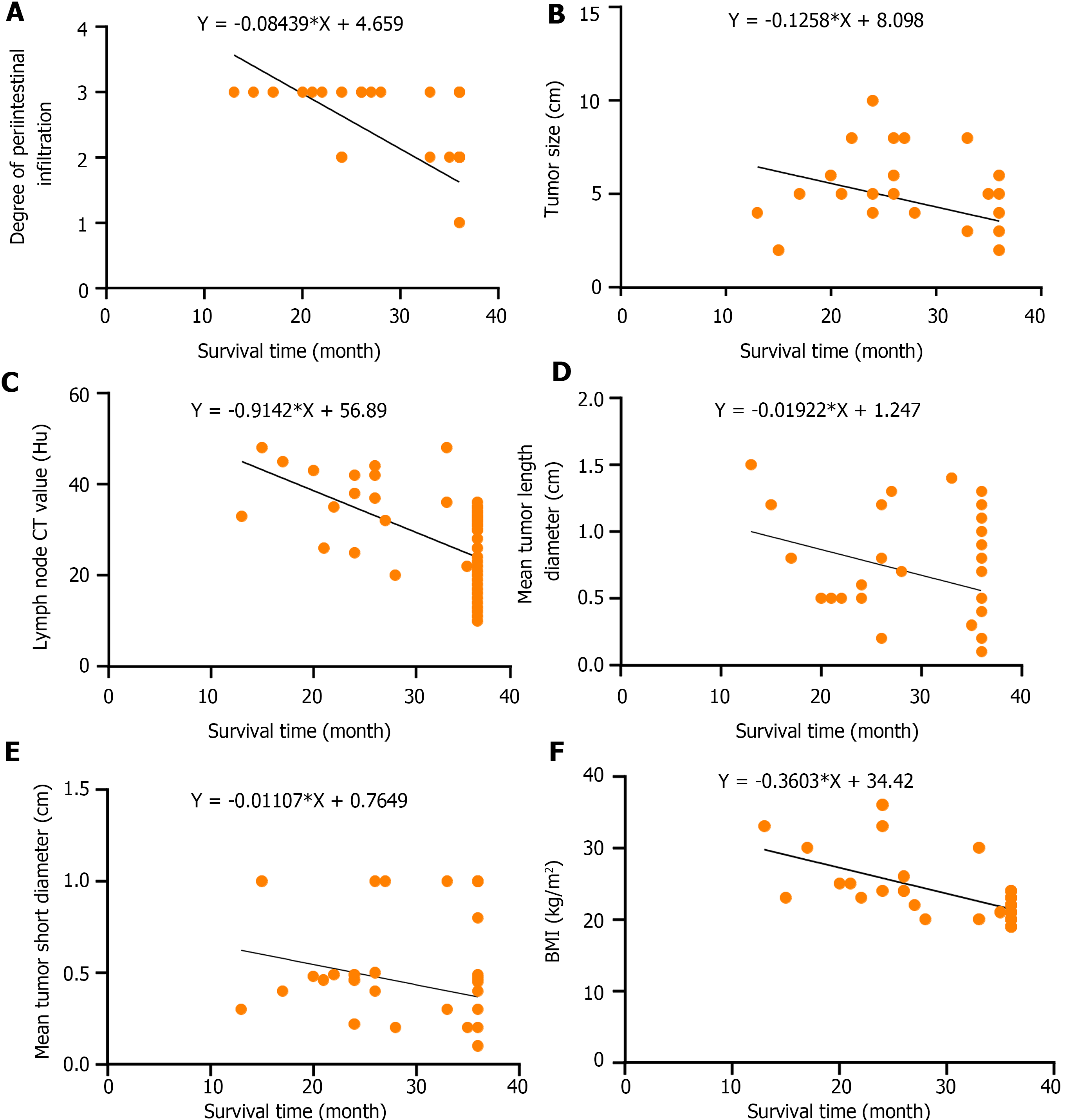
The survival time of patients with CRC was negatively correlated with BMI, degree of periintestinal infiltration, tumor size, lymph node CT value, mean tumor long-axis diameter, and mean tumor short-axis diameter (r = -0.559, 0.679, -0.430, -0.585, -0.425, and -0.385, respectively, P < 0.05 for all). BMI was positively correlated with the degree of periintestinal invasion, lymph node CT value, and mean tumor short-axis diameter (r = 0.303, 0.431, and 0.437, P < 0.05 for all), as shown in Table 5 and Figure 5.
| Independent variable | Survival time | BMI | Degree of periintestinal infiltration | Tumor size | Lymph nodes CT value | Mean tumor long-axis diameter | Mean tumor short-axis diameter | |
| Survival time | r value | - | -0.559 | -0.679 | -0.430 | -0.585 | -0.425 | -0.385 |
| P value | - | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| BMI | r value | - | - | 0.303 | 0.254 | 0.431 | 0.211 | 0.437 |
| P value | - | - | 0.004 | 0.017 | < 0.001 | 0.049 | < 0.001 | |
| Degree of periintestinal infiltration | r value | - | - | - | 0.199 | 0.246 | 0.332 | 0.264 |
| P value | - | - | - | 0.063 | 0.021 | 0.002 | 0.013 | |
| Tumor size | r value | - | - | - | - | 0.352 | 0.303 | 0.276 |
| P value | - | - | - | - | 0.001 | 0.004 | 0.009 | |
| Lymph node CT value | r value | - | - | - | - | - | 0.390 | 0.420 |
| P value | - | - | - | - | - | < 0.001 | < 0.001 | |
| Mean tumor long-axis diameter | r value | - | - | - | - | - | - | 0.587 |
| P value | - | - | - | - | - | - | < 0.001 | |
| Mean tumor short-axis diameter | r value | - | - | - | - | - | - | - |
| P value | - | - | - | - | - | - | - |
The high mortality rate of CRC in China is primarily attributed to the local recurrence or metastasis of CRC, and surgery cannot prolong patient survival. Patients with CRC of different stages exhibit different abdominal CT signs and, therefore, require different treatment plans. Clarifying the relationship between abdominal CT signs associated with CRC and patient prognoses is crucial for the early detection of CRC and the improvement of the quality of life of patients[9].
The three-year mortality rate of the 88 patients with CRC was 26.14%, which is lower than the 36% (95% confidence interval: 0.99-0.70) reported by Maajani et al[10]; the difference may be related to the different regions where the patients lived in. A higher proportion of dead patients with CRC had a BMI > 24 kg/m2, pathological stage IV, highly differentiated tumor, severe periintestinal infiltration, tumor size ≥ 4 cm, lymph node CT value ≥ 30 HU, mean tumor long-axis diameter ≥ 0.6 cm, and mean short-axis diameter ≥ 0.45 cm. Additional multivariate Cox regression analysis showed that BMI, degree of intestinal infiltration, tumor size, and lymph node CT value were independent factors influencing the likelihood of postoperative death in patients with CRC. Recent studies have found that obesity affects not only the progression of tumors but also the long-term prognosis of patients[11]. International studies have shown that, compared to CRC patients with a normal BMI, CRC patients with a higher BMI have a higher risk of death, recurrence, and a poorer prognosis[12]. Moreover, obesity is associated with hyperinsulinemia and insulin resistance. High insulin levels can increase the biological activity of insulin-like growth factors and stimulate the proliferation and metastasis of tumor cells. In addition, insulin induces angiogenesis and enhances tumor aggressiveness. Obesity can also promote cancer progression through chronic inflammatory pathways. Several studies have reported that obesity is often accompanied by immunosuppression, and relevant inflammatory factors can exacerbate the systemic inflammatory response (increasing the risk of disease) and further promote a pro-inflammatory and oxidative environment in the body, leading to tumor recurrence or lesion reformation after CRC surgery[13,14]. Doleman et al[15] conducted a systematic review and meta-analysis of observational studies to examine the relationship between BMI and CRC outcomes. The authors found that obese patients had an increased risk of all-cause death [relative risk (RR) = 1.14] and cancer-specific mortality (RR = 1.14). Compared with patients with normal weights, obese patients with CRC had a higher risk of all-cause and cancer-specific deaths.
Invasion and metastasis are important features of malignant tumors and are key factors affecting tumor prognosis. CRC can infiltrate and grow along all layers of the intestinal wall and narrow the intestinal cavity, manifesting phenomena such as tubular intestinal wall stiffness, thickening, poor intestinal expansion, mucosal surface hyperemia and edema, and superficial ulcers[16]. Huang et al[17] showed that tumor-associated macrophage (M2-TAM) infiltration driven by the SPON2 gene significantly contributes to the growth and metastasis of CRC. Therefore, the higher the degree of periintestinal infiltration, the wider the scope of CRC infiltration, the more likely early metastasis will occur, the worse the prognosis, and the higher the risk of death[18]. Furthermore, multiple studies have confirmed that polyp size is one of the most important criteria for assessing benign and malignant lesions, and more than 56% of patients with polyp sizes larger than 2.0 cm are likely to have malignant lesions[19]. Additionally, tumor overload may promote the spread and metastasis of cancer cells[20]. Tumor size is one of the indices used to ascertain the stage of the tumor and can assist in establishing a prognosis. We discovered that tumor size was an independent factor affecting CRC prognoses, which is consistent with the results of previous studies. Lymph nodes, a part of the lymphatic system, filter and remove waste, bacteria, and viruses from the body. CT value is a measure of the density of tissues or organs. A lower CT value for lymph nodes indicates that the lymph node tissues are primarily water sample dense. Moreover, it implies that more cystic components in the lesions are present, and the possibility of benign lesions is higher. In contrast, a high lymph node CT value and dense lymph nodes may be caused by cancer cell metastasis or the infiltration of a malignancy into local tissues[21]. Hence, a high lymph node CT value may predict the progression of CRC, suggesting a poor prognosis and an increased risk of death.
We analyzed in-depth the relationship between risk factors (primarily abdominal CT signs) and CRC prognosis. We found that in CRC patients with a high degree of periintestinal infiltration, tumor size ≥ 4 cm, lymph node CT value ≥ 30 HU, and BMI > 24 kg/m2, the survival rate was significantly lower. In particular, BMI was positively correlated with the degree of periintestinal infiltration, CT value of lymph nodes, and mean short diameter. This further elucidates the relationship between CT signs and surgical prognosis and physical indicators of CRC patients. A high BMI is a predictor related to increased body fat. Suzuki et al[22] showed that BMI was positively correlated with CRC risk. Sensitivity analysis showed that for every unit increase in genetically predicted BMI, the odds ratio for CRC increased. The higher the BMI, the further the development of CRC and the shorter the survival time of patients. The degree of periintestinal invasion, tumor size, CT value of lymph nodes, mean long diameter, mean short diameter, and other CT signs are risk factors leading to high pathological grade in patients with CRC[23]. Zhang et al[24] showed that tumor size is significantly related to the prognosis of CRC. The high degree of periintestinal invasion, tumor size, CT value of lymph nodes, mean long diameter, and mean short diameter predicted the high malignant degree of CRC tumor and reduced the survival time of patients. Bähr et al[25] suggest that obesity promotes the progression and metastasis of cancer, which may be related to the increased expression of MACC1 antibody, which is related to CRC metastasis caused by obesity; however, more studies are required to confirm this conclusion. Our study also showed that high BMI would aggravate CT signs in CRC patients, which were manifested as an increased degree of periintestinal invasion, CT value of lymph nodes, and mean short diameter, which is consistent with previous studies. Therefore, we suggest that in clinical practice, the postoperative prognosis of patients with CRC can be evaluated according to the degree of periintestinal infiltration, tumor size, lymph node CT value, and BMI.
This study discovered that abdominal CT examination of patients with CRC can fully reveal the internal state of the tumor. It can also reveal the relationship between the tumor and adjacent tissues and determine the degree of periintestinal infiltration, tumor size, lymph node CT value, and other signs, which are crucial for evaluating the prognosis of patients with CRC. Therefore, understanding the abdominal CT signs of patients with CRC can enable relatively accurate prognosis prediction for the appropriate surgical treatment of CRC and provide a reliable basis for determining clinical treatment plans. However, this study had certain limitations. Specifically, the sample size was relatively small, and the study was conducted in a single center. Thus, the accuracy of the relationship between abdominal CT signs and the postoperative prognosis of patients with CRC should be confirmed by considering a large number of studies with larger sample sizes.
| 1. | Tiffon C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 2. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47087] [Article Influence: 3363.4] [Reference Citation Analysis (5)] |
| 3. | Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (2)] |
| 4. | Watson DK, McWilliams-Smith MJ, Kozak C, Reeves R, Gearhart J, Nunn MF, Nash W, Fowle JR 3rd, Duesberg P, Papas TS. Conserved chromosomal positions of dual domains of the ets protooncogene in cats, mice, and humans. Proc Natl Acad Sci U S A. 1986;83:1792-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Lee JW, Baek MJ, Ahn TS, Lee SM. Fluorine-18-fluorodeoxyglucose uptake of bone marrow on PET/CT can predict prognosis in patients with colorectal cancer after curative surgical resection. Eur J Gastroenterol Hepatol. 2018;30:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Veit-Haibach P, Kuehle CA, Beyer T, Stergar H, Kuehl H, Schmidt J, Börsch G, Dahmen G, Barkhausen J, Bockisch A, Antoch G. Diagnostic accuracy of colorectal cancer staging with whole-body PET/CT colonography. JAMA. 2006;296:2590-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Shida D, Inoue M, Tanabe T, Moritani K, Tsukamoto S, Yamauchi S, Sugihara K, Kanemitsu Y. Prognostic impact of primary tumor location in Stage III colorectal cancer-right-sided colon versus left-sided colon versus rectum: a nationwide multicenter retrospective study. J Gastroenterol. 2020;55:958-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Nieto Y, Nawaz F, Jones RB, Shpall EJ, Nawaz S. Prognostic significance of overexpression and phosphorylation of epidermal growth factor receptor (EGFR) and the presence of truncated EGFRvIII in locoregionally advanced breast cancer. J Clin Oncol. 2007;25:4405-4413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Lv L, Xin B, Hao Y, Yang Z, Xu J, Wang L, Wang X, Song S, Guo X. Radiomic analysis for predicting prognosis of colorectal cancer from preoperative (18)F-FDG PET/CT. J Transl Med. 2022;20:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Maajani K, Khodadost M, Fattahi A, Shahrestanaki E, Pirouzi A, Khalili F, Fattahi H. Survival Rate of Colorectal Cancer in Iran: A Systematic Review and Meta-Analysis. Asian Pac J Cancer Prev. 2019;20:13-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Petrelli F, Cortellini A, Indini A, Tomasello G, Ghidini M, Nigro O, Salati M, Dottorini L, Iaculli A, Varricchio A, Rampulla V, Barni S, Cabiddu M, Bossi A, Ghidini A, Zaniboni A. Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021;4:e213520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 305] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 12. | Arnold M, Charvat H, Freisling H, Noh H, Adami HO, Soerjomataram I, Weiderpass E. Adult Overweight and Survival from Breast and Colorectal Cancer in Swedish Women. Cancer Epidemiol Biomarkers Prev. 2019;28:1518-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Kulkarni A, Bowers LW. The role of immune dysfunction in obesity-associated cancer risk, progression, and metastasis. Cell Mol Life Sci. 2021;78:3423-3442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Sanchez-Pino MD, Gilmore LA, Ochoa AC, Brown JC. Obesity-Associated Myeloid Immunosuppressive Cells, Key Players in Cancer Risk and Response to Immunotherapy. Obesity (Silver Spring). 2021;29:944-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Doleman B, Mills KT, Lim S, Zelhart MD, Gagliardi G. Body mass index and colorectal cancer prognosis: a systematic review and meta-analysis. Tech Coloproctol. 2016;20:517-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Wang S, Qu Y, Xia P, Chen Y, Zhu X, Zhang J, Wang G, Tian Y, Ying J, Fan Z. Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res. 2020;30:610-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 17. | Huang C, Ou R, Chen X, Zhang Y, Li J, Liang Y, Zhu X, Liu L, Li M, Lin D, Qiu J, Liu G, Zhang L, Wu Y, Tang H, Liu Y, Liang L, Ding Y, Liao W. Tumor cell-derived SPON2 promotes M2-polarized tumor-associated macrophage infiltration and cancer progression by activating PYK2 in CRC. J Exp Clin Cancer Res. 2021;40:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 18. | Yang Z, Liu Z. The efficacy of (18)F-FDG PET/CT-based diagnostic model in the diagnosis of colorectal cancer regional lymph node metastasis. Saudi J Biol Sci. 2020;27:805-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Horning AM, Wang Y, Lin CK, Louie AD, Jadhav RR, Hung CN, Wang CM, Lin CL, Kirma NB, Liss MA, Kumar AP, Sun L, Liu Z, Chao WT, Wang Q, Jin VX, Chen CL, Huang TH. Single-Cell RNA-seq Reveals a Subpopulation of Prostate Cancer Cells with Enhanced Cell-Cycle-Related Transcription and Attenuated Androgen Response. Cancer Res. 2018;78:853-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Greenlee JD, King MR. A syngeneic MC38 orthotopic mouse model of colorectal cancer metastasis. Biol Methods Protoc. 2022;7:bpac024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 21. | Cheng Y, Yu Q, Meng W, Jiang W. Clinico-Radiologic Nomogram Using Multiphase CT to Predict Lymph Node Metastasis in Colon Cancer. Mol Imaging Biol. 2022;24:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Suzuki S, Goto A, Nakatochi M, Narita A, Yamaji T, Sawada N, Katagiri R, Iwagami M, Hanyuda A, Hachiya T, Sutoh Y, Oze I, Koyanagi YN, Kasugai Y, Taniyama Y, Ito H, Ikezaki H, Nishida Y, Tamura T, Mikami H, Takezaki T, Suzuki S, Ozaki E, Kuriki K, Takashima N, Arisawa K, Takeuchi K, Tanno K, Shimizu A, Tamiya G, Hozawa A, Kinoshita K, Wakai K, Sasaki M, Yamamoto M, Matsuo K, Tsugane S, Iwasaki M. Body mass index and colorectal cancer risk: A Mendelian randomization study. Cancer Sci. 2021;112:1579-1588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Bülbül HM, Burakgazi G, Kesimal U. Preoperative assessment of grade, T stage, and lymph node involvement: machine learning-based CT texture analysis in colon cancer. Jpn J Radiol. 2024;42:300-307. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Zhang Q, Li B, Zhang S, Huang Q, Zhang M, Liu G. Prognostic impact of tumor size on patients with metastatic colorectal cancer: a large SEER-based retrospective cohort study. Updates Surg. 2023;75:1135-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 25. | Bähr I, Jaeschke L, Nimptsch K, Janke J, Herrmann P, Kobelt D, Kielstein H, Pischon T, Stein U. Obesity, colorectal cancer and MACC1 expression: A possible novel molecular association. Int J Oncol. 2022;60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |