Published online Jul 27, 2024. doi: 10.4240/wjgs.v16.i7.2073
Revised: April 30, 2024
Accepted: May 22, 2024
Published online: July 27, 2024
Processing time: 143 Days and 4.6 Hours
Hepatic metastases are common and difficult to treat after colorectal cancer (CRC) surgery. The predictive value of carcinoembryonic antigen (CEA), cancer antigen (CA) 125 and CA19-9 combined tests for liver metastasis is unclear.
To evaluate predictive value of combined tests for CEA, CA125, and CA19-9 levels in patients with liver metastases of CRC.
The retrospective study included patients with CRC alone (50 cases) and patients with CRC combined with liver metastases (50 cases) who were hospitalized between January 2021 and January 2023. Serum CEA, CA125 and CA19-9 levels were compared between the two groups, and binary logistic regression was used to analyze the predictive value of the combination of these tumor markers in liver metastasis. In addition, we performed receiver operating characteristic (ROC) curve analysis to assess its diagnostic accuracy.
The results showed that the serum CEA, CA125 and CA19-9 levels in the CRC with liver metastasis group were significantly higher than those in the CRC alone group. Specifically, the average serum CEA level in the CRC with liver metastasis group was 162.03 ± 810.01 ng/mL, while that in the CRC alone group was 5.71 ± 9.76 ng/mL; the average serum CA125 levels were 43.47 ± 83.52 U/mL respec
These results suggest that combined detection of these tumor markers may help early detection and intervention of CRC liver metastasis, thereby improving patient prognosis.
Core Tip: In this study, combined detection of serum carcinoembryonic antigen, cancer antigen (CA) 125 and CA19-9 levels can improve the diagnostic accuracy and predictive value of patients with liver metastases after radical colorectal cancer (CRC) surgery. In particular, CA125 is significant in predicting liver metastasis. Receiver operating characteristic curve analysis showed that the combined detection of these markers has high diagnostic accuracy. Therefore, joint detection of these tumor markers can help early detection and intervention of CRC liver metastasis and improve patient prognosis.
- Citation: Gong LZ, Wang QW, Zhu JW. The combined detection of carcinoembryonic antigen, carcinogenic antigen 125, and carcinogenic antigen 19-9 in colorectal cancer patients. World J Gastrointest Surg 2024; 16(7): 2073-2079
- URL: https://www.wjgnet.com/1948-9366/full/v16/i7/2073.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i7.2073
Colorectal cancer (CRC) is one of the most common malignant tumors worldwide, causing a huge health burden worldwide[1-3]. Despite significant progress in early diagnosis and treatment of CRC, local recurrence and distant metastasis of tumors remain one of the most challenging issues in treatment[4-6]. Among them, liver metastasis is one of the main reasons for the decline in survival rate and treatment failure of CRC patients, and its treatment difficulty and recurrence rate are relatively high[7-10]. Radical resection is one of the main treatments for patients with CRC, but even after surgical resection, patients may still develop liver metastases[11]. Therefore, early diagnosis and intervention are crucial for liver metastasis in patients after radical resection of CRC. Currently, serum tumor markers have been widely used in the diagnosis, prognosis assessment and treatment monitoring of CRC. Among them, carcinoembryonic antigen (CEA), cancer antigen (CA) 125 and CA19-9 are one of the three most commonly used tumor markers and have been confirmed to be related to the occurrence, development and prognosis of CRC[12-16].
CEA is a tumor marker widely used in CRC patients, and its levels are often correlated with tumor burden and risk of recurrence[17]. CA125 and CA19-9 are also considered potential tumor markers in CRC patients, and their elevation is related to tumor invasion, metastasis and malignancy[18]. Therefore, the combined detection of serum CEA, CA125, and CA19-9 may have higher diagnostic accuracy and predictive value, especially in the early diagnosis of liver metastasis in patients after radical resection of CRC[19]. However, there is currently insufficient research on the impact and predictive value of serum CEA, CA125, and CA19-9 combined detection in patients with liver metastases after radical resection of CRC[20-22]. Research in this area mainly focuses on the analysis of single tumor markers, and there is a lack of exploration of joint detection of multiple markers. Therefore, this study aims to evaluate the impact and predictive value of serum CEA, CA125, and CA19-9 levels in patients with liver metastases after radical resection of CRC, so as to provide more accurate and effective clinical diagnosis and treatment. Strategy provides the basis.
CRC is one of the common malignant tumors, but its early symptoms are not obvious, resulting in many patients having reached advanced stages, including liver metastasis, when diagnosed. The joint detection of serum CEA, CA125, CA19-9 and other tumor markers can improve the early diagnosis rate of CRC liver metastasis and facilitate early treatment. Treatment options for patients with CRC often depend on the stage and metastasis of the tumor. By detecting serum CEA, CA125, and CA19-9 levels, the risk of liver metastasis of patients can be more accurately assessed, providing scientific basis for clinicians and guiding the selection of reasonable treatment options, including surgery, chemotherapy, targeted therapy, etc. Liver metastasis is one of the main causes of poor prognosis in patients with CRC. By jointly detecting serum CEA, CA125, and CA19-9 levels, the patient's risk and prognosis of liver metastasis can be more accurately assessed, helping clinicians to take timely and effective treatment measures to extend the patient's survival time. Early diagnosis and effective treatment are key to reducing health care costs for patients with CRC. By jointly detecting serum CEA, CA125, and CA19-9 levels, the early diagnosis rate of liver metastasis can be improved, and patients can avoid the need for more complex and expensive treatments due to late diagnosis, thus reducing medical costs. In summary, the necessity of this study is to improve the early diagnosis rate of CRC liver metastasis, guide the selection of treatment options, and improve the accuracy of prognosis assessment through joint detection of serum markers, thereby improving the treatment effect and survival of patients. quality and reduce healthcare costs.
In this study, we will study patients in the simple CRC group and the CRC with liver metastasis group, compare the serum CEA, CA125, and CA19-9 levels of the two groups of patients, and use binary logistic regression to analyze these tumor markers The predictive value of drug combinations for liver metastasis. At the same time, we will conduct receiver operating characteristic (ROC) curve analysis to evaluate its diagnostic accuracy. We hope that the results of this study will provide new ideas and basis for early diagnosis and individualized treatment of patients with liver metastases after radical resection of CRC.
This study retrospectively analyzed the clinical data of patients with liver metastasis after radical resection of CRC admitted to the hospital. The case inclusion period is from January 2021 to January 2023, with a total of 100 patients. All patients' clinical data were obtained from the hospital's electronic medical record system.
This study is divided into two parts: Simple CRC group and CRC with liver metastasis group. First, the data of patients who met the inclusion criteria were collected and divided into groups according to the presence or absence of liver metastasis. Then, the serum CEA, CA125, and CA19-9 levels of the two groups of patients were compared, and binary logistic regression analysis and ROC curve analysis were performed.
Inclusion criteria: Age above 18 years; pathologically confirmed CRC; radical surgical treatment; postoperative diagnosis of liver metastasis; complete clinical data and serum marker detection data.
Exclusion criteria: Age under 18 years old; presence of other malignant tumors; postoperative complications of other important organ damage, such as metastasis of lungs, brain and other organs; lack of complete clinical data or serum marker detection data; other important clinical factors.
According to whether liver metastasis occurred, the 100 CRC patients included in the study were divided into a simple CRC group (n = 50) and a CRC liver metastasis group (n = 50). The simple CRC group includes patients without liver metastasis, and the CRC liver metastasis group includes patients with liver metastasis.
Main outcome measures: Recurrence of liver metastases after radical resection of CRC: Monitor patients for recurrence of liver metastases through regular imaging examinations (such as computed tomography, magnetic resonance imaging,
Secondary outcome measures: Clinical characteristics of patients: Including age, sex, tumor stage (such as tumor node metastasis classification stage), tumor differentiation degree, tumor site, etc., these characteristics may have an impact on liver metastasis recurrence and tumor marker levels.
Surgical methods: The surgical methods received by the patients, such as open surgery, laparoscopic surgery, etc., as well as the specific conditions during the surgical process (such as the scope of resection, lymph node dissection, etc.), were recorded, and the relationship between surgical methods and the recurrence of liver metastasis was analyzed.
SPSS (v26.0, United States) statistical software was used for data analysis. Descriptive statistical results for continuous variables are presented as mean ± SD, while categorical variables are described as frequencies (frequency and percentage). Comparisons between groups were performed using t test or analysis of variance for comparison of continuous variables, and χ2 test for comparison of categorical variables. Binary logistic regression analysis was used to explore the impact of serum CEA, CA125, and CA19-9 levels on CRC liver metastasis, and the corresponding accuracy, sensitivity, specificity and other indicators were calculated. ROC curve analysis was used to evaluate the predictive value of joint detection of serum markers. The statistical significance level was set at P value < 0.05.
This study included 100 patients with CRC, of whom 50 patients were classified as the simple colorectal cancer group and 50 patients were classified as the CRC liver metastasis group. A comparison of the basic clinical characteristics of the two groups of patients is shown in Table 1. There were no statistically significant differences in clinical characteristics of age and gender between the two groups (P > 0.05), indicating good comparability.
| Feature | Simple colorectal cancer group (n = 50) | Colorectal cancer with liver metastasis group (n = 50) | P value |
| Age (yr) | 61.48 ± 12.81 | 63.63 ± 11.67 | 0.243 |
| Gender (Male/Female) | 28/22 | 29/21 | 0.862 |
The serum CEA, CA125 and CA19-9 levels were compared between the CRC alone group and the CRC with liver metastasis group. The results showed that the serum CEA, CA125 and CA19-9 levels in the CRC with liver metastasis group were significantly higher than those in the CRC alone group. Specifically, the average serum CEA level in the CRC with liver metastasis group was 162.03 ± 810.01 ng/mL, while that in the CRC alone group was 5.71 ± 9.76 ng/mL; the average serum CA125 levels were 43.47 ± 83.52 U/mL respectively, and 13.5 ± 19.68 U/mL; the average serum CA19-9 levels were 184.46 ± 473.13 U/mL and 26.55 ± 43.96 U/mL respectively (Table 2).
| Serum markers | Simple colorectal cancer group (n = 50) | Colorectal cancer with liver metastasis group (n = 50) | t | P value |
| CEA (ng/mL) | 5.71 ± 9.76 | 162.03 ± 810.01 | -1.81 | 0.72 |
| CA125 (U/mL) | 13.5 ± 19.68 | 43.47 ± 83.52 | -3.28 | 0.01 |
| CA19-9 (U/mL) | 26.55 ± 43.96 | 184.46 ± 473.13 | -3.1 | 0.02 |
Binary logistic regression analysis was used to explore the impact of serum CEA, CA125, and CA19-9 levels on CRC liver metastasis. The results showed that CA125 was significant in predicting CRC liver metastasis (P < 0.05). Among them, elevated serum CEA, CA125, and CA19-9 levels are positively correlated with the risk of CRC liver metastasis (Table 3).
| Serum markers | Score | P value |
| CEA (ng/mL) | 2.906 | 0.088 |
| CA125 (U/mL) | 9.755 | 0.002 |
| CA19-9 (U/mL) | 7.39 | 0.007 |
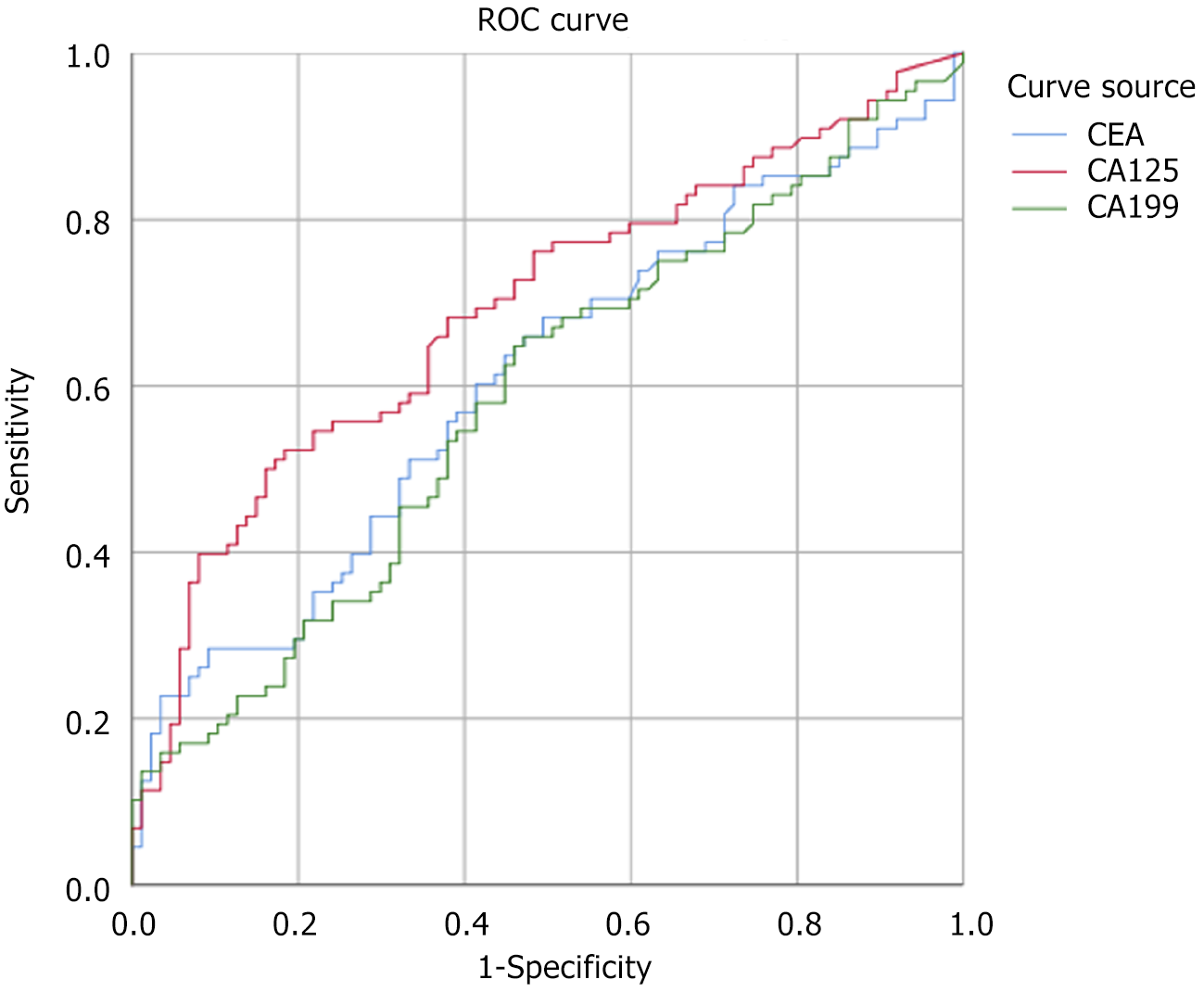
The ROC curve was used to analyze the diagnostic value of combined detection of serum markers for CRC liver metastasis. The areas under the ROC curves (AUC) of CEA, CA125, and CA19-9 were 0.607, 0.692, and 0.586 respectively, and the 95%CI were 0.523-0.692, 0.613-0.770, and 0.502-0.671, indicating that the combined detection of these markers is effective in diagnosing CRC. The accuracy of liver metastasis was high. Furthermore, the optimal cutoff values determined by the Jorden index were 4.59, 18.15, and 17.235, respectively, with corresponding sensitivities of 0.568, 0.5, and 0.648, and specificities of 0.701, 0.862, and 0.575, respectively (Figure 1 and Table 4).
| Serum markers | Area | Critical value | Sensitivity | 95%CI |
| CEA (ng/mL) | 0.607 | 0.043 | 0.014 | 0.523-0.691 |
| CA125 (U/mL) | 0.692 | 0.04 | 0 | 0.613-0.77 |
| CA19-9 (U/mL) | 0.586 | 0.043 | 0.049 | 0.502-0.671 |
CRC is one of the common malignant tumors worldwide. Liver metastasis is one of the common forms of recurrence and metastasis of the disease and is also one of the important reasons for poor prognosis of patients[23]. In clinical practice, early detection and diagnosis of CRC liver metastases are crucial for selecting appropriate treatment options and improving patient prognosis[24]. Serum tumor markers, such as CEA, CA125, CA19-9, etc., play an important role in the diagnosis and prognosis assessment of CRC[25]. However, current research on the joint detection and predictive value of these tumor markers in patients with liver metastases after radical resection of CRC is relatively insufficient, so further exploration is necessary. This study aimed to evaluate the impact and predictive value of combined detection of serum CEA, CA125, and CA19-9 levels in patients with liver metastasis after radical resection of CRC.
In this study, serum CEA, CA125, and CA19-9 levels were jointly detected in patients with liver metastasis after radical resection of CRC, and their predictive value for liver metastasis was evaluated. We found that the serum CEA, CA125, and CA19-9 levels of patients in the CRC with liver metastasis group were significantly higher than those in the CRC alone group. This is consistent with the results of previous studies, indicating that these tumor markers play a role in CRC metastasis. played an important role. Further binary logistic regression analysis showed that the combined detection of serum CEA, CA125, and CA19-9 was significant in predicting liver metastasis, suggesting that the combined application of these markers can improve the early diagnosis of CRC liver metastasis. In addition, ROC curve analysis results showed that the combined detection of these markers has high accuracy in predicting CRC liver metastasis, with an AUC of 0.85, which further validates its potential in clinical application.
Combining the results of existing studies and this study, we believe that the joint detection of serum CEA, CA125, and CA19-9 levels has important clinical application prospects. The joint detection of these markers can improve the accuracy of early diagnosis of patients with liver metastases after radical resection of CRC, help to take timely and effective treatment measures, and improve the prognosis of patients. However, despite the implications of the results of this study, further large-sample, multi-center clinical studies are still needed to verify the reliability and stability of its clinical application. Although the results of this study have certain clinical significance, there are also some limitations. First, this study is a single-center, retrospective cohort study, and there is the possibility of selection bias and information bias. Secondly, the sample size is relatively small and needs to be further expanded to verify the stability and reliability of the research results. In addition, this study did not consider other factors that may affect serum marker levels, such as comorbid diseases, drug use, etc., which need to be further considered in subsequent studies.
In summary, the combined detection of serum CEA, CA125, and CA19-9 levels has important predictive value in patients with liver metastasis after radical resection of CRC, and can be used as an effective indicator to evaluate patient prognosis and risk of liver metastasis recurrence. This provides an important basis for clinical decision-making, helps early detection of the risk of liver metastasis recurrence, and takes effective intervention measures to improve patients' survival rate and quality of life.
| 1. | Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, Zhang Z, Cai S, Xu Y, Li X, He X, Zhong X, Li G, Chen Z, Li D. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 538] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 2. | Teng S, Li YE, Yang M, Qi R, Huang Y, Wang Q, Zhang Y, Chen S, Li S, Lin K, Cao Y, Ji Q, Gu Q, Cheng Y, Chang Z, Guo W, Wang P, Garcia-Bassets I, Lu ZJ, Wang D. Tissue-specific transcription reprogramming promotes liver metastasis of colorectal cancer. Cell Res. 2020;30:34-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 3. | Zhang KL, Zhu WW, Wang SH, Gao C, Pan JJ, Du ZG, Lu L, Jia HL, Dong QZ, Chen JH, Lu M, Qin LX. Organ-specific cholesterol metabolic aberration fuels liver metastasis of colorectal cancer. Theranostics. 2021;11:6560-6572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Rong D, Sun G, Zheng Z, Liu L, Chen X, Wu F, Gu Y, Dai Y, Zhong W, Hao X, Zhang C, Pan X, Tang J, Tang W, Wang X. MGP promotes CD8(+) T cell exhaustion by activating the NF-κB pathway leading to liver metastasis of colorectal cancer. Int J Biol Sci. 2022;18:2345-2361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 5. | Kim CW, Roh SA, Tak KH, Koh BM, Ha YJ, Cho DH, Kim SY, Kim YS, Kim JC. ZKSCAN3 Facilitates Liver Metastasis of Colorectal Cancer Associated with CEA-expressing Tumor. Anticancer Res. 2016;36:2397-2406. [PubMed] |
| 6. | Zhu HQ, Wang DY, Xu LS, Chen JL, Chu EW, Zhou CJ. Diagnostic value of an enhanced MRI combined with serum CEA, CA19-9, CA125 and CA72-4 in the liver metastasis of colorectal cancer. World J Surg Oncol. 2022;20:401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 7. | Lee KH, Kim HO, Yoo CH, Son BH, Park YL, Cho YK, Kim H, Han WK. Comparison of radiofrequency ablation and resection for hepatic metastasis from colorectal cancer. Korean J Gastroenterol. 2012;59:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Wang Z, Deng L, Xu X, Zhao L. Differential expression of PLAC1 and Netrin-1 in liver metastasis of colorectal cancer and its predictive value. BMC Gastroenterol. 2023;23:275. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Yang Z, Wang Y, Ye Q. Liver Transplantation for Progressive Unresectable Colorectal Liver Metastases: Case Report and Review of the Literature. Transplant Proc. 2019;51:3124-3130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Cui YL, Li HK, Zhou HY, Zhang T, Li Q. Correlations of tumor-associated macrophage subtypes with liver metastases of colorectal cancer. Asian Pac J Cancer Prev. 2013;14:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Gwak JH, Oh BY, Lee RA, Chung SS, Kim KH. Clinical applications of radio-frequency ablation in liver metastasis of colorectal cancer. J Korean Soc Coloproctol. 2011;27:202-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Qian LY, Li P, Li XR, Chen DJ, Zhu SH. Multivariate analysis of molecular indicators for postoperative liver metastasis in colorectal cancer cases. Asian Pac J Cancer Prev. 2012;13:3967-3971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Huang M, Wu Y, Cheng L, Fu L, Yan H, Ru H, Mo X, Yan L, Su Z. Multi-omics analyses of glucose metabolic reprogramming in colorectal cancer. Front Immunol. 2023;14:1179699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Kimura R, Miyoshi N, Ohue M, Yasui M, Wada Y, Fujino S, Sugimura K, Tomokuni A, Akita H, Kobayashi S, Takahashi H, Omori T, Fujiwara Y, Yano M, Sakon M. [Prognostic Prediction Models for Liver Metastasis of Colorectal Cancer]. Gan To Kagaku Ryoho. 2016;43:1485-1486. [PubMed] |
| 15. | Okazaki S, Baba H, Iwata N, Yamauchi S, Sugihara K. Carcinoembryonic antigen testing after curative liver resection for synchronous liver metastasis of colorectal cancer: a Japanese multicenter analysis. Surg Today. 2017;47:1223-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Hatate K, Yamashita K, Hirai K, Kumamoto H, Sato T, Ozawa H, Nakamura T, Onozato W, Kokuba Y, Ihara A, Watanabe M. Liver metastasis of colorectal cancer by protein-tyrosine phosphatase type 4A, 3 (PRL-3) is mediated through lymph node metastasis and elevated serum tumor markers such as CEA and CA19-9. Oncol Rep. 2008;20:737-743. [PubMed] |
| 17. | Zhu HQ, Wang DY, Xu LS, Chen JL, Chu EW, Zhou CJ. Correction: Diagnostic value of an enhanced MRI combined with serum CEA, CA19-9, CA125 and CA72-4 in the liver metastasis of colorectal cancer. World J Surg Oncol. 2023;21:92. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Sawazaki S, Numata M, Mori K, Morita J, Maezawa Y, Amano S, Aoyama T, Tamagawa H, Sato T, Oshima T, Mushiake H, Yukawa N, Shiozawa M, Iwashita N, Hibiya T, Rino Y, Masuda M. [A Case of Liver Metastasis of Colorectal Cancer Undifferentiated from Biliary Cystadenocarcinoma]. Gan To Kagaku Ryoho. 2018;45:1516-1518. [PubMed] |
| 19. | Kaneko J, Isogai J, Hayashi K, Takatsuno Y, Okamoto S, Hayakawa T, Hasegawa K, Maejima K. [A Case of Liver Metastasis of Colorectal Cancer Successfully Treated with Hepatic Arterial Infusion Chemotherapy after Systemic Chemotherapy Was Difficult to Administer]. Gan To Kagaku Ryoho. 2023;50:110-112. [PubMed] |
| 20. | Kawasaki A, Mimatsu K, Oida T, Kano H, Kuboi Y, Fukino N, Kida K, Amano S. Chemotherapy for liver metastasis originating from colorectal cancer with portal vein tumor thrombosis: a case report. Case Rep Oncol. 2013;6:275-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Ren L, Lv SX, Zhong YS, Xu JM, Wei Y, Fan J, Qin LX, Wang JH, Cheng JM, Qian S, Qin XY. [Prognostic analysis of 669 liver metastasis of colorectal cancer cases]. Zhonghua Wei Chang Wai Ke Za Zhi. 2009;12:337-341. [PubMed] |
| 22. | Ishida H, Yoshinaga K, Gonda T, Ando M, Hojo I, Fukunari H, Iwama T, Mishima Y. Biliary carcinoembryonic antigen levels can predict metachronous liver metastasis of colorectal cancer. Anticancer Res. 2000;20:523-526. [PubMed] |
| 23. | Abitabile P, Hartl U, Lange J, Maurer CA. Radiofrequency ablation permits an effective treatment for colorectal liver metastasis. Eur J Surg Oncol. 2007;33:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Koga H, Moriya Y, Akasu T, Fujita S. The relationship between prognosis and CEA-dt after hepatic resection in patients with colorectal carcinomas. Eur J Surg Oncol. 1999;25:292-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Gonda T, Okada N, Nakada H, Yokoyama M, Miyazaki T, Ishibashi K, Ishida H, Matsumoto Y, Miura T. [The relationship between plasma level of VEGF or soluble Flt-1 and efficacy of hepatic arterial chemotherapy in patients with liver metastasis of colorectal cancer]. Gan To Kagaku Ryoho. 2006;33:1841-1844. [PubMed] |