Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1918
Revised: March 20, 2024
Accepted: April 17, 2024
Published online: June 27, 2024
Processing time: 153 Days and 0.1 Hours
Myopericytoma is a benign tumor that typically occurs within subcutaneous tissue and most often involves the distal extremities, followed by the proximal extremities, neck, thoracic vertebrae and oral cavity. Complete resection is often curative. Malignant myopericytoma is extremely rare and has a poor prognosis. Here, we report for the first time a case of malignant myopericytoma originating from the colon.
A 69-year-old male was admitted to our hospital with right upper quadrant pain for five days. Imaging suggested a liver mass with hemorrhage. A malignant hepatic tumor was the initial diagnosis. Surgical resection was performed after a complete preoperative work up. Initial postoperative pathology suggested that the mass was a malignant myoblastoma unrelated to the liver. Four months after the first surgery, an enhanced computed tomography (CT) scan revealed a recurrence of the tumor. The diagnosis of malignant myopericytoma derived from the colon was confirmed on histopathological examination of the specimen from the second surgery. The patient did not return to the hospital regularly for surveillance. The first postoperative abdominal CT examination six months after the second surgery demonstrated multiple liver metastases. Survival time between the diagnosis of the tumor to death was approximately one year.
Malignant myopericytoma is a rare cancer. Preoperative diagnosis may be difficult. Due to a lack of treatment options, prognosis is poor.
Core Tip: The incidence of malignant myopericytoma is very low and prognosis is poor. Presently, there is no ideal intervention. In this case, the patient was initially admitted to hospital with pain secondary to a hemorrhage into a liver tumor. After two surgeries, the patient was definitively diagnosed with a malignant myopericytoma originating in the colon. Due to the lack of comprehensive antitumor therapy and poor patient compliance, the tumor progressed rapidly. The patient’s survival was only one year. In view of the lack of diagnostic and treatment guidelines, future clinical and basic science research on myopericytomas is warranted and can hopefully improve prognosis.
- Citation: Zhang HL, Zhang M, Guo JQ, Wu FN, Zhu JD, Tu CY, Lv XL, Zhang K. Malignant myopericytoma originating from the colon: A case report. World J Gastrointest Surg 2024; 16(6): 1918-1925
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1918.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1918
Myopericytoma was formally defined by Granter et al[1] in 1998. It is a novel perivascular tumor which shares several histopathologic similarities with angioleiomyoma, myofibromatosis, glomus tumors and infantile haemangiopericytoma, all of which comprise a morphological spectrum of tumours that show differentiation towards perivascular myoid[2,3]. Despite the histological and morphological overlap with these tumors, myopericytomas have distinct characteristics. Microscopically, they are composed of myoid-appearing oval or spindle-shaped cells with a concentric perivascular arrangement, often with diffuse expression of smooth muscle actin (SMA)[4,5].
In the majority of cases, myopericytoma are benign tumors generally arising in the subcutaneous and superficial soft tissues of the extremities. Recurrence is very rare after complete surgical resection[6,7]. Malignant myopericytoma is rare with only a few cases reported in the literature. The rarity and poor prognosis of the disease warranted us to identify more cases in the literature and conduct in-depth research. Herein, we report for the first time a case of malignant myopericytoma originating in the colon, whose imaging results suggested a hepatic neoplasm leading to difficulty in diagnosis and treatment.
A 69-year-old male presented to the emergency department with right upper quadrant pain.
He described the pain as paroxysmal, dull, located in his right upper abdomen and present for five days. He went to the emergency department due to a continued worsening of the pain. He denied nausea, vomiting, melena, change to his bowel habit and weight loss.
The patient reported no past history of specific illness, including jaundice, hepatitis, gallbladder pathology or renal, cardiac, or pulmonary disease.
The patient was not taking any medication or herbal supplements and had no allergies. Also, he had no significant family history of carcinoma or hepatobiliary pathology. He had not undergone a colonoscopy in the past or during his treatment.
The patient’s vital signs were within normal limits. No obvious abnormality was observed on pulmonary or cardiac examination. His abdomen was non-distended and soft. Mild tenderness was found on deep palpation of the right upper quadrant without rebound tenderness.
Routine blood laboratory tests showed no obvious abnormalities in the patient’s liver function, kidney function, coagulation, electrolytes, blood sugar, blood lipids, hepatitis B virus DNA quantification and tumor marker levels.
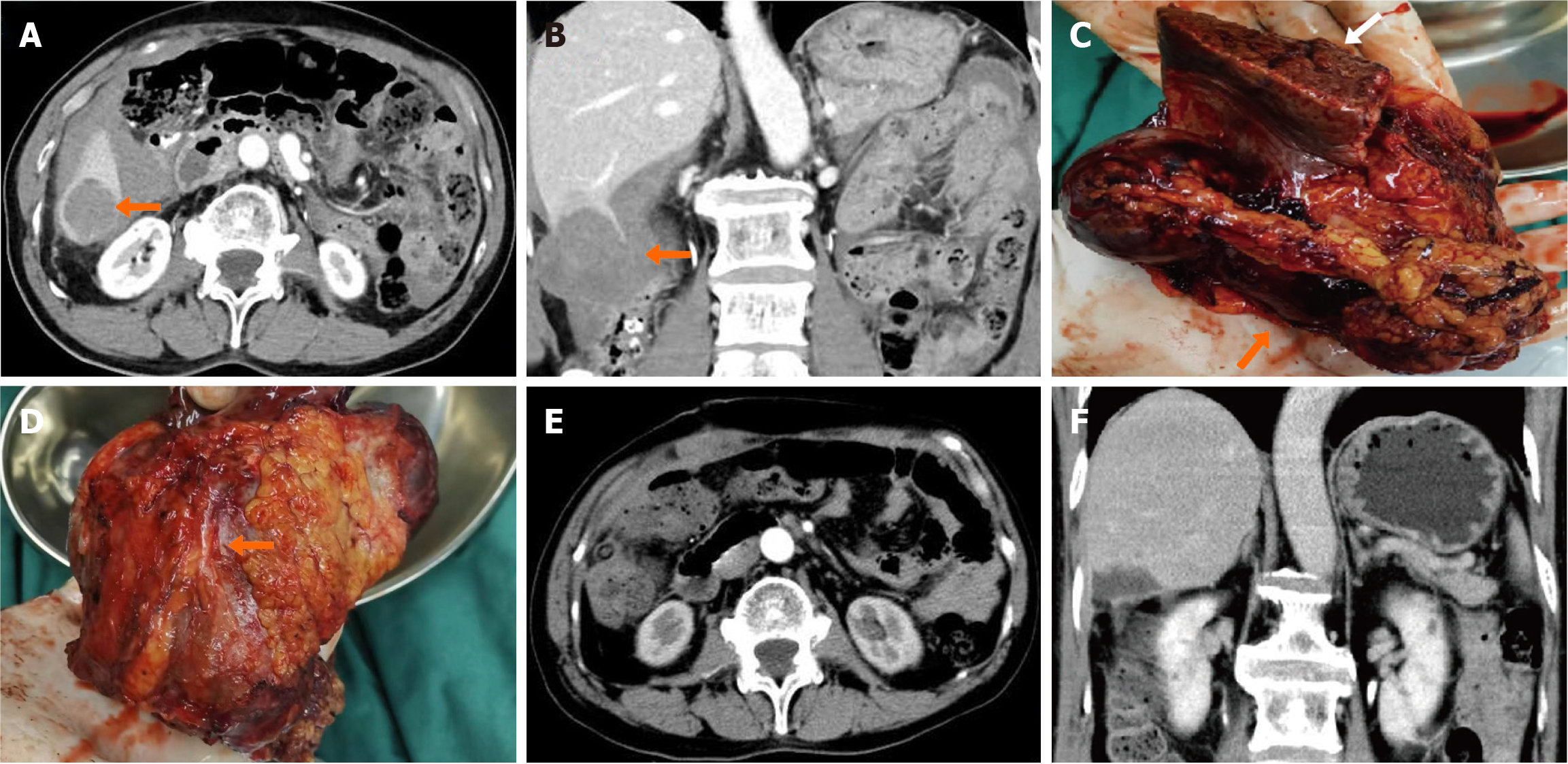
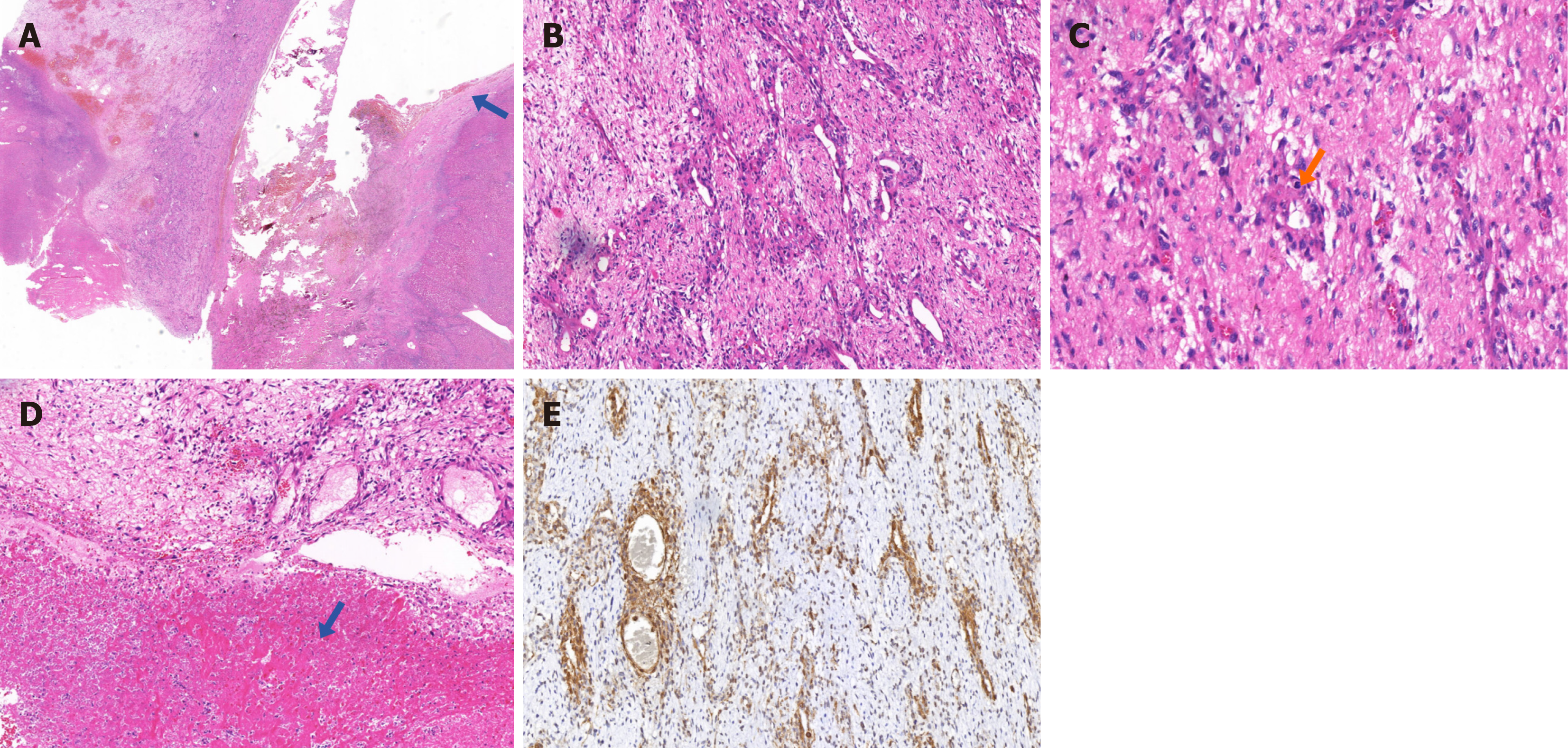
Abdominal computed tomography (CT) scanning with contrast demonstrated an exogenous mass in the lower segment of the right lobe of the liver measuring 4.5 cm × 5.4 cm and moderate ascites. Based on the CT, a rupture and hemorrhage of a hepatoma was the primary diagnosis (Figure 1A and B). The tumor invaded the lower segment of right posterior lobe of liver and the ascending colon. Surgical resection was performed after a complete preoperative work up. Part of the liver and the serosa and mesenteric tissue of the colon were excised during the first surgery (Figure 1C and D). Postoperative CT examination showed no residual mass (Figure 1E and F). Histopathological examination of the resection specimen revealed that the lesion was a malignant myopericytoma without invasion of the surrounding liver tissue. The primary lesion was considered to be in the colon (Figure 2).
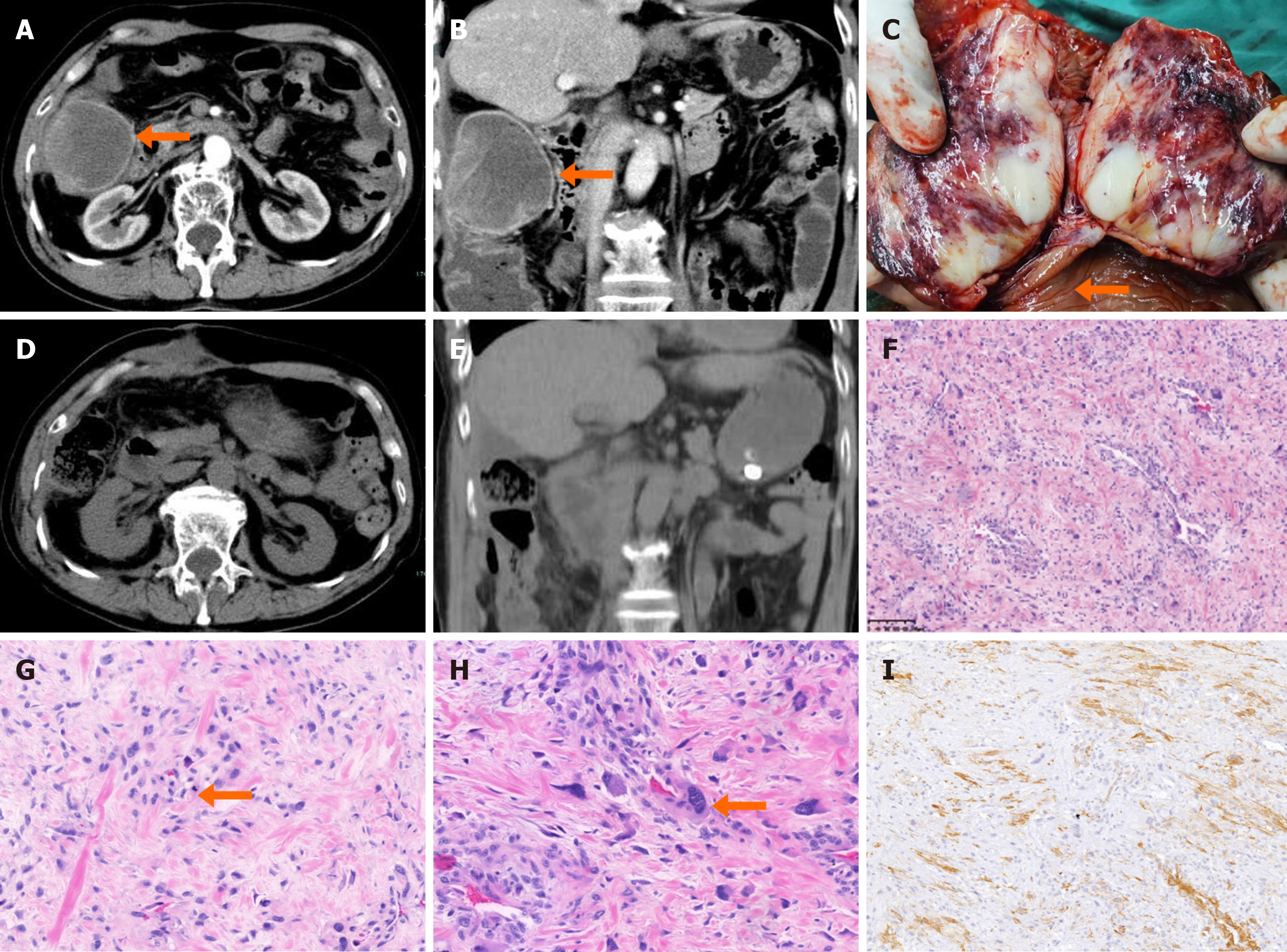
Four months after the first surgery, a surveillance abdominal CT scan revealed the recurrence of the colonic tumor (Figure 3A and B). A right hemicolectomy with curative intent was performed for the tumor recurrence. During the second surgery, the tumor was found within the mucosal layer of the transverse colon and protrude into the lumen. On examination of the resection specimen, pale tissue was seen on its anatomical surface (Figure 3C). Postoperative imaging indicated complete resection of the tumor (Figure 3D and E). The pathological section obtained in the second operation was correlative with a malignant myopericytoma again. The tumor was eventually diagnosed as a malignant myopericytoma originating in the colon and growing around the liver (Figure 3F-I).
The patient underwent two open surgeries. During the first surgery, we set the route of excision in liver tissue to about 2 cm from the tumor boundary and the right liver tumor was completely resected along with a 2 cm margin of macroscopically unaffected tissue. The adhesions between the tumor and the colon were carefully separated and part of the serous layer and mesenteric tissue of the colon were concomitantly resected the patient recovered well after the first operation, with no postoperative complications such as bleeding, biliary leakage or hepatic failure. A right hemicolectomy with curative intent was performed for the tumor recurrence. The resection included distal ileum 15 cm away from the ileocecal junction to the proximal third of the transverse colon. The bowel containing the intact mass, and part of mesentery and greater omentum were removed. Finally, an end-to-end ileocoleostomy was performed. One week after the second operation, the patient experienced anastomotic leakage. This was treated with an abdominal drain and antibiotics. The patient recovered smoothly and was discharged from hospital. Due to the lack of effective treatment methods other than surgical resection and the patient’s poor treatment compliance, adjuvant therapy such as radiotherapy and/or chemotherapy were not received after surgery.
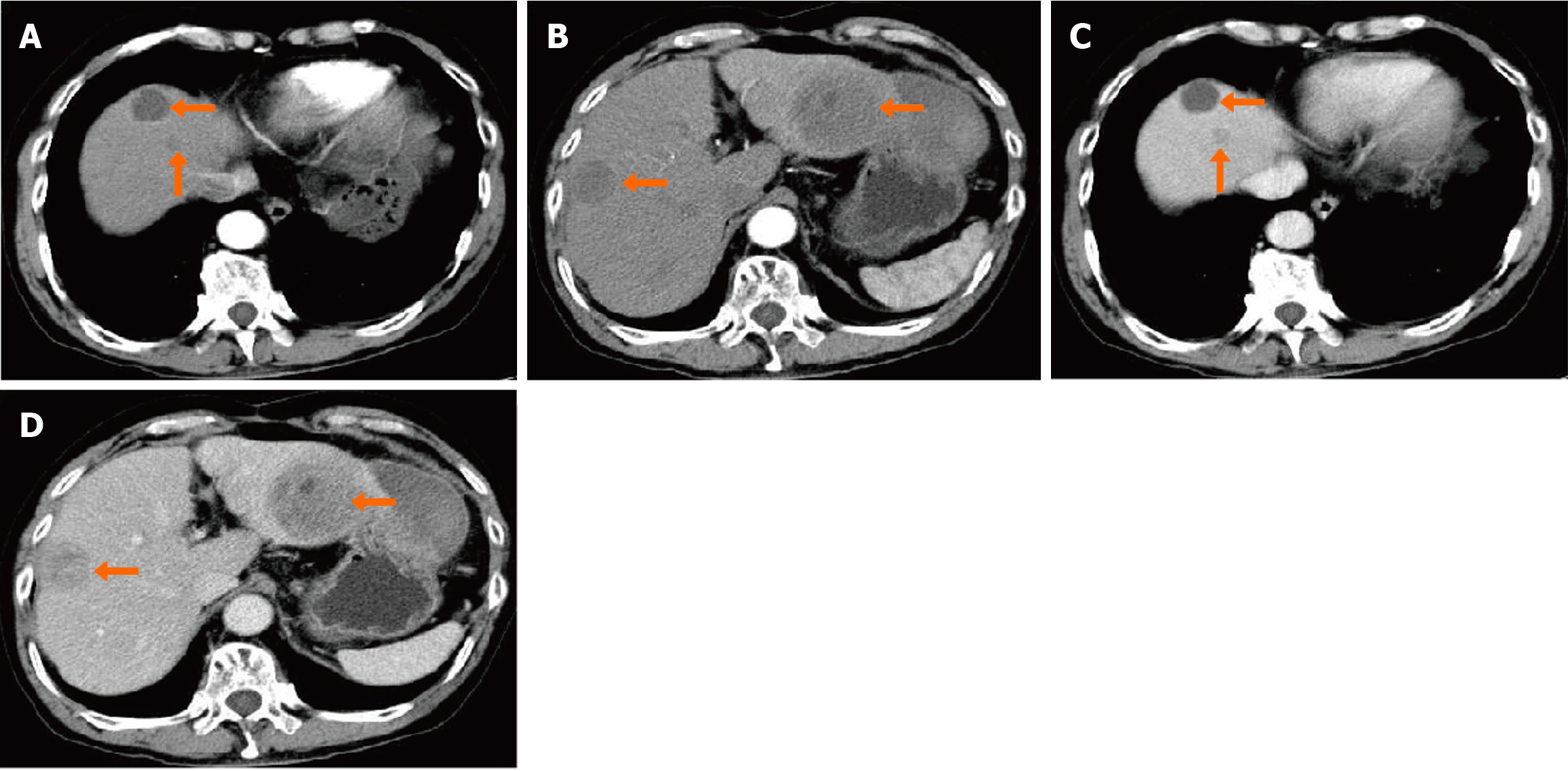
The patient did not return for early, routine surveillance as requested; however, six months after the second operation, he presented for surveillance. An abdominal CT showed multiple masses in the liver (Figure 4). Due to his past medical history, hepatic metastasis from colonic carcinoma were considered. The patient and his family declined surgery and opted for palliation. The overall survival time from initial tumor diagnosis to death was approximately one year.
Requena et al[8] suggested the term myopericytoma as an alternative name for some cutaneous adult myofibromas, basing their argument on the myopericytic differentiation seen in these tumors. Later, Granter et al[1] described a group of benign tumors showing a distinct histologic pattern characterized by a concentric, perivascular cellular proliferation with myoid differentiation.
Myopericytoma often present as incidental, painless, superficial masses, which have a predilection for subcutaneous sites in the extremities. Histologically, they have clear boundaries and are composed of round or oval cells arranged in the shape of concentric circles and surrounded by blood vessels. Due to the characteristics secondary to the myoid differentiation of tumor cells, they demonstrate a powerful immune response to SMA[9-11]. In recent years, increasing numbers of myopericytoma have been reported. Most cases have a low recurrence rate and a favorable prognosis. Unfortunately, myopericytoma carries a risk of malignant transformation. Malignant myopericytoma, like other malignancies, exhibits increased mitotic activity, nuclear atypia, pleomorphism, necrosis, infiltration, and metastasis[12,13].
Cases diagnosed as malignant myopericytoma are very rare. The keywords “malignant myopericytoma” were used to search relevant studies in PubMed, Web of Science, GeenMedical, and Google scholar literature databases. Additional literature was identified through hand searches of the references of retrieved literature. After review, we identified fourteen cases of malignant myopericytoma, the characteristics of which are summarized in Table 1[13-19]. By extracting the clinical manifestations, diagnoses and treatment of each case, we noticed that the majority of cases found the lump at first unintentionally or the patient felt mild pain and had symptoms due to the mass pressing on the surrounding organs or nerve tissue. Pathology is the main criterion for the diagnosis of malignant myopericytoma, but the lack of unified diagnostic criteria in imaging and laboratory tests poses a great challenge to the early diagnosis of the disease. In these cases, enhanced magnetic resonance imaging (MRI) and CT are the most commonly used imaging methods. Contrast-enhanced T1-weighted MRI showed that the mass was irregular or lobulated with clear edges, and some cases were accompanied by annular enhancement of the edge of the mass or partial necrosis. The imaging findings of CT are similar to MRI, and the combination of the two may be helpful to diagnosis. Ultrasonography is more commonly used for masses that occur in superficial tissue and present as well-demarcated heterogeneous solid mass. Surgery is currently the only effective intervention. Of the fourteen patients who underwent surgery, seven had varying degrees of recurrence and metastases, and four patients died within one year, indicating a high degree of malignancy and poor prognosis of the tumor. Similar to benign tumors, malignant myopericytomas originating in the extremities are the most common, while the liver appears to be a common site for metastases. Amongst the seven patients with metastasis, four had lesions in the liver. This is likely due to hematogenous spread via the abundant blood supply to the liver. All histopathology showed concentric growth of tumor cells around blood vessels, intense expression of SMA, accompanied by increased mitotic activity, nuclear atypia, necrosis and other malignant features.
| Case | Sex/age | Site | Presenting symptoms | Treatment | Follow up | Ref. |
| 1 | F/81 | Left side of neck | Rapidly growing painless mass | Excision | Liver metastasis 9 months after diagnosis. Alive with disease at 24 months | McMenamin and Fletcher[14], 2002 |
| 2 | M/46 | Left posterior thigh | Painful deep-seated intermuscular mass | Excision, postoperative adjuvant radiotherapy | Metastasis to the heart, brain, liver, and bone after 6 months. Death from the disease at 7 months | McMenamin and Fletcher[14], 2002 |
| 3 | M/19 | Dermis/subcutaneous tissue of the right heel | Painful mass | Below-knee amputation | Metastatic disease and death within 1 yr | McMenamin and Fletcher[14], 2002 |
| 4 | F/80 | Superficial mass of the left upper arm | Superficial painless mass | Marginal excision followed by wide excision | No metastasis. Discharged after 8 months of follow-up | McMenamin and Fletcher[14], 2002 |
| 5 | F/67 | Mass in the superior mediastinum | Short history of superior vena cava obstruction | Excision of cutaneous metastatic deposit | Metastasis disease to the skin and subcutaneous tissue. Died of respiratory failure within 1 month | McMenamin and Fletcher[14], 2002 |
| 6 | M/61 | Lower leg | Subcutaneous painless mass | Marginal excision, recurrence after 12 months treated by wide excision | Disease free a 3 yr | Mentzel et al[9], 2006 |
| 7 | M/30 | Lower leg | Obstructive periampullary mass with jaundice | Biopsy and pancreaticoduodenectomy | Liver metastasis 8 months after diagnosis | Ramdial et al[15], 2011 |
| 8 | M/52 | Floor of the left atrial wall | Sudden decrease in left vision | Excision of cardiac mass. Excision of metastatic brain tumor followed by γ-knife radiotherapy. Laminectomy and vertebral fixation due to vertebral metastases | Alive with metastatic disease at 8 months | Mainville et al[12], 2015 |
| 9 | M/38 | Intradural tumor of the lower spine (L5/S1) | Progressing painful in the right calf during sport | Excision | Disease free after 18 months | Holling et al[16], 2015 |
| 10 | M/65 | Dermis/subcutaneous tissue of the right arm | Enlarging painless mass | Excision | Disease free after 5 months | Patrick et al[13], 2016 |
| 11 | F/15 | Left shoulder region | Slowly growing mass | Excision, postoperative chemotherapy and radiotherapy | Disease free after 18 months | Binesh et al[17], 2016 |
| 12 | M/56 | Subcutaneous tissue left armpit | Pain of left shoulder mass | Preoperative chemotherapy, excision | Disease free after several years | Chen et al[18], 2017 |
| 13 | M/33 | Intracranial | New onset seizures | Excision | Disease free after 12 months | Conradie et al[19], 2021 |
| 14 | M/65 | Colon | Right upper quadrant pain | Excision | Metastatic disease to the liver and death within 1 yr | This case |
Here, we report a case of malignant myopericytoma that was indistinguishable on imaging from a liver tumor, which we finally determined actually occurred in the colon. There was a lack of concordance between diagnostic imaging (from both axial and coronal planes) and histopathologic results. Imaging showed that the mass was located within the liver parenchyma and had enhancing edges. Therefore, even if no cirrhosis and/or tumor index anomalies were found, in retrospect, we prefer a hepatic malignancy as the primary diagnosis. During surgery, we found that the mass was grossly adherent to both the liver and colon. We excised portions of liver and colonic tissue en bloc to include the intact mass. It was still considered to be an exogenous liver tumor at the end of the operation until histopathology overturned our diagnosis. The pathological report showed that the tumor cells grew around the blood vessels in a typical concentric circle pattern, with increased mitoses, atypia and diffuse expression of SMA. The lesion did not invade the surrounding liver tissue. It was considered as a malignant myopericytoma originating from the colon and surrounding the liver. Due to the rarity of malignant myopericytoma, there is no effective treatment and complete surgical resection is regarded as the best option. Adjuvant therapy is being tried for some patients. One patient received postoperative radiotherapy and six courses of chemotherapy (ifosfamide-etoposide/cyclophosphamide, vincristine-doxorubicin), no recurrence after surgery. But the other patient had no tumor response after receiving two standard courses of theprubicin combined with ifosfamide chemotherapy regimen[14,18]. There are no reports of receiving immunotherapy and targeted therapy at present. As more cases emerge, the possibility of diversified adjuvant therapy will be further explored.
The pathophysiological mechanism of malignant myopericytoma is unclear. This is the main reason for the lack of treatment. Some studies have indicated that BRAF and PDGFRB may be involved in the occurrence and progression of myopericytoma. BRAF is an important component in the regulation of MAPK signaling pathway. Sadow et al[20] discovered BRAFV600E mutation in a comprehensive genetic test of myopericytoma. BRAFV600E mutation greatly enhanced the adhesion, migration and angiogenesis of tumor cells by upregulating the expression of some key molecules that influence extracellular matrix remodeling, angiogenesis and tumor microenvironment, which ultimately may lead to invasiveness. PDGFRB is a receptor tyrosine kinase and plays an important role in cell proliferation, migration and vascular development. Its abnormal expression is considered to be a significant cause of various forms of potentially malignant diseases. In a study by Hung et al[21], genetic testing found that all myopericytoma cases harbored PDGFRB mutations, strongly suggesting the role of PDGFRB signaling in the pathogenesis of myopericytoma. However, due to their small sample size, these results have not been replicated in other studies[22,23]. The genetic drivers of myopericytoma remain poorly understood. Further basic science and clinical studies of this rare neoplasm are warranted in the future in order to better understand the etiology, improve the diagnosis and determine the optimal clinical management of this rare pathology.
We report for the first time a rare case of malignant myopericytoma originating from the colon, which was difficult to differentiate radiographically from a liver neoplasm. The patient had no background of hepatitis or gastrointestinal symptoms and presented only with abdominal pain after hemorrhage into the tumor occurred. Exogenous malignant myopericytomas occurring in deep tissue are asymptomatic or cause only mild symptoms, which are easy to ignore when they are not ruptured. In light of both a lack of established treatment pathways and successful adjuvant treatments, complete surgical excision is the currently preferred treatment. Ongoing molecular studies may uncover key signaling pathways that could provide better diagnostic and therapeutic approaches in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology & hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Lomeli SM, Mexico S-Editor: Chen YL L-Editor: A P-Editor: Zhang XD
| 1. | Granter SR, Badizadegan K, Fletcher CD. Myofibromatosis in adults, glomangiopericytoma, and myopericytoma: a spectrum of tumors showing perivascular myoid differentiation. Am J Surg Pathol. 1998;22:513-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 246] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Matsuyama A, Hisaoka M, Hashimoto H. Angioleiomyoma: a clinicopathologic and immunohistochemical reappraisal with special reference to the correlation with myopericytoma. Hum Pathol. 2007;38:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Ide F, Mishima K, Yamada H, Saito I, Horie N, Shimoyama T, Kusama K. Perivascular myoid tumors of the oral region: a clinicopathologic re-evaluation of 35 cases. J Oral Pathol Med. 2008;37:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Inoue T, Misago N, Asami A, Tokunaga O, Narisawa Y. Myopericytoma proliferating in an unusual anastomosing multinodular fashion. J Dermatol. 2016;43:557-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Orenay OM, Sarifakioglu E, Yildirim U. A perivascular tumour on the wrist; myopericytoma. J Eur Acad Dermatol Venereol. 2016;30:709-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Zhang Z, Yu D, Shi H, Xie D. Renal myopericytoma: A case report with a literature review. Oncol Lett. 2014;7:285-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Agrawal N, Nag K. Myopericytoma of the thoracic spine: a case report and review of literature. Spine J. 2013;13:e23-e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Requena L, Kutzner H, Hügel H, Rütten A, Furio V. Cutaneous adult myofibroma: a vascular neoplasm. J Cutan Pathol. 1996;23:445-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 101] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Mentzel T, Dei Tos AP, Sapi Z, Kutzner H. Myopericytoma of skin and soft tissues: clinicopathologic and immunohistochemical study of 54 cases. Am J Surg Pathol. 2006;30:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 214] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Agustí J, Peñas L, Bosch N. Intravascular Myopericytoma of the Plantar Region: Case Report and Discussion of the Probable Origin From a Cutaneous Vascular Malformation. Am J Dermatopathol. 2016;38:546-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Yang JC, Venteicher AS, Koch MJ, Stapleton CJ, Friedman GN, Venteicher EM, Shin JH. Myopericytoma at the Craniocervical Junction: Clinicopathological Report and Review of a Rare Perivascular Neoplasm. Neurosurgery. 2019;85:E360-E365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Mainville GN, Satoskar AA, Iwenofu OH. Primary malignant myopericytoma of the left atrium--a tumor of aggressive biological behavior: report of the first case and review of literature. Appl Immunohistochem Mol Morphol. 2015;23:464-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Patrick A, Soares-de-Almeida L, Heinz K. Malignant Myopericytoma: Report of a New Case and Review of the Literature. Am J Dermatopathol. 2016;38:307-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | McMenamin ME, Fletcher CD. Malignant myopericytoma: expanding the spectrum of tumours with myopericytic differentiation. Histopathology. 2002;41:450-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Ramdial PK, Sing Y, Deonarain J, Singh B, Allopi L, Moodley P. Periampullary Epstein-Barr virus-associated myopericytoma. Hum Pathol. 2011;42:1348-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Holling M, Wildförster U, Verheggen R, Müller K, Stummer W, Jeibmann A. Myopericytoma: A Series of 5 Cases Affecting the Nervous System. World Neurosurg. 2015;84:1493.e5-1493.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Binesh F, Moghadam RN, Shabani M, Mortazavizadeh MR, Zare S. Malignant Myopericytoma of Shoulder: A Rare Lesion. APSP J Case Rep. 2016;7:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Chen W, Han L, Pang H, Duan L, Zhao Z. Primary malignant myopericytoma with cancer cachexia: Report of the first case and review of literature. Medicine (Baltimore). 2017;96:e9064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Conradie JM, Bonnet EG, Thomas A, Van der Linde G. Intracranial malignant myopericytoma: case report and literature review. Br J Neurosurg. 2021;1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (1)] |
| 20. | Sadow PM, Priolo C, Nanni S, Karreth FA, Duquette M, Martinelli R, Husain A, Clohessy J, Kutzner H, Mentzel T, Carman CV, Farsetti A, Henske EP, Palescandolo E, Macconaill LE, Chung S, Fadda G, Lombardi CP, De Angelis AM, Durante O, Parker JA, Pontecorvi A, Dvorak HF, Fletcher C, Pandolfi PP, Lawler J, Nucera C. Role of BRAFV600E in the first preclinical model of multifocal infiltrating myopericytoma development and microenvironment. J Natl Cancer Inst. 2014;106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Hung YP, Fletcher CDM. Myopericytomatosis: Clinicopathologic Analysis of 11 Cases With Molecular Identification of Recurrent PDGFRB Alterations in Myopericytomatosis and Myopericytoma. Am J Surg Pathol. 2017;41:1034-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Mito JK, Jo VY. BRAF V600E is not a consistent feature of myopericytoma. J Cutan Pathol. 2016;43:1248-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Agaimy A, Bieg M, Michal M, Geddert H, Märkl B, Seitz J, Moskalev EA, Schlesner M, Metzler M, Hartmann A, Wiemann S, Mentzel T, Haller F. Recurrent Somatic PDGFRB Mutations in Sporadic Infantile/Solitary Adult Myofibromas But Not in Angioleiomyomas and Myopericytomas. Am J Surg Pathol. 2017;41:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |