Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1775
Revised: April 30, 2024
Accepted: May 17, 2024
Published online: June 27, 2024
Processing time: 110 Days and 2.4 Hours
Hepatitis is a systemic disease that often results in various comorbidities. Meta-bolic disorders, the most common comorbidities in clinical practice, were selected for this study.
To investigate the causal relationship between comorbidities and hepatitis trea-tment outcomes.
A total of 23583378 single nucleotide polymorphisms from 1248743 cases and related summaries of genome-wide association studies were obtained from online public databases. A two-sample Mendelian randomization (MR) was performed to investigate causality between exposure [type 2 diabetes mellitus (T2D), hyperlipidemia, and hypertension] and outcome (chronic hepatitis B or C in-fections).
The data supported the causal relationship between comorbidities and hepatitis infections, which will affect the severity of hepatitis progression and will also provide a reference for clinical researchers. All three exposures showed a link with progression of both hepatitis B (T2D, P = 0.851; hyperlipidemia, P = 0.596; and hypertension, P = 0.346) and hepatitis C (T2D, P = 0.298; hyperlipidemia, P = 0.141; and hypertension, P = 0.035).
The results of MR support a possible causal relationship between different ex-posures (T2D, hyperlipidemia, and hypertension) and chronic hepatitis progression; however, the potential mechanisms still need to be elucidated.
Core Tip: In our study, the randomization model was well defined for the exposures [type 2 diabetes mellitus (T2D), hyperlipidemia, and hypertension] and outcomes (chronic hepatitis B and chronic hepatitis C) by two-sample Mendelian randomization (MR) analysis, and they showed capabilities for interaction with chronic hepatitis infection. The results of our MR support a possible causal relationship between different exposures (T2D, hyperlipidemia, and hypertension) and chronic hepatitis progression.
- Citation: Liang LB, Liu XP, Mao TR, Su QL. Metabolic disorders and hepatitis: Insights from a Mendelian randomization study. World J Gastrointest Surg 2024; 16(6): 1775-1790
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1775.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1775
According to the Global Hepatitis Report 2017 by the World Health Organization, more than 1.34 million people died of hepatitis virus infection worldwide in 2015; more than half of the patients died because of progression to cirrhosis and the other half died due to hepatocellular carcinoma[1]. Hepatitis can be caused by infections with different viruses: Hepatitis A virus, hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), and hepatitis E virus. However, only HBV, HCV, and HDV can induce chronic hepatitis, leading to severe cirrhosis and hepatocellular carcinoma[1]. Because chronic hepatitis is mainly caused by HBV and HCV[2], we investigated the comorbidities of these two types in this study.
HBV is a DNA virus, whereas HCV is an RNA virus. HBV can activate severe immune responses in patients and can be removed by the human body in the short term[3]. However, HBV can also induce a chronic form in the long term; approximately 40% of HBV patients progress to cirrhosis[4], which could be attributable to the long half-life of HBV covalently closed circular DNA[5,6]. Meanwhile, other risk factors such as aging[5], other concomitant diseases, or consumption of alcohol, could significantly increase the probability of being diagnosed with cirrhosis, based on the theory of Sagnelli et al[7]. Similar to HBV, the chances of progression to cirrhosis and hepatocellular carcinoma among patients with HCV infection also increase with age[8]. Other potential risk factors, such as gastrointestinal diseases of the esophagus, stomach, and duodenum (41.7%), could induce comorbidities with hepatitis[9].
Researchers have recently explored the relationship between hepatitis and related comorbidities. According to Hsu et al[10], comorbidities accompanying hepatitis are often caused by hypertension, diabetes, and ischemic heart disease. In addition, circulatory diseases[9], renal diseases, and non-liver cancers can worsen hepatitis progression accompanied by comorbidities[8]. Therefore, in this study, we investigated the role of the following typical comorbidities in influencing hepatitis: Type 2 diabetes mellitus (T2D), hyperlipidemia, and hypertension.
As the risk factors and pathology of hepatitis comorbidities vary, analyzing the relationship between clinical treatment and hepatitis progression among different comorbidities becomes challenging. Meanwhile, knowledge in this field is still rather limited; therefore, we employed a Mendelian randomization (MR) protocol to explore this pair of causations. Currently, MR is a widely used research method based on genome-wide association studies (GWAS) and the theory of nucleotide polymorphisms. As a newly developed tool, MR inherits the principles of the equal, random, and independent distribution method, which adopts genetic variants to reveal causal relationships[11,12], thus providing more reliable and authentic results[13,14]. Regarding hepatitis research, MR will help establish the causal linkage between hepatitis comorbidities and disease outcomes. In this study, patients with different comorbidities were regarded as having a functional variation of specific genes, and MR analysis will guide researchers to better understand hepatitis comorbidities and progression.
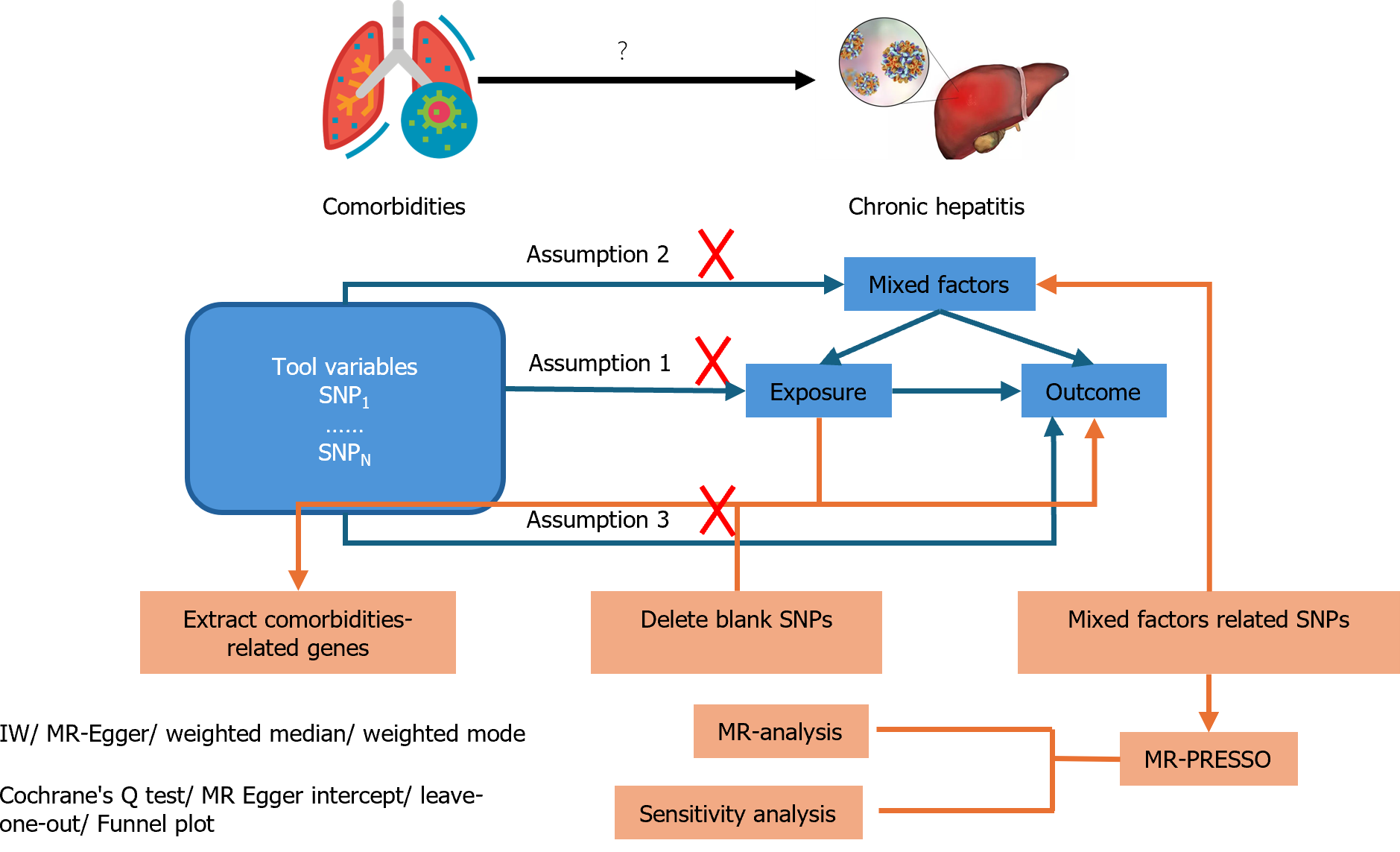
In this study, a two-sample MR analysis was employed to investigate the causal relationship between comorbidities and hepatitis treatment outcomes. Meanwhile, the inverse variance weighting (IVW) method was applied to determine the causal relationship between exposures and outcomes, where comorbidities were regarded as exposures and indicators from various aspects were regarded as outcomes. Consequently, single nucleotide polymorphisms (SNPs) associated with hepatitis comorbidities were used as instrumental variables, while their clinical conditions were used as outcome variables. Based on this condition, three important assumptions should be satisfied.
Assumption 1: The selected SNPs should be significantly related to the exposure variable. Assumption 2: SNPs should remain independent of factors that could play a role in exposure and outcomes. Assumption 3: SNPs should not have a direct impact on inducing changes in hepatitis status but can only alter the status via comorbidities to exhibit a causal relationship.
Based on the descriptions above, we conducted an MR analysis as depicted in Figure 1.
The included SNPs and related GWAS summaries were obtained from the online public databases IEU OpenGWAS (https://gwas.mrcieu.ac.uk/) and FinnGen Biobank (https://r8.finngen.fi/), which provide genetic insights from a well-phenotyped isolated population[15]. Online calculations were performed using the MR-Base platform (http://app.mrbase.org/, version 1.4.3 8a77eb; accessed on 08 January 2024)[11]. In this study, different pairs of exposures and outcomes were formed to examine their relationships, where T2D (total 50,533 samples, 16677 cases/33856 controls), hyperlipidemia (total 9714 samples, 3310 cases/6404 controls), and hypertension (total 484598 samples, 129909 cases/354689 controls) comprised the exposures, and chronic hepatitis B (CHB; total 351885 samples, 145 cases/351740 controls) and chronic hepatitis C (CHC) infections (total 352013 samples, 273 cases/351740 controls). Table 1 lists the characteristics of these pairs. These data were accessed and investigated on 06 January 2024.
| Group | Trait | ID | Sample size | Year | Case/control | SNPs |
| Exposures | T2D | ebi-a-GCST90093109 | 50533 | 2022 | 16677/33856 | 13403040 |
| Hyperlipidemia | ebi-a-GCST90090994 | 9714 | 2022 | 3310/6404 | 592502 | |
| Hypertension | ebi-a-GCST90038604 | 484598 | 2021 | 129909/354689 | 9587836 | |
| Outcomes | CHB | ebi-a-GCST90018804 | 351885 | 2021 | 145/351740 | 19079722 |
| CHC | ebi-a-GCST90018805 | 352013 | 2021 | 273/351740 | 19074546 |
MR analysis was deployed to investigate the association between metabolic disorders and chronic hepatitis, which leveraged genetic variants as instrumental variables to infer causal relationships. The MR-base GWAS catalog served as a crucial tool for selecting appropriate SNPs as instrumental variables during the MR analysis. Meanwhile, rigorous criteria were adopted to ensure the high authenticity of the SNP statistics: P value threshold of less than 5 × 10-8 and LD Rsq threshold of 0.001.
Meanwhile, we utilized various models to test the theory that comorbidities affect chronic hepatitis. For instance, the IVW method as a commonly used approach in MR analysis, was used to estimate the causal effect between exposure and outcome. Additionally, the MR-Egger model was utilized to detect and correct for potential horizontal pleiotropy with a non-zero P value indicating statistical significance (P < 0.05). To ensure a convincing conclusion, the Cochrane Q test was applied with the IVW and MR-Egger tests to test for heterogeneity.
To verify the robustness of this MR analysis, sensitivity analyses were conducted by using various MR methods, including MR-Egger regression, weighted median, and weighted mode methods. The weighted mode was examined by leave-one-out analysis, where the impact of each instrumental SNP on exposures and outcomes could be detected. In addition, the odds ratio (OR) and its 95% confidence interval (95%CI) were calculated using the MR results to predict the integrity of the causal relationship between exposure and outcome factors.
Presentation of the results was meticulously executed through the creation of comprehensive visualizations, including forest plots, funnel plots, leave-one-out plots, and scatter plots. These plots provided insights into the effects of each SNP on both exposure and outcome variables, facilitating a deeper understanding of the observed associations.
The statistical analyses were conducted using R (version 4.0.3) and R/TwoSampleMR (https://mrcieu.github.io/TwoSampleMR, version 0.5.5) were used with the online analysis tool for computational efficiency. By employing these rigorous statistical approaches, the study aimed to provide robust evidence regarding the relationship between metabolic disorders and chronic hepatitis, contributing to the broader understanding of disease etiology and potential therapeutic interventions.
We chose three typical comorbidities among patients with chronic hepatitis, namely T2D, hyperlipidemia, and hypertension, as exposure factors and found their GWAS summary for detailed MR. In this study, we found that these factors were significantly associated with the progression of chronic hepatitis. As shown in Table 1, the overall SNP exposure was 23583378 and the total sample accounted for 1248743 cases. In the outcome group, the number of patients with hepatitis B and C infections was 418 of 703898 cases. These data were retrieved from public research worldwide and, therefore, present extensive human research.
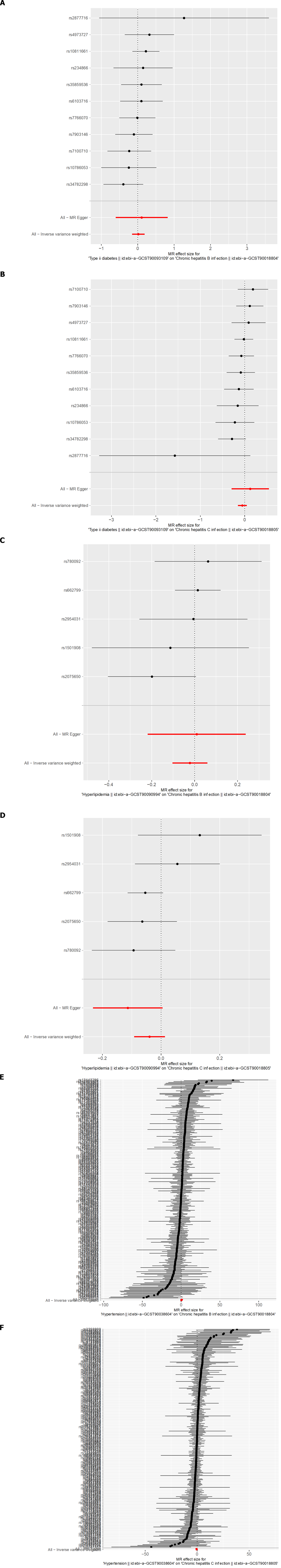
Detailed data of the two-sample MR are presented in Table 2. All three exposures exhibited a role in promoting the progress of hepatitis: T2D showed a link with hepatitis B progression examined by IVW (OR = 1.01676, 95%CI = 0.854–1.210, P = 0.851), as did hyperlipidemia (OR = 0.97827, 95%CI = 0.902–1.061, P = 0.596), and hypertension (OR = 1.35243, 95%CI = 0.721–2.535, P = 0.346). For the other outcomes of CHC infection, T2D promoted its progression with OR = 0.94825, 95%CI = 0.858–1.048, P = 0.298; hyperlipidemia with OR = 0.96140, 95%CI = 0.912–1.013, P = 0.141; and hypertension with OR = 0.67301, 95%CI = 0.465–0.973, P = 0.035).
| Outcome | Exposure | Number of instruments | Method | OR | 95%CI | P value | Heterogeneity | Pleiotropy | |||
| Q | Q_df | Q p | Intercept | P value | |||||||
| CHB infection | T2D | 11 | MR Egger | 1.11338 | 0.546-2.271 | 0.774 | 6.861 | 9 | 0.6516 | -0.011 | 0.803 |
| Weighted median | 1.08022 | 0.860-1.357 | 0.504 | - | - | - | |||||
| IVW | 1.01676 | 0.854-1.210 | 0.851 | 6.927 | 10 | 0.7323 | |||||
| Weighted mode | 1.10321 | 0.815-1.493 | 0.576 | - | - | - | |||||
| Hyperlipidemia | 5 | MR Egger | 1.00996 | 0.804-1.269 | 0.938 | 3.888 | 3 | 0.2738 | -0.013 | 0.784 | |
| Weighted median | 1.00910 | 0.918-1.110 | 0.857 | - | - | - | |||||
| IVW | 0.97827 | 0.902-1.061 | 0.596 | 4.004 | 4 | 0.4055 | |||||
| Weighted mode | 1.01660 | 0.912-1.133 | 0.765 | - | - | - | |||||
| Hypertension | 277 | MR Egger | 1.845401 | 0.350-9.728 | 0.471 | 279.1 | 258 | 0.1751 | -0.0028 | 0.693 | |
| Weighted median | 1.56894 | 0.604-4.077 | 0.346 | - | - | - | |||||
| IVW | 1.35243 | 0.721-2.535 | 0.346 | 279.3 | 259 | 0.1846 | |||||
| Weighted mode | 1.81921 | 0.519-6.379 | 0.359 | - | - | - | |||||
| CHC infection | T2D | 11 | MR Egger | 1.13338 | 0.744-1.726 | 0.574 | 9.143 | 9 | 0.4242 | -0.022 | 0.414 |
| Weighted median | 0.94250 | 0.825-1.077 | 0.391 | - | - | - | |||||
| IVW | 0.94825 | 0.858-1.048 | 0.298 | 9.887 | 10 | 0.4504 | |||||
| Weighted mode | 0.94916 | 0.810-1.112 | 0.550 | - | - | - | |||||
| Hyperlipidemia | 5 | MR Egger | 0.89288 | 0.793-1.005 | 0.158 | 3.222 | 3 | 0.3587 | 0.03 | 0.274 | |
| Weighted median | 0.94627 | 0.898-0.997 | 0.038 | - | - | - | |||||
| IVW | 0.96140 | 0.912-1.013 | 0.141 | 5.134 | 4 | 0.2738 | |||||
| Weighted mode | 0.94262 | 0.891-0.998 | 0.108 | - | - | - | |||||
| Hypertension | 260 | MR Egger | 0.59067 | 0.223-1.566 | 0.291 | 289.5 | 258 | 0.08644 | 0.0012 | 0.777 | |
| Weighted median | 0.69088 | 0.391-1.220 | 0.206 | - | - | - | |||||
| Inverse variance weighted | 0.67301 | 0.465-0.973 | 0.035 | 289.6 | 259 | 0.09284 | |||||
| Weighted mode | 0.83477 | 0.408-1.707 | 0.617 | - | - | - | |||||
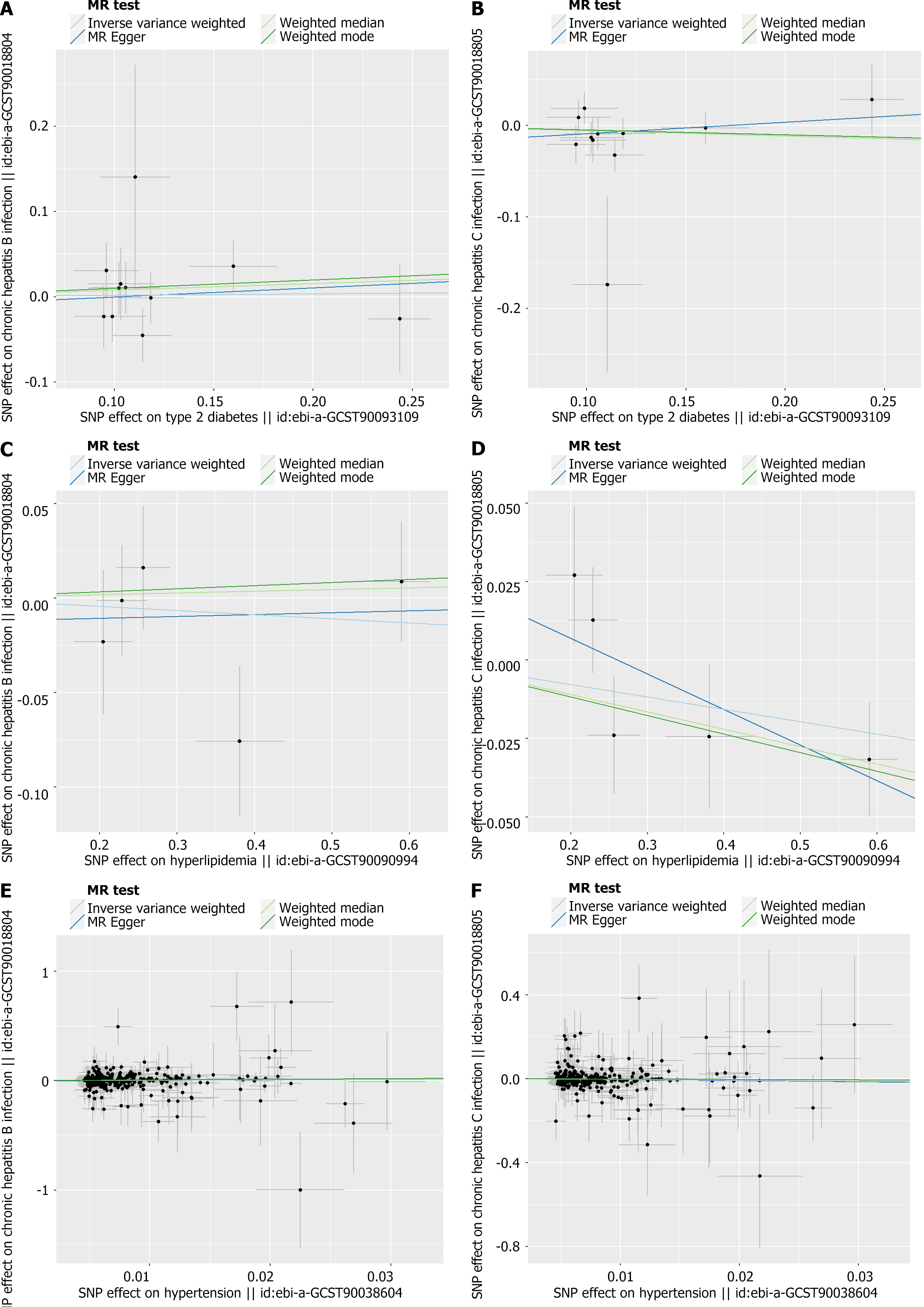
Consistent with the findings in Table 2, the effects of the SNPs on exposure and outcomes are illustrated below (Figure 2).
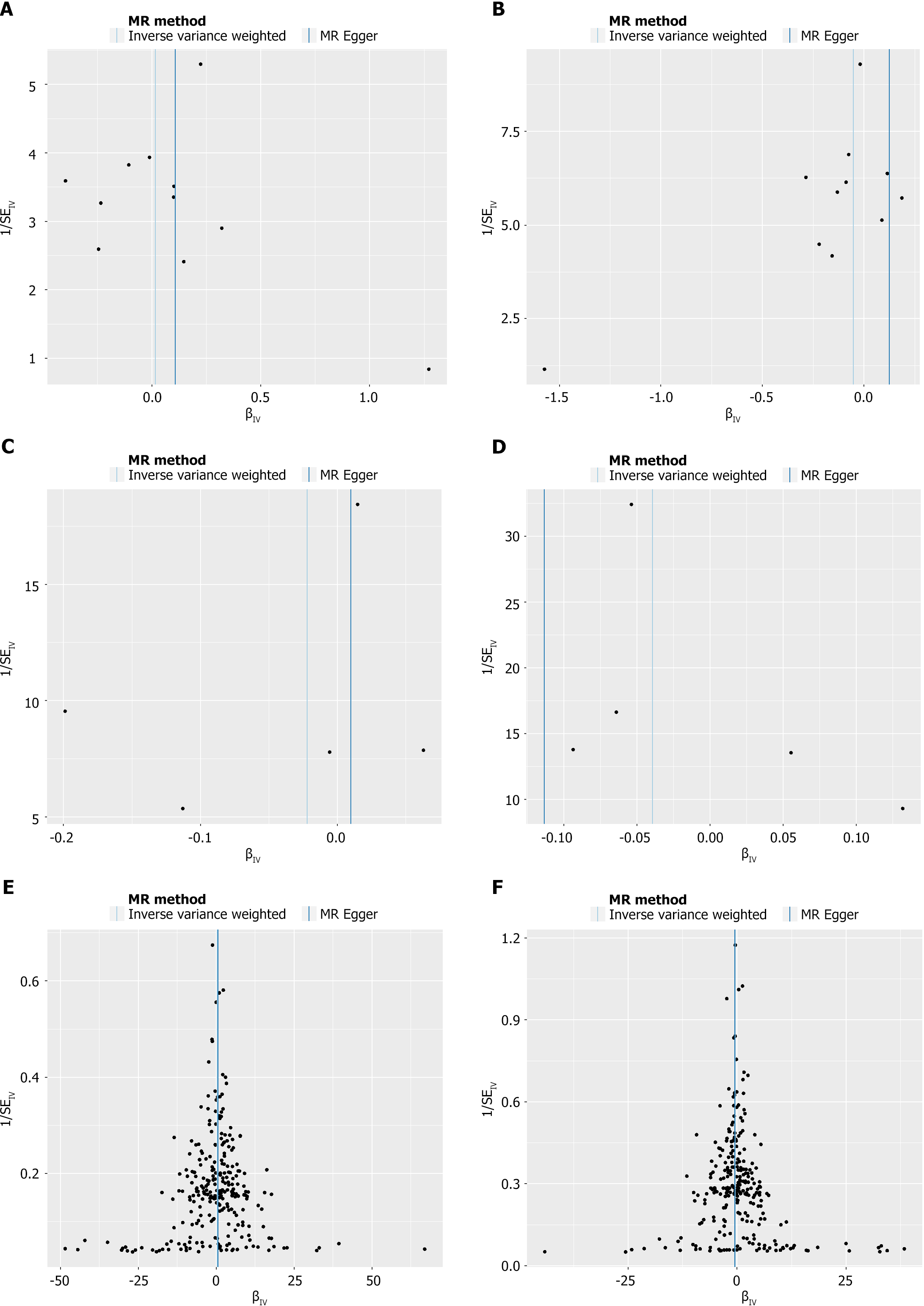
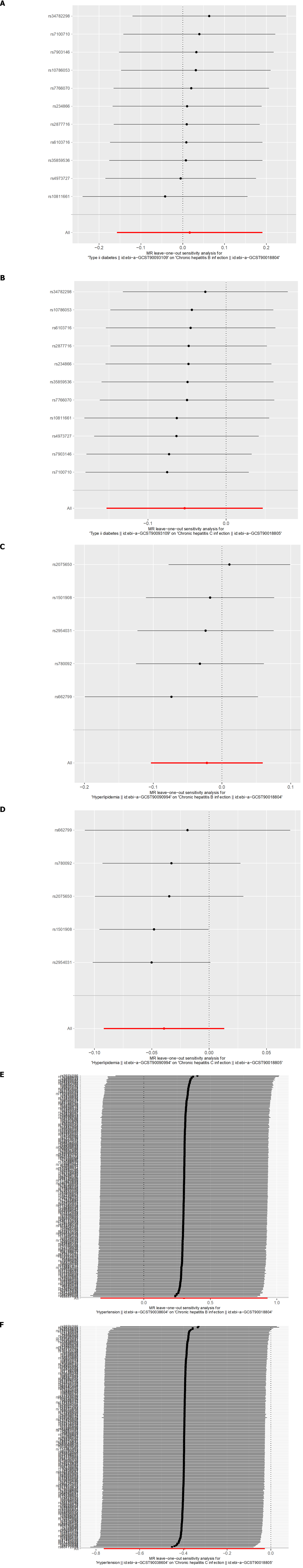
As mentioned in the MR section, different statistical models were applied to examine the quality of the analysis. For example, to examine the horizontal pleiotropy of this MR, the MR-Egger intercept was calculated based on the mode with P > 0.05, suggesting a correct scientific basis for this analysis. Directional horizontal pleiotropy was drawn in funnel plots (Figure 3), where the data dots were distributed in a symmetrical form. In addition, no heterogeneity was found in this study because the P values were greater than 0.05. Meanwhile, the leave-one-out analysis results indicated a significant relationship between different exposures and outcomes (Figures 4 and 5).
Currently, with faster transportation and a changeable lifestyle in modern societies, the probability of a person diagnosed with two or more diseases are increasing[16]. Since the mortality rate for patients with chronic hepatitis remains low, and the situations for the patients carrying an additional disease become complicated, we performed this MR to analyze the potential effects of comorbidities during hepatitis and the disease progression among the patients, and the first category went to cardiovascular disease after we looked up the related findings.
According to our findings, comorbidities associated with metabolic disorders and cardiovascular disease promote the progression of chronic hepatitis. A meta-analysis by Naing et al[17], which included 17 studies with 286084 patients demonstrated a strong relationship between T2D and CHC infection. This correlation was first proposed by Allison et al[18] in 1994, based on the finding of abnormalities in carbohydrate metabolism, such as glucose intolerance, in cirrhosis. The exact molecular mechanisms remain to be further explored, but MR could help answer this question since the underlying mechanisms are related to genetic specificity[19]. In addition, a meta-analysis found that T2D is associated with hepatitis B infection, and that T2D could promote hepatitis B progression into hepatocellular carcinoma[20]. The pathology remains to be further explored, but studies have indicated that altered microbiome caused by HBV affect both lipid and glucose metabolism, thus increasing the severity of the chronic hepatitis infection until non-alcoholic fatty liver disease (NAFLD) develops[21]. Multiple studies have revealed that parenteral viral hepatitis can affect insulin resistance in the body, thus increasing the severity of hepatitis progression in patients[22]. Hepatitis infection with comorbid T2D eventually progresses to NAFLD[21,23], hepatic steatosis[24], hepatocarcinoma, and fibrosis[25].
Hyperlipidemia is also a frequently observed comorbidity of chronic hepatitis infections in clinical practice[26]. According to a cohort study of 1927 patients from 2005 to 2015, hyperlipidemia was correlated with hepatitis progression into hepatocarcinoma without cirrhosis[27]. Meanwhile, a new type of HBV infection, occult HBV (HBV DNA-positive, but HBV surface antigen-negative), is more likely to occur in patients with hyperlipidemia[28]. The molecular mechanisms by which hyperlipidemia interacts with hepatitis remain unknown; however, an interesting phenomenon between hyperlipidemia and HBV infection has been reported. Statins, which were originally used to treat hyperlipidemia, have been widely used in clinical hepatitis treatment, and they could reduce the risks of progression to cirrhosis[29] and hepatocarcinoma[30,31].
Hypertension, which shares most instrumental SNPs with hepatitis, exhibits a strong association with hepatitis B and C infections. In clinical research, hypertension has been reported to be a risk factor for HCV patients with severe progression[32], and the overall effect in patients (e.g., ascites) with both hypertension and HCV is dependent on the severity of liver damage[33]. However, if patients receive antihypertensive treatment, they present with a mild viral syndrome upon exposure to HBV infection[34]. Researchers have attempted to explain this relationship, and one recent finding is that among patients with hypertension, the estimated glomerular filtration rate (eGFR) is lower in patients with HCV than those without HCV infection[35]. eGFR and other pathways may link hepatitis progression to hypertension; however, further research is required to support this theory.
In our study, the randomization model was well defined for the exposures (T2D, hyperlipidemia, and hypertension) and outcomes (CHB and CHC) by two-sample MR analysis, and they showed capabilities for interaction with chronic hepatitis infection; however, this study lacks clinical experimental data and other supporting materials to strengthen this theory. However, this shortage does not hinder this finding from being further explored, and as the next step in continued research, in-depth research on both clinical and molecular levels will be conducted to determine the exact molecular mechanisms and pathology of this linkage.
The results of our MR support a possible causal relationship between different exposures (T2D, hyperlipidemia, and hypertension) and chronic hepatitis progression; however, the potential mechanisms still need to be elucidated, and more supported data should come together to support the theory that these common comorbidities will surely affect the clinical treatment of chronic hepatitis infections.
The author thanks to all those who support assistance during the writing of this thesis.
| 1. | World Health Organization. Global hepatitis report 2017. Apr 19, 2017. [cited 30 April 2024]. Available from: https://www.who.int/publications/i/item/9789241565455. |
| 2. | Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol. 2016;16:509-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 3. | Ferri C, Govoni M, Calabrese L. The A, B, Cs of viral hepatitis in the biologic era. Curr Opin Rheumatol. 2010;22:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1951] [Article Influence: 216.8] [Reference Citation Analysis (0)] |
| 5. | Zhu JY. Analysis of the correlation between CONUT score and hepatitis B cirrhosis. M.Sc. Thesis, Shandong University. 2023. Available from: https://kns.cnki.net/kcms2/article/abstract?v=BQVG6Ge829Y-qHI3Y5tUWGw8m9QqwGqqup_xu90qFn3ceRLdP2CUfKGZnQ7s_vjK9dWWGzOfnnAt6mVLuwZ34Qr4c1jgF_YpOfJpheuTFEjV7mh4XYXKwpjLnAanFjIIyY9mygto1_ykLHm3Ms9Cvw==&uniplatform=NZKPT&language=CHS. |
| 6. | Zhang H, Tu T. Approaches to quantifying hepatitis B virus covalently closed circular DNA. Clin Mol Hepatol. 2022;28:135-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Sagnelli E, Stroffolini T, Mele A, Imparato M, Sagnelli C, Coppola N, Almasio PL. Impact of comorbidities on the severity of chronic hepatitis B at presentation. World J Gastroenterol. 2012;18:1616-1621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, Wang CH, Chen WJ, Chen CJ; R. E.V.E.A.L.-HCV Study Group. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 425] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 9. | Ruzicka DJ, Tetsuka J, Fujimoto G, Kanto T. Comorbidities and co-medications in populations with and without chronic hepatitis C virus infection in Japan between 2015 and 2016. BMC Infect Dis. 2018;18:237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Hsu PY, Wei YJ, Liang PC, Lee JJ, Niu SW, Huang JC, Hsu CT, Jang TY, Huang CI, Lin YH, Hsieh MY, Hsieh MH, Chen SC, Dai CY, Lin ZY, Huang JF, Chang JM, Yeh ML, Huang CF, Chiu YW, Hwang SJ, Chuang WL, Yu ML. Comorbidities in patients with chronic hepatitis C and hepatitis B on hemodialysis. J Gastroenterol Hepatol. 2021;36:2261-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4747] [Cited by in RCA: 4774] [Article Influence: 682.0] [Reference Citation Analysis (0)] |
| 12. | Goto A, Yamaji T, Sawada N, Momozawa Y, Kamatani Y, Kubo M, Shimazu T, Inoue M, Noda M, Tsugane S, Iwasaki M. Diabetes and cancer risk: A Mendelian randomization study. Int J Cancer. 2020;146:712-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Hartwig FP, Borges MC, Horta BL, Bowden J, Davey Smith G. Inflammatory Biomarkers and Risk of Schizophrenia: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry. 2017;74:1226-1233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 14. | Lee K, Lim CY. Mendelian Randomization Analysis in Observational Epidemiology. J Lipid Atheroscler. 2019;8:67-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 15. | Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, Reeve MP, Laivuori H, Aavikko M, Kaunisto MA, Loukola A, Lahtela E, Mattsson H, Laiho P, Della Briotta Parolo P, Lehisto AA, Kanai M, Mars N, Rämö J, Kiiskinen T, Heyne HO, Veerapen K, Rüeger S, Lemmelä S, Zhou W, Ruotsalainen S, Pärn K, Hiekkalinna T, Koskelainen S, Paajanen T, Llorens V, Gracia-Tabuenca J, Siirtola H, Reis K, Elnahas AG, Sun B, Foley CN, Aalto-Setälä K, Alasoo K, Arvas M, Auro K, Biswas S, Bizaki-Vallaskangas A, Carpen O, Chen CY, Dada OA, Ding Z, Ehm MG, Eklund K, Färkkilä M, Finucane H, Ganna A, Ghazal A, Graham RR, Green EM, Hakanen A, Hautalahti M, Hedman ÅK, Hiltunen M, Hinttala R, Hovatta I, Hu X, Huertas-Vazquez A, Huilaja L, Hunkapiller J, Jacob H, Jensen JN, Joensuu H, John S, Julkunen V, Jung M, Junttila J, Kaarniranta K, Kähönen M, Kajanne R, Kallio L, Kälviäinen R, Kaprio J; FinnGen, Kerimov N, Kettunen J, Kilpeläinen E, Kilpi T, Klinger K, Kosma VM, Kuopio T, Kurra V, Laisk T, Laukkanen J, Lawless N, Liu A, Longerich S, Mägi R, Mäkelä J, Mäkitie A, Malarstig A, Mannermaa A, Maranville J, Matakidou A, Meretoja T, Mozaffari SV, Niemi MEK, Niemi M, Niiranen T, O Donnell CJ, Obeidat ME, Okafo G, Ollila HM, Palomäki A, Palotie T, Partanen J, Paul DS, Pelkonen M, Pendergrass RK, Petrovski S, Pitkäranta A, Platt A, Pulford D, Punkka E, Pussinen P, Raghavan N, Rahimov F, Rajpal D, Renaud NA, Riley-Gillis B, Rodosthenous R, Saarentaus E, Salminen A, Salminen E, Salomaa V, Schleutker J, Serpi R, Shen HY, Siegel R, Silander K, Siltanen S, Soini S, Soininen H, Sul JH, Tachmazidou I, Tasanen K, Tienari P, Toppila-Salmi S, Tukiainen T, Tuomi T, Turunen JA, Ulirsch JC, Vaura F, Virolainen P, Waring J, Waterworth D, Yang R, Nelis M, Reigo A, Metspalu A, Milani L, Esko T, Fox C, Havulinna AS, Perola M, Ripatti S, Jalanko A, Laitinen T, Mäkelä TP, Plenge R, McCarthy M, Runz H, Daly MJ, Palotie A. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613:508-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1241] [Cited by in RCA: 1988] [Article Influence: 994.0] [Reference Citation Analysis (0)] |
| 16. | Nettleton S. The sociology of health and illness. [cited 30 April 2024]. Available from: https://www.wiley.com/en-us/The+Sociology+of+Health+and+Illness%2C+4th+Edition-p-9781509512737. |
| 17. | Naing C, Mak JW, Ahmed SI, Maung M. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: meta-analysis. World J Gastroenterol. 2012;18:1642-1651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 316] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15:1537-1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Tan Y, Wei S, Zhang W, Yang J, Yan L. Type 2 diabetes mellitus increases the risk of hepatocellular carcinoma in subjects with chronic hepatitis B virus infection: a meta-analysis and systematic review. Cancer Manag Res. 2019;11:705-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Han W, Huang C, Ji Y, Zhou L, Chen J, Hou J. Alterations in the Gut Microbiota and Hepatitis-B-Virus Infection in Southern Chinese Patients With Coexisting Non-Alcoholic Fatty Liver Disease and Type-2 Diabetes Mellitus. Front Med (Lausanne). 2021;8:805029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Merza MA. Seroprevalence and risk factors of hepatitis B and C viruses among diabetes mellitus patients in Duhok province, Iraqi Kurdistan. J Family Med Prim Care. 2020;9:642-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Zhu L, Jiang J, Zhai X, Baecker A, Peng H, Qian J, Zhou M, Song C, Zhou Y, Xu J, Liu H, Hang D, Hu Z, Shen H, Zhang ZF, Zhu F. Hepatitis B virus infection and risk of non-alcoholic fatty liver disease: A population-based cohort study. Liver Int. 2019;39:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 24. | Yu MW, Lin CL, Liu CJ, Huang YW, Hu JT, Wu WJ, Wu CF. Hepatic steatosis and development of type 2 diabetes: Impact of chronic hepatitis B and viral specific factors. J Formos Med Assoc. 2022;121:1478-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Mak LY, Hui RW, Lee CH, Mao X, Cheung KS, Wong DK, Lui DT, Fung J, Yuen MF, Seto WK. Glycemic burden and the risk of adverse hepatic outcomes in patients with chronic hepatitis B with type 2 diabetes. Hepatology. 2023;77:606-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 26. | Wang X, Xie Q. Metabolic Dysfunction-associated Fatty Liver Disease (MAFLD) and Viral Hepatitis. J Clin Transl Hepatol. 2022;10:128-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Phan J, Ng V, Sheinbaum A, French S, Choi G, El Kabany M, Durazo F, Saab S, Tong M, Busuttil R, Han SH. Hyperlipidemia and Nonalcoholic Steatohepatitis Predispose to Hepatocellular Carcinoma Development Without Cirrhosis. J Clin Gastroenterol. 2019;53:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Yang L, Li T, Li W, Tang X, Li J, Long R, Fu Y, Allain JP, Li C. Occult Hepatitis B Virus Infection in Hyperlipidemia Patients. Tohoku J Exp Med. 2017;241:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Chang FM, Wang YP, Lang HC, Tsai CF, Hou MC, Lee FY, Lu CL. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: A population-based study. Hepatology. 2017;66:896-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Goh MJ, Sinn DH, Kim S, Woo SY, Cho H, Kang W, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Statin Use and the Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B. Hepatology. 2020;71:2023-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 31. | Simon TG, Duberg AS, Aleman S, Hagstrom H, Nguyen LH, Khalili H, Chung RT, Ludvigsson JF. Lipophilic Statins and Risk for Hepatocellular Carcinoma and Death in Patients With Chronic Viral Hepatitis: Results From a Nationwide Swedish Population. Ann Intern Med. 2019;171:318-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 32. | Fujinaga K, Usui M, Yamamoto N, Ishikawa E, Nakatani A, Kishiwada M, Mizuno S, Sakurai H, Tabata M, Isaji S. Hypertension and hepatitis C virus infection are strong risk factors for developing late renal dysfunction after living donor liver transplantation: significance of renal biopsy. Transplant Proc. 2014;46:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Valla D, Flejou JF, Lebrec D, Bernuau J, Rueff B, Salzmann JL, Benhamou JP. Portal hypertension and ascites in acute hepatitis: clinical, hemodynamic and histological correlations. Hepatology. 1989;10:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Parrilli G, Manguso F, Orsini L, Coccoli P, Vecchione R, Terracciano L, De Luca N, Cirillo N, Abazia C, Budillon G, Marchesini G. Essential hypertension and chronic viral hepatitis. Dig Liver Dis. 2007;39:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Gantumur G, Batsaikhan B, Huang CI, Yeh ML, Huang CF, Lin YH, Lin TC, Liang PC, Liu TW, Lee JJ, Lin YC, Lin IL, Huang JF, Chuang WL, Yu ML, Tu HP, Dai CY. The association between hepatitis C virus infection and renal function. J Chin Med Assoc. 2021;84:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |