Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1825
Revised: April 22, 2024
Accepted: May 6, 2024
Published online: June 27, 2024
Processing time: 168 Days and 19.1 Hours
Application of indocyanine green (ICG) fluorescence has led to new develop
To investigate the effect of ICG in the diagnosis of anastomotic leakage in a sur
Sixteen rats were divided into two groups: GL group (n = 8) and sham group (n = 8). Approximately 0.5 mL of ICG (2.5 mg/mL) was intravenously injected post
The peritoneal fluids from the GL group showed ICG-dependent green fluore
The postoperative ICG test in a drainage tube is a valuable and simple technique for the diagnosis of GL. Hence, it should be employed in clinical settings in patients with suspected GL.
Core Tip: This study demonstrates the effectiveness of the peritoneal fluid indocyanine green (ICG) test in detecting postoperative gut leakage (GL) using rat models of surgical GL. The ICG test is a highly useful tool for diagnosing GL in patients with colorectal surgery. Our proposed method is a simple technique that can be used for both diagnosing and ruling out GL.
- Citation: Huang Y, Li TY, Weng JF, Liu H, Xu YJ, Zhang S, Gu WL. Peritoneal fluid indocyanine green test for diagnosis of gut leakage in anastomotic leakage rats and colorectal surgery patients. World J Gastrointest Surg 2024; 16(6): 1825-1834
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1825.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1825
Gut leakage (GL) is a serious yet common postoperative complication of gastrointestinal (GI) surgery. It has an incidence rate of 2.4%–27.0% and a mortality rate of up to 18% among patients with resected colorectal carcinoma[1-4]. Postope
Despite these serious adverse effects of postoperative GL, no simple method for its definitive diagnosis has yet been developed. Patients with GL generally display systemic inflammatory response symptoms, such as severe abdominal pain, high fever, rapid heart and respiratory rates, peritonitis, fecal matter in the drainage tube, and increased leukocytes postoperative day (POD) 5–8[9,10]. However, it is not easy to diagnose GL based on clinical symptoms alone. Postope
Indocyanine green (ICG) fluorescence has helped advance the field of GI surgery. Intraoperative usage of ICG for assessing anastomosis perfusion has been well studied[16,17]. However, little is known about using ICG for diagnosing postoperative GL. In the present study, we design a GL rat model by conducting surgical abscission of the sigmoid colon and test the effectiveness of ICG for diagnosing postoperative GL in rat models. In addition, we investigate whether ICG can be used for the diagnosis of postoperative GL in human patients who have undergone colorectal cancer resections.
We designed an enterostomy test to identify any possible leakage of ICG from the peripheral veins to the enterostomy stoma. In addition, we developed a diagnostic test to explore the diagnostic effect of the ICG test on GL in patients with colorectal surgery. The human study was approved by the Research Ethics Committee of the Guangzhou First People’s Hospital (Approval No. K-2019-173-01). This study was registered in the Chinese Clinical Trial Registry (registration number: ChiCTR1900028537).
We employed the following criteria to diagnose leakage: (1) Feces or pus discharged from an abdominal drainage tube, the rectum, or rectovaginal fistula[18]; and (2) peritonitis with sepsis, or with one of the following clinical features: Leukocytosis (> 12 × 109/L), elevated CRP (> 10 mg/L), PCT (> 0.5 ng/mL), abnormal drainage volume (> 100 mL lasting 3 d), significant increase in reduced drainage (increased > 30 mL from the previous day), or abnormal drainage color (turbid or brown)[18]. The ICG index test for the diagnosis of leakage involved the detection of green fluorescence emitted by the collected peritoneal fluid under near-infrared light.
The sample size was estimated as follows: n = Z2P (1-P)/d2[19]. P is a pre-determined value of sensitivity ascertained based on our previous clinician experience, and d is the marginal error. Here, P = 0.95, Z = 1.96, a = 0.05, d = 0.1. Hence, the total required sample size was calculated as follows: n = 1.962 × 0.95 × (1-0.95) / 0.12 = 18.
Six patients were enrolled in the enterostomy test. We used the following inclusion criteria for enrollment: (1) Patients that underwent a temporary or permanent enterostomy, including a colostomy or ileostomy for the first time; (2) patients should not have any postoperative intestinal obstructions and have smooth draining from the stoma; and (3) patients should be ≥ 18 years of age. Patients with a history of adverse reactions or known allergies to ICG, iodine, or iodine dyes were excluded from the test.
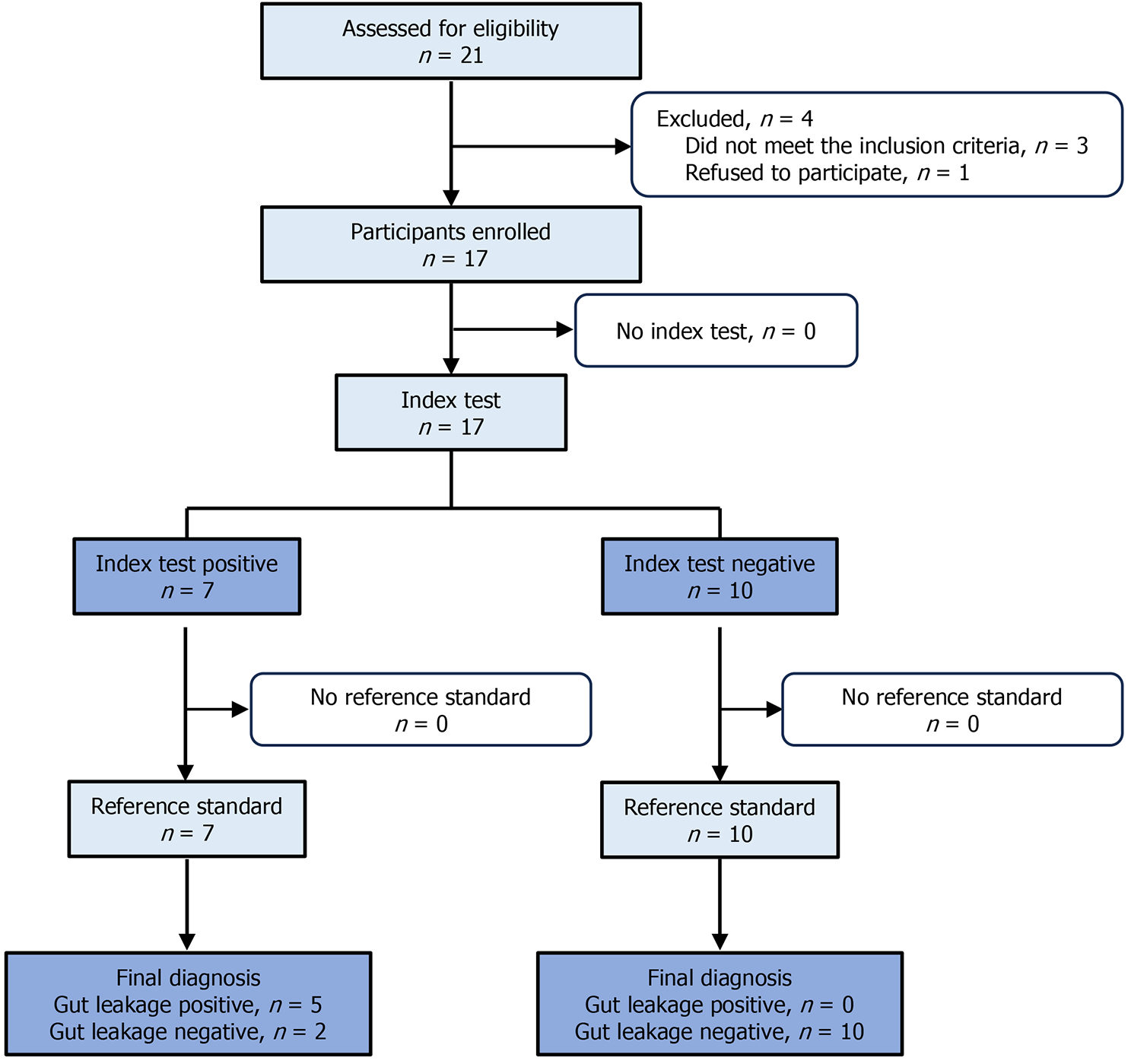
For the diagnostic test, we assessed 21 patients for eligibility. However, we enrolled only 17. Patients aged ≥ 18 years who had undergone GI reconstruction surgery, including colectomy or rectal cancer resection, were enrolled in the diag
Sixteen male Sprague–Dawley rats aged 7 wk and weighing 220 ± 20 g were purchased from Guangdong Medical La
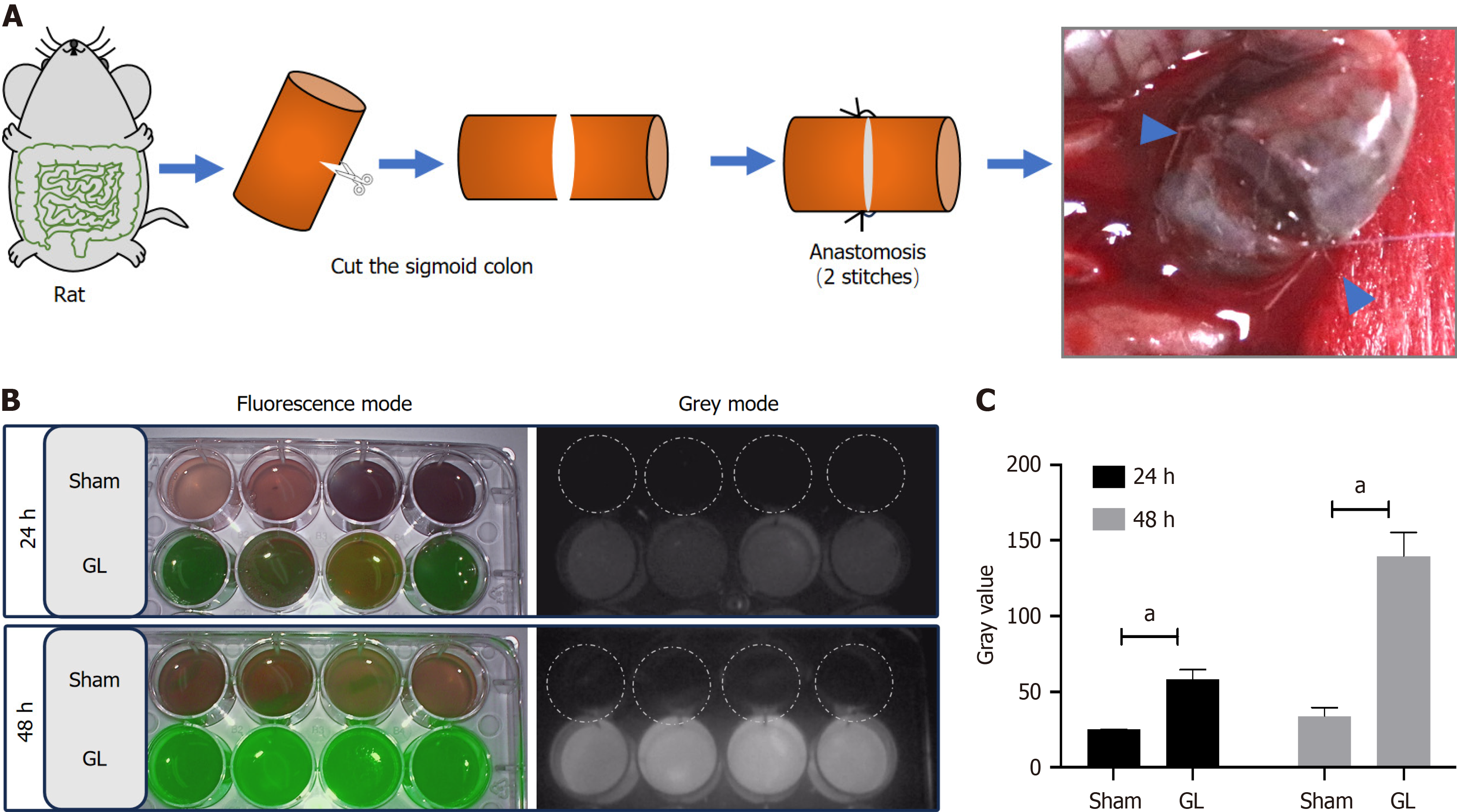
Eight rats were first anesthetized with inhaled isoflurane and then a laparotomy was performed in their inferior middle abdomen. Their colon was exposed approximately 6–8 cm from the anus and incised with scissors. The disassociated ends of the colon were then anastomosed with non-absorbable monofilament sutures at two diagonal points (GL group, n = 8). The abdomen was closed using 5-0 braided silk sutures in layers. The remaining eight rats did not undergo colon surgery for leakage and were used as controls (sham group, n = 8).
ICG was obtained from the Department of Pharmacy of Guangzhou First People’s Hospital. The ICG solution was diluted to 2.5 mg/mL, according to the instruction manual. Approximately 1.25 mg (0.5 mL) of diluted ICG was intravenously injected into the penile vein of the rats.
ICG was administered immediately post-surgery to human patients. On POD 1, 25 mg (10 mL) of diluted ICG was intravenously injected into six patients who had undergone enterostomy with a stoma. Next, 17 patients diagnosed with or suspected to have GL were intravenously injected with 25 mg (10 mL) of diluted ICG at the following times: POD 1 for 4 cases, POD 2 for 8 cases, POD 4 for 1 case, and POD 6 for 4 cases.
The rats were anesthetized with inhaled isoflurane at 24 and 48 h after post-surgical ICG injection. Their abdominal cavity was opened, and their intestines at the surgical points were exposed. After photographing the site, the abdominal cavity was washed with 5 mL of saline solution containing 2% v/v of fetal bovine serum albumin. The wash solution containing leaked stool, ingested food, bile, and digestive juices was collected and preserved at 4°C until fluorescence detection.
In human patients, the ostomy fluid or drainage liquid was collected from the enterostomy stoma or abdominal drain
This test was performed using a fluorescence laparoscope equipped with a near-infrared fluorescence imaging system (OptoMedic Technologies Inc., Guangzhou, China). The images were collected in three modes: white light, fluorescence, and gray. The fluorescence intensities were measured by ImageJ software with the gray-mode pictures.
The data were presented as the mean ± SD or the actual number of cases. A two-sided P value of < 0.05 was considered statistically significant. Intergroup comparisons of continuous variables were conducted using a two-sided Student’s t test. Statistical analysis was performed using SPSS 26.0, and the figures were generated using GraphPad Prism.
To simulate clinical postoperative GL in the colon, we established a GL rat model by surgically incising the sigmoid colon with an incomplete colon anastomosis sutured by only two stitches (Figure 1A). The incomplete anastomosis caused a state of intestinal discontinuity for at least 2 d postoperative. During this period, the stool comprising ingested food, bile, and digestive juices leaked into the abdominal cavity. The sham group showed no fluorescence, while the GL group showed green fluorescence in the collected peritoneal fluids at 24 and 48 h (Figure 1B). The semiquantitative analysis of gray values in the images showed that the GL group exhibited increased fluorescence intensity at 24 and 48 h compared to that shown by the sham group (Figure 1C).
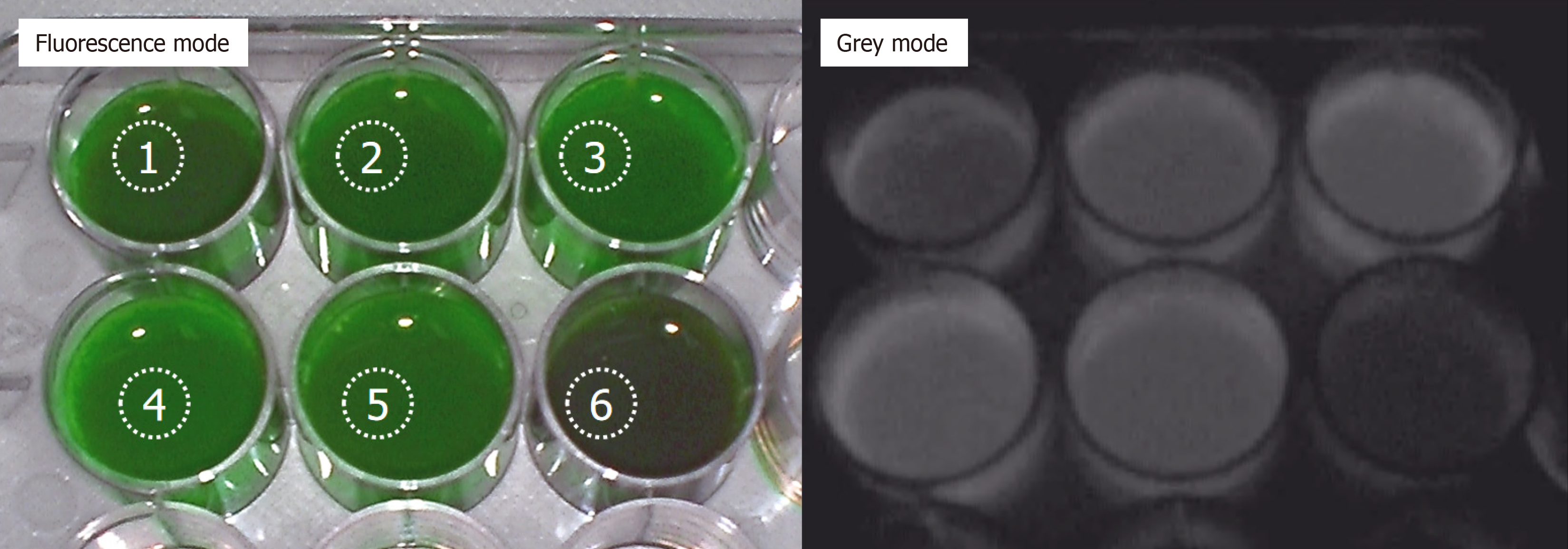
To test the status of ICG from the peripheral veins to the enterostomy port in patients, six patients (four women and two men), with an average age of 66.3 ± 10.6 years, who had undergone laparoscopic rectal cancer resection along with enterostomies, were selected for ICG injection on POD 1 (Table 1). The ostomy fluid at the stoma was collected on POD 2 and used to detect the ICG-dependent fluorescence (Figure 2). Five samples of stoma fluids from the aforementioned six cases showed strong green fluorescence, while one exhibited weak green fluorescence (Figure 2).
| Patients | n = 6 |
| Age (yr), mean ± SD | 66.3 ± 10.6 |
| Gender (male/female) | 2/4 |
| Diagnose | 6 (100%) |
| Rectal cancer | |
| Surgery approach | 6 (100%) |
| Laparoscopic rectal cancer resection plus ileostomy |
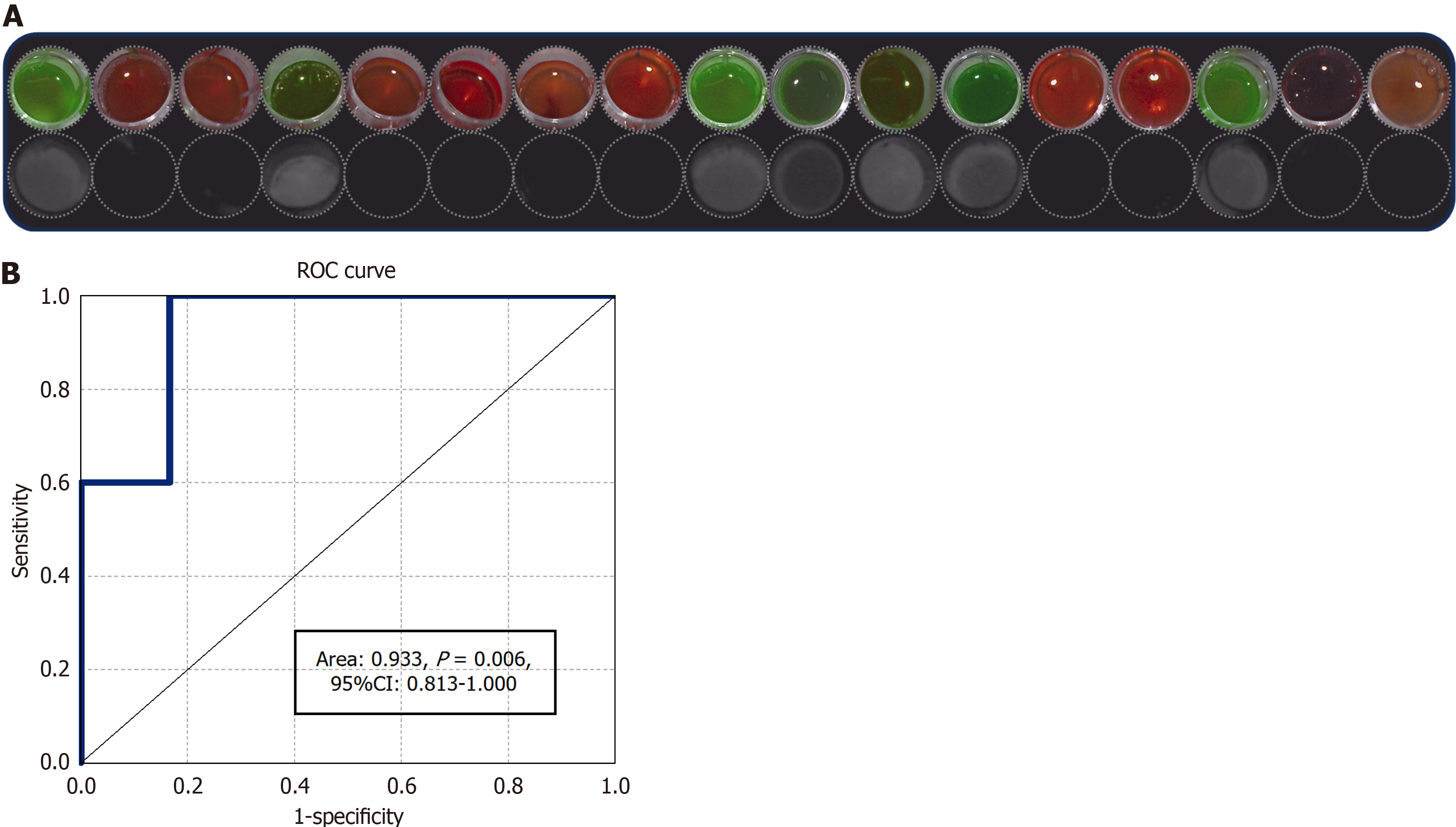
In the diagnostic test (Figure 3), 21 patients were assessed for eligibility. Of these, four patients were excluded. Three of these excluded patients did not meet the inclusion criteria, while one patient refused to participate. Finally, 17 were enrolled for the ICG test analysis. The baseline demographic and clinical characteristics of the participants are listed in Table 2. In the ICG test, seven of the 17 patients showed green fluorescence, while the other did not (Figure 4A). When tested with a reference standard, five of the seven patients who tested positive in the index test were diagnosed as GL positive, while all the other 10 who tested negative were diagnosed as GL negative (Table 3). The ICG test exhibited 100% sensitivity and 83.3% specificity for the diagnosis of GL. The positive predictive value was 71.4% and the negative pre
| Characteristic | Value (n = 17) (percentage) |
| Age (yr), mean ± SD | 58.6 ± 11.4 |
| Gender (male/female) | 10/7 |
| Diagnosis, n (%) | |
| Ascending colon cancer | 3 (17.6) |
| Descending colon cancer | 2 (11.8) |
| Sigmoid colon cancer | 4 (23.5) |
| Rectal cancer | 8 (47.1) |
| Surgery, n (%) | |
| Laparoscopic right hemicolectomy | 3 (17.6) |
| Laparoscopic left hemicolectomy | 2 (11.8) |
| Laparoscopic sigmoidectomy | 4 (23.5) |
| Laparoscopic rectal cancer resection | 8 (47.1) |
| Enrollment criteria | |
| Feces or pus discharged from drainage tube, n (%) | 2 (11.8) |
| Peritonitis with leukocytosis, n (%) | 5 (29.4) |
| Peritonitis with procalcitonin, n (%) | 5 (29.4) |
| Peritonitis with C-reactive protein, n (%) | 3 (17.6) |
| Abnormal drainage volume (> 100 mL lasting 3 d), n (%) | 2 (11.8) |
| Postoperative fever (> 38°C), n (%) | 7 (41.2) |
| Intraoperative blood loss > 50 mL, n (%) | 3 (17.6) |
| Operative time > 3 h, n (%) | 6 (35.3) |
| Tachycardia POD 1 (≥ 100 bpm), n (%) | 2 (11.8) |
| Clinical T stage (T3/T4) | 7/3 |
| ICG test | Postoperative gut leakage | Total | |
| Positive | Negative | ||
| Positive | 5 | 2 | 7 |
| Negative | 0 | 10 | 10 |
| Total | 5 | 12 | 17 |
We demonstrated the effectiveness of the peritoneal fluid ICG test in identifying postoperative GL in surgical GL rat models. We also showed the diagnostic value of the ICG test for diagnosing GL in patients undergoing colorectal surgery. Experimental models have become essential for verifying the effectiveness of ICG in diagnosing postoperative leakage. Previously reported GL models have mainly been used to study the mechanisms of intestinal wound healing[21]. Us
The intravenously injected ICG was taken up by hepatocytes. It was then initially excreted into the bile and later into the bowel. If no leakage occurred, there would be no green fluorescence in the peritoneal fluid when exposed to near-infrared light. Inversely, green peritoneal fluid is indicative of leakage, because the ICG cannot enter the enterohepatic circulation. Our animal experiment demonstrated that the ICG test can be used to detect postoperative GL because all peritoneal fluids of GL model rats showed green fluorescence. Furthermore, the ICG test of the ostomy fluids showed that this test can be used to detect postoperative GL in human patients. These results support the high sensitivity (nearly 100%) of ICG for detecting leakage.
Leakage is traditionally diagnosed based on clinical symptoms; laboratory tests such as markers of leukocytosis, CRP, and PCT; or imaging with an abdominopelvic CT scan or endoscopy[18]. Several biomarkers have been employed for diagnosing GL, such as postoperative fever, time to first defecation after operation[20], gut microbiota[23], MMP9[14], cytokines IL6 and TNFα[24,25], and ischemia biomarkers such as lactic acid and pH[26]. Usually, the most effective evidence of postoperative GL is a direct clinical manifestation, such as feces or pus discharged from an abdominal dra
The intraoperative use of ICG for assessing anastomosis perfusion has been well documented[16,17]. It has been re
Our study has some limitations as well. The rat model of anastomotic leakage has not been previously reported. To verify the leakage, we used only two stitches for anastomosis in the rat model, which is not a common practice in clinical surgery for patients. Nonetheless, it was still acceptable and appropriate for this study because we needed 100% leakage. In addition, the fluorescence was detected by collecting the drainage liquid. However, this method can be applied only in patients with an abdominal drainage tube. Finally, the sample size for the diagnostic test was small. In addition, all the enrolled patients were from a single center. Therefore, it is necessary to conduct multicenter studies with a large sample size.
In conclusion, our study showed that the postoperative ICG test using the drainage tube is a valuable and simple te
We thank Dr. Di Huang for biostatistics review of the study.
| 1. | Platell C, Barwood N, Dorfmann G, Makin G. The incidence of anastomotic leaks in patients undergoing colorectal surgery. Colorectal Dis. 2007;9:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Matsubara N, Miyata H, Gotoh M, Tomita N, Baba H, Kimura W, Nakagoe T, Simada M, Kitagawa Y, Sugihara K, Mori M. Mortality after common rectal surgery in Japan: a study on low anterior resection from a newly established nationwide large-scale clinical database. Dis Colon Rectum. 2014;57:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007;246:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 795] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 4. | Matthiessen P, Strand I, Jansson K, Törnquist C, Andersson M, Rutegård J, Norgren L. Is early detection of anastomotic leakage possible by intraperitoneal microdialysis and intraperitoneal cytokines after anterior resection of the rectum for cancer? Dis Colon Rectum. 2007;50:1918-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 206] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 6. | Smith JD, Paty PB, Guillem JG, Temple LK, Weiser MR, Nash GM. Anastomotic leak is not associated with oncologic outcome in patients undergoing low anterior resection for rectal cancer. Ann Surg. 2012;256:1034-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Ha GW, Kim JH, Lee MR. Oncologic Impact of Anastomotic Leakage Following Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. Ann Surg Oncol. 2017;24:3289-3299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Shearer R, Gale M, Aly OE, Aly EH. Have early postoperative complications from laparoscopic rectal cancer surgery improved over the past 20 years? Colorectal Dis. 2013;15:1211-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Zhang YB, Pan XF, Chen J, Cao A, Zhang YG, Xia L, Wang J, Li H, Liu G, Pan A. Combined lifestyle factors, incident cancer, and cancer mortality: a systematic review and meta-analysis of prospective cohort studies. Br J Cancer. 2020;122:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 10. | Kulu Y, Ulrich A, Bruckner T, Contin P, Welsch T, Rahbari NN, Büchler MW, Weitz J; International Study Group of Rectal Cancer. Validation of the International Study Group of Rectal Cancer definition and severity grading of anastomotic leakage. Surgery. 2013;153:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Italian ColoRectal Anastomotic Leakage (iCral) Study Group. Anastomotic leakage after elective colorectal surgery: a prospective multicentre observational study on use of the Dutch leakage score, serum procalcitonin and serum C-reactive protein for diagnosis. BJS Open. 2020;4:499-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Giaccaglia V, Salvi PF, Antonelli MS, Nigri G, Pirozzi F, Casagranda B, Giacca M, Corcione F, de Manzini N, Balducci G, Ramacciato G. Procalcitonin Reveals Early Dehiscence in Colorectal Surgery: The PREDICS Study. Ann Surg. 2016;263:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Sparreboom CL, Komen N, Rizopoulos D, Verhaar AP, Dik WA, Wu Z, van Westreenen HL, Doornebosch PG, Dekker JWT, Menon AG, Daams F, Lips D, van Grevenstein WMU, Karsten TM, Bayon Y, Peppelenbosch MP, Wolthuis AM, D'Hoore A, Lange JF. A multicentre cohort study of serum and peritoneal biomarkers to predict anastomotic leakage after rectal cancer resection. Colorectal Dis. 2020;22:36-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Edomskis P, Goudberg MR, Sparreboom CL, Menon AG, Wolthuis AM, D'Hoore A, Lange JF. Matrix metalloproteinase-9 in relation to patients with complications after colorectal surgery: a systematic review. Int J Colorectal Dis. 2021;36:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Doeksen A, Tanis PJ, Vrouenraets BC, Lanschot van JJ, Tets van WF. Factors determining delay in relaparotomy for anastomotic leakage after colorectal resection. World J Gastroenterol. 2007;13:3721-3725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | De Nardi P, Elmore U, Maggi G, Maggiore R, Boni L, Cassinotti E, Fumagalli U, Gardani M, De Pascale S, Parise P, Vignali A, Rosati R. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: results of a multicenter randomized controlled trial. Surg Endosc. 2020;34:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (1)] |
| 17. | Alekseev M, Rybakov E, Shelygin Y, Chernyshov S, Zarodnyuk I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: results of the FLAG randomized trial. Colorectal Dis. 2020;22:1147-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 18. | Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 1026] [Article Influence: 68.4] [Reference Citation Analysis (4)] |
| 19. | Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform. 2014;48:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 641] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 20. | Fukada M, Matsuhashi N, Takahashi T, Imai H, Tanaka Y, Yamaguchi K, Yoshida K. Risk and early predictive factors of anastomotic leakage in laparoscopic low anterior resection for rectal cancer. World J Surg Oncol. 2019;17:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Pommergaard HC, Rosenberg J, Schumacher-Petersen C, Achiam MP. Choosing the best animal species to mimic clinical colon anastomotic leakage in humans: a qualitative systematic review. Eur Surg Res. 2011;47:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Bachmann R, Van Hul M, Leonard D, Delzenne NM, Kartheuser A, Cani PD. The colonoscopic leakage model: a new model to study the intestinal wound healing at molecular level. Gut. 2020;69:2071-2073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Hajjar R, Santos MM, Dagbert F, Richard CS. Current evidence on the relation between gut microbiota and intestinal anastomotic leak in colorectal surgery. Am J Surg. 2019;218:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Su'a BU, Mikaere HL, Rahiri JL, Bissett IB, Hill AG. Systematic review of the role of biomarkers in diagnosing anastomotic leakage following colorectal surgery. Br J Surg. 2017;104:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Sparreboom CL, Wu Z, Dereci A, Boersema GS, Menon AG, Ji J, Kleinrensink GJ, Lange JF. Cytokines as Early Markers of Colorectal Anastomotic Leakage: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2016;2016:3786418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Wright EC, Connolly P, Vella M, Moug S. Peritoneal fluid biomarkers in the detection of colorectal anastomotic leaks: a systematic review. Int J Colorectal Dis. 2017;32:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Arezzo A, Bonino MA, Ris F, Boni L, Cassinotti E, Foo DCC, Shum NF, Brolese A, Ciarleglio F, Keller DS, Rosati R, De Nardi P, Elmore U, Fumagalli Romario U, Jafari MD, Pigazzi A, Rybakov E, Alekseev M, Watanabe J, Vettoretto N, Cirocchi R, Passera R, Forcignanò E, Morino M. Intraoperative use of fluorescence with indocyanine green reduces anastomotic leak rates in rectal cancer surgery: an individual participant data analysis. Surg Endosc. 2020;34:4281-4290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Song M, Liu J, Xia D, Yao H, Tian G, Chen X, Liu Y, Jiang Y, Li Z. Assessment of intraoperative use of indocyanine green fluorescence imaging on the incidence of anastomotic leakage after rectal cancer surgery: a PRISMA-compliant systematic review and meta-analysis. Tech Coloproctol. 2021;25:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |