Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1765
Revised: April 3, 2024
Accepted: April 22, 2024
Published online: June 27, 2024
Processing time: 155 Days and 20.2 Hours
Malnutrition is common in critically ill patients, and it is associated with an increased risk of complications. Early enteral nutrition with adequate caloric and protein intake is critical nevertheless it is difficult to achieve. Peptide-based for
To determine the effects of a high-protein peptide formulation on gastrointestinal tolerance, nutritional status, biochemical changes, and adverse events in patients in the surgery intensive care unit (SICU) compared to an isocaloric isonitrogenous standard polymeric formulation.
This study was a multi-center double-blind, randomized controlled trial. We enrolled adult patients in the surgical intensive care unit, age ≥ 15 years and expected to receive enteral feeding for at least 5-14 d post-operation. They were randomly assigned to receive either the high-protein peptide-based formula or the isocaloric isonitrogenous standard formula for 14 d. Gastric residual volume (GRV), nutritional status, body composition and biochemical parameters were assessed at baseline and on days 3, 5, 7, 9, 11, and 14.
A total of 19 patients were enrolled, 9 patients in the peptide-based formula group and 10 patients in the standard formula group. During the study period, there were no differences of the average GRV, body weight, body composition, nutritional status and biochemical parameters in the patients receiving peptide-based formula, compared to the standard regimen. However, participants in the standard formula lost their body weight, body mass index (BMI) and skeletal muscle mass significantly. While body weight, BMI and muscle mass were maintained in the peptide-based formula, from baseline to day 14. Moreover, the participants in the peptide-based formula tended to reach their caloric target faster than the standard formula.
The study emphasizes the importance of early nutritional support in the SICU and showed the efficacy and safety of a high-protein, peptide-based formula in meeting caloric and protein intake targets while maintaining body weight and muscle mass.
Core Tip: This study is to focus on early nutrition support by novel high protein, peptide-based formula in various surgical intensive care patients. The formula could help maintaining body weight and muscle mass of patients and help them to meet calories and protein requirement. In addition, this nutrition support improved serum albumin, prealbumin and retinol binding protein which lead to decrease risk of malnutrition. Besides nutritional status outcomes evaluation, we investigate wound healing improvement by plasma fibronectin which is protein for cell adhesion, wound healing and blood clotting in this study. This finding would be helpful for recovery surgical patients after operation.
- Citation: Sumritpradit P, Shantavasinkul PC, Ungpinitpong W, Noorit P, Gajaseni C. Effect of high-protein peptide-based formula compared with isocaloric isonitrogenous polymeric formula in critically ill surgical patient. World J Gastrointest Surg 2024; 16(6): 1765-1774
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1765.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1765
Malnutrition is common in critically ill patients, and it is associated with an increased risk of complications, particularly in surgical intensive care (SICU) patients. Malnutrition increases infectious complications, delayed wound healing, prolonged hospital stays, and increased hospital cost and overall mortality[1,2]. Early enteral nutrition with adequate caloric and protein intake have been shown to successfully handle the metabolic demands that arise during the acute phase of critical illness, especially in surgical patients which increased metabolic needs for recovery and wound healing. Early enteral feeding could reduce length of hospital stay, ICU stay, ventilator days and mortality in critically ill patients[3]. Moreover, high protein intake during peri-operative period results in less negative nitrogen balance and decrease risk of muscle wasting. Nevertheless, high caloric and protein intake is difficult to achieve. Since the greater volumes and concentrations of enteral nutrition may be required, which may raise the risk of feeding intolerance. Using high volume enteral feeding may increase gastric residual volume (GRV) which leads to increased risk of aspiration pneumonia[4,5]. Fibronectin is a protein that is essential for cell adhesion, wound healing, and blood clotting. Plasma fibronectin is essential for host defense in critically ill patients, especially during sepsis. Low plasma fibronectin levels can promote phagocytic failure, reticuloendothelial system malfunction, and multiple organ failure[6,7].
Peptide-based formula is an enteral formula that incorporates partially or totally hydrolyzed protein in the form of dipeptides or tripeptides. Additionally, a peptide-based formula usually contains a significant amount of medium chain triglyceride (MCT) which is easier to absorb and utilize[8]. This form of enteral formula has several advantages over other forms of enteral nutrition. It contains smaller protein fragments that can be absorbed and utilize more efficiently[9]. It is also a beneficial nutrition support for patients with tube feeding-related diarrhea, feeding intolerance or malabsorption[10]. However, there are limited studies showing the efficacy and safety of high-protein peptide-based formula in critically ill surgical patients. Thus, this study aimed to determine the effect of high-protein peptide-based formula, compared to the isocaloric isonitrogenous standard polymeric formula, on the gastrointestinal (GI) tolerability and changes of fibronectin levels in patients who were admitted to the SICU. The secondary objective was to investigate the nutritional status, biochemical changes (serum albumin, prealbumin, and retinol-binding protein) and adverse events of this peptide-based formula compared to the standard regimen. The study's findings will be beneficial and guide physicians in choosing the appropriate nutritional regimen for critically ill surgical patients.
This study was a multi-center double-blind, randomized controlled trial. It was conducted at SICU of Ramathibodi Hospital (Mahidol University), Chonburi Hospital and Surin Hospital, Thailand. We enrolled adult patients, age 15 years or older, who were admitted to the SICU and expected to receive enteral feeding for at least 5-14 d post-operation. Any acute surgical conditions patients, not the high-risk postoperative observation, could be enrolled to cover clinical scenarios which have problem to achieve goal of calories by enteral feeding. They were randomly assigned to receive either the high-protein peptide-based formula or the isocaloric isonitrogenous standard formula. Block randomization was generated from computerized system by statistical center and transferred to study site location as opaque sealed envelopes at a ratio of 1:1. We excluded patients who required parenteral nutrition, high doses sedative agents (fentanyl > 2 mg/kg/h or morphine > 0.05 mg/kg/h), history of aspiration pneumonia, thyroid disease, severe hepatic or renal impairment, abdominal hypertension, fluid overload, allergy to any research food components, end stage cancer, and severe burn (grade 2 or grade 3 burn with a lesion greater than 50% of the body surface area).
The study was approved by the Institutional Review Board from all institutes and the study was registered at the Thai Clinical Trials Registry (TCTR20220507003) before the first patient’s enrollment. All patients voluntarily signed and dated the written informed consent.
The study formula is a high-protein peptide-based formula with a Protein: Carbohydrate: Fat ratio of 20:45:35. It contains whey protein hydrolysate and leucine as protein sources and MCT, fish oil and canola oil as fat sources. The formula is fiber-free and it has low osmotic properties. For the control group, casein is added to the standard polymeric formula to pro
| Peptide-based formula | Standard formula | |
| Caloric distribution (Protein: Carbohydrate: Fat) | 20:45:35 | 20:52:28 |
| Source of protein | Whey protein hydrolysate (96%); Leucine (4%) | Sodium caseinate (8%); Soy protein isolate (92%) |
| Source of carbohydrate | Maltodextrin (79%); Potato starch (21%) | Maltodextrin (64%); Sucrose (32%); Fructo-oligosaccharide (4%) |
| Source of fat | Fish oil (5%); Canola oil (43%); MCT oil (52%) | Rice bran oil (92%); MCT oil (8%) |
| Osmolality (mOsm/kg H2O) | 290 | 364 |
| Osmolarity (mOsm/L) | 242 | 307 |
The feeding was started within 48 h after ICU admission and delivered continuously through NG tube. The daily calorie intake for the first 7 d was 20-25 kcal/kg body weight/d, then it was gradually increased to 25-30 kcal/kg of body weight/d over the following 8-14 d. The feeding started by providing at least 50% of the patient’s total daily energy requirement, and then gradually increased the feeding rate until the protocol-set feeding rate is reached.
GRV was measured before feeding, 6 times daily at 4-h intervals. If the GRVs was less than 250 mL, feeding was gradually increased until the target is reached. If the GRV was between 250-400 mL, the feeding was withholded for 1 h before reassessing GRV. If the amount is still over 250 mL, the feeding was stopped for another hour before reassessing the gastric content and the prokinetic agents was administered according to the physician judgement. If the GRV exceeded 400 mL, feeding was stopped, and medication was administered. Symptoms of GI intolerance including diar
Serum fibronectin concentrations were analyzed using a Human Fibronectin Detection kit, PerkinElmer, Inc. The data was generated by white OptiplatedTM 384 microplate and the Envision® plate reader 2103, PerkinElmer, Inc. At baseline (day 1) and on days 3, 5, 11, and 14. The other blood samplings such as complete blood count, blood urea nitrogen, creatinine, glomerular filtration rate, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, albumin, prealbumin, retinol binding protein (RBP), total lymphocyte count (TLC), prothrombin time, total bilirubin, international normalized ratio, fasting blood glucose, free thyroxine, free triiodothyronine, thyroid stimulating hormone, serum electrolyte were also performed.
The nutritional status was determined on days 1, 3, 5, 7, 9, 11, and 14 using Bhumibol Nutrition Triage (BNT) which was endorsed by Society of Parenteral and Enteral Nutrition of Thailand[10]. Body composition using Bioelectrical Impedance Analysis (Inbody®, Korea), and Sequential Organ Failure Assessment score on day 1 and 14 and Glasgow Coma Scale were also recorded on day 1, 3, 5, 7, 9, 11 and 14.
The sample size calculation was based on estimated serum fibronectin amount of ICU patients who received small-peptide and whole protein enteral feeding[11]. Considering standard deviation of serum fibronectin in post-operative patients as 20[12], a 95% confidence level, 80% power of study, and a 10% drop-out rate, 10 subjects were required in each group.
Statistical analysis was carried out using the SPSS version 18.0 (SPSS Inc, Chicago, IL, United States). Continuous data are presented as mean ± SD or median (interquartile range) while categorical data are shown as number (percentage). Independence sample t-test or Mann-Whitney U test were used to determine the differences of continuous variables between groups. While dependence sample t-test or Wilcoxon signed-rank test were used to compare within-subject parameters. Test results of categorical variables were evaluated by Chi-square and Fisher exact tests as appropriate. Results were deemed statistically significant at P-value < 0.05.
A total of 19 patients were enrolled, 9 patients were randomized to the peptide-based formula and 10 patients were randomized to the standard formula. Most of the subjects (77.8%) were men. Mean (SD) age of the participants in the peptide-based formula was slightly higher than the standard formula (60.1 ± 21.6 vs 49.1 ± 26.0 years, P = 0.333). Eighty percent of participants were classified as having a risk of malnutrition or mild malnutrition according to the BNT. Baseline characteristics were similar between groups (Table 2). Causes of ICU admission and type of surgery are shown in Table 3.
| Peptide-based formula (n = 9) | Standard formula (n = 10) | P value | |
| Demographic | |||
| Age (yr) | 60.1 ± 21.6 | 49.1 ± 26.0 | 0.333 |
| Gender (Male) | 7 (77.8) | 9 (90.0) | 0.466 |
| Weight (kg) | 66.2 ± 14.3 | 62.6 ± 10.4 | 0.533 |
| BMI (kg/m2) | 24.2 ± 3.1 | 22.4 ± 3.8 | 0.275 |
| GCS score | 10.7 ± 3.0 | 10.0 ± 3.1 | 0.864 |
| SOFA score | 4.0 ± 2.0 | 4.2 ± 2.0 | 0.769 |
| 1Nutrition status assessment | |||
| Risk of malnutrition | 4 (44.4) | 3 (30.0) | 0.752 |
| Mild malnutrition | 4 (44.4) | 5 (50.0) | |
| Moderate malnutrition | 0 (0.0) | 1 (10.0) | |
| Severe malnutrition | 1 (11.1) | 1 (10.0) | |
| Biochemistry | |||
| Hemoglobin (g/dL) | 9.8 ± 2.4 | 10.1 ± 1.8 | 0.793 |
| White blood cells count (× 103/μL) | 14.8 ± 4.2 | 15.0 ± 7.5 | 0.955 |
| Platelets count (× 103/μL) | 218.8 ± 112.7 | 175.5 ± 85.5 | 0.253 |
| Neutrophils | 84.3 ± 8.5 | 83.5 ± 5.8 | 0.826 |
| Monocytes | 6.3 ± 3.5 | 7.2 ± 3.8 | 0.513 |
| Total lymphocytes count (cell/μL) | 1267.1± 1164.8 | 1006.0 ± 1005.6 | 0.288 |
| Blood urea nitrogen (mg/dL) | 22.3 ± 8.7 | 21.3 ± 20.6 | 0.327 |
| Creatinine (mg/dL) | 1.1 ± 0.4 | 1.0 ± 0.9 | 0.102 |
| Urine urea nitrogen | 12.0 ± 6.4 | 15.4 ± 22.2 | 0.441 |
| GFR (mL/min/1.73 m²) | 74.7± 31.6 | 103.5 ± 35.8 | 0.082 |
| Fasting plasma glucose (mg/dL) | 128.0 ± 26.7 | 128.1 ± 49.1 | 0.374 |
| Alkaline phosphatase (U/L) | 92.1 ± 41.2 | 96.3 ± 64.6 | 0.859 |
| Aspartate transaminase (U/L) | 84.5 ± 52.0 | 104.3 ± 66.5 | 0.501 |
| Alanine transaminase (U/L) | 32.5 ± 26.0 | 86.8 ± 99.8 | 0.154 |
| Nutritional status | |||
| Albumin (g/dL) | 2.6 ± 0.6 | 2.5 ± 0.5 | 0.821 |
| Prealbumin (mg/dL) | 10.2 ± 5.3 | 14.1 ± 8.2 | 0.236 |
| Retinol binding protein (mg/dL) | 2.1 ± 1.7 | 2.0 ± 1.3 | 1.000 |
| Subject number | Cause of ICU admission | Type of surgery |
| 1 | Traumatic subdural hematoma | Non operative management, close neurological observation |
| 2 | Acute calculus cholecystitis, adult respiratory distress syndrome | Open cholecystectomy |
| 3 | Closed fracture of shaft of right of humerus, closed fracture of right tibia and fibula severe head injury, maxillofacial injury, traumatic subdural hematoma | Open reduction of fracture with internal fixation humerus. Closed reduction of fracture without internal fixation tibia and fibula. Non operative management, close neurological observation |
| 4 | AAST grade II liver injury severe head injury, traumatic subdural hematoma thoracic blunt aortic injury | Craniotomy, tracheostomy, thoracic endovascular repair of the aorta |
| 5 | Splenic injury grade 5 left hemothorax | Exploratory laparotomy with splenectomy |
| 6 | Large gastric ulcer perforation | Exploratory laparotomy, simple suture with omental patch |
| 7 | Omental twist causing omental infarction | Exploratory laparotomy, omentectomy with drainage |
| 8 | Sigmoid colon diverticulitis with perforation | Exploratory laparotomy c sigmoidectomy with Hartmann operation |
| 9 | Gastric ulcer perforation | Exploratory laparotomy, simple suture with omental patch |
| 10 | Traumatic subdural hematoma | Craniotomy |
| 11 | AAST grade 4 pancreatic injury | Exploratory laparotomy, distal pancreatectomy, total splenectomy |
| 12 | Malignant neoplasm of adrenal gland, gallstone | Unilateral adrenalectomy, total cholecystectomy |
| 13 | Acute calculus cholecystitis | Laparoscopic cholecystectomy |
| 14 | Closed fracture of acetabulum, fracture of right radius and ulnar bones | Open reduction of fracture with internal fixation of radius and ulnar bones |
| 15 | Severe head injury, traumatic subdural hematoma | Craniectomy for clot removal |
| 16 | Epidural hematoma, fracture zygoma, fracture orbit, fracture rib with hemothorax right | Craniotomy with clot removal, open reduction with internal fixation facial fractures |
| 17 | Distal rectal cancer | Abdominoperineal resection |
| 18 | Necrotizing fasciitis with septic shock | Debridement right leg |
| 19 | Necrotizing fasciitis right leg | Debridement right leg |
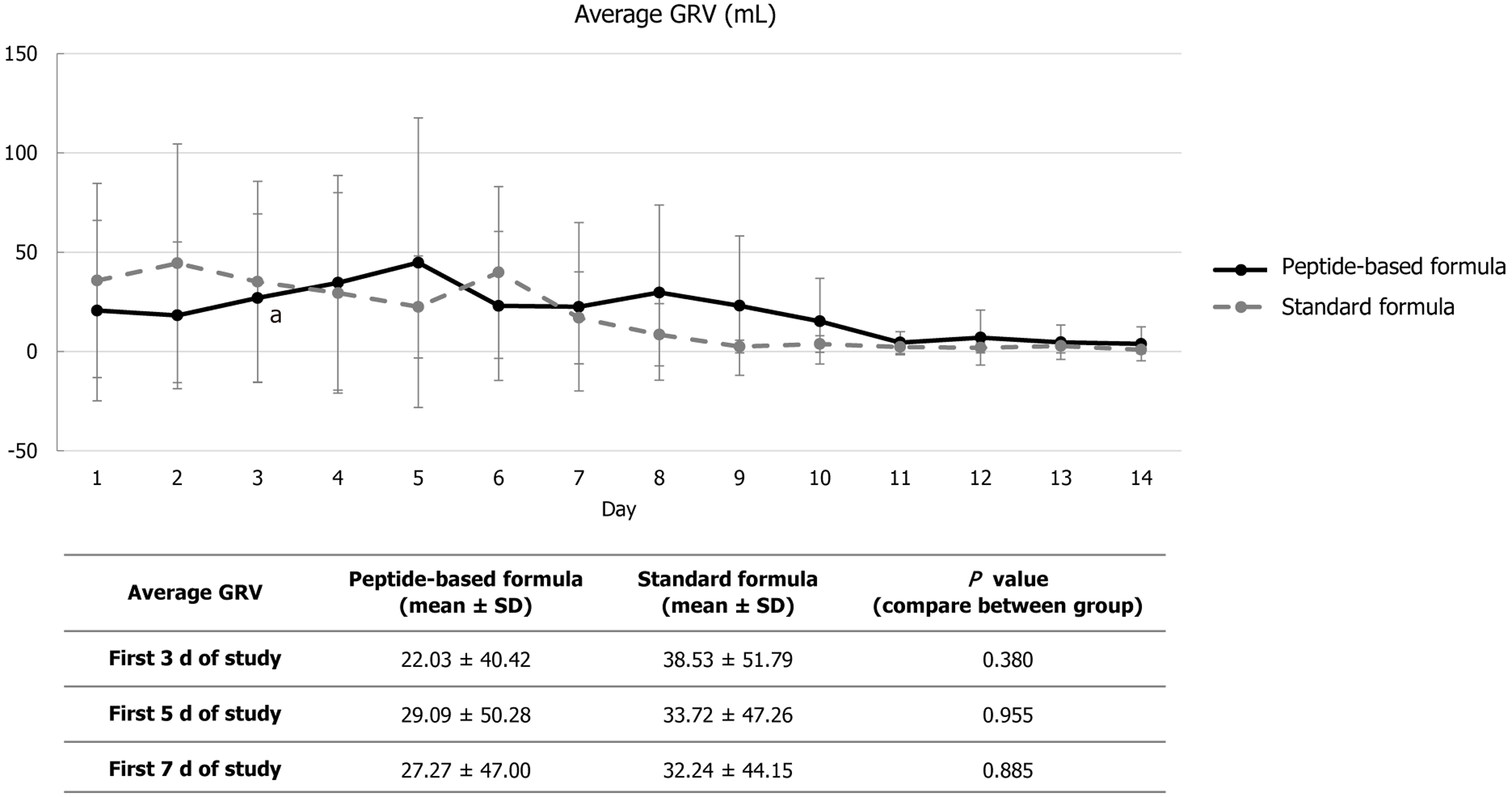
GRV was measured before feeding, 6 times a day then it was calculated as a daily average GRV. There were no significant differences between groups in average GRV measurement over the first three, five, or seven days of ICU admission (Figure 1). There was no difference between the two groups’ percentage changes in serum fibronectin levels between days 1 and 3, 5, 11, and 14 (Table 4).
| Peptide-based formula | Standard formula | P value | |
| Day 3 vs Day 1 | 17.8 (39.5) | 17.5 (100.4) | 0.790 |
| Day 5 vs Day 1 | 21.4 (41.9) | 63.4 (150.4) | 0.290 |
| Day 11 vs Day 1 | 65.1 (106.8) | 59.1 (211.8) | 0.641 |
| Day 14 vs Day 1 | 11.0 (115.6) | 80.5 (357.0) | 0.157 |
During the SICU admission, body weight, body mass index (BMI), skeletal muscle index were not significantly different between groups at day 14 after ICU admission. Mean calorie intake were slightly higher in the control group (peptide-based formula 25.6 ± 4.1 vs standard formula 27.3 ± 3.7 kcal/kg/d, P = 0.402). However, on day 14, participants in the standard formula lost their body weight, BMI and skeletal muscle mass significantly. While body weight, BMI and muscle mass were maintained in the peptide-based formula, from baseline to day 14 (Table 5).
| Peptide-based formula | Standard formula | P value (compare between group for day 14) | |||||
| Day 1 | Day 14 | P value | Day 1 | Day 14 | P value | ||
| Body weight (kg) | 66.2 ± 14.3 | 65.2 ± 13.1 | 0.389 | 62.6 ± 10.4 | 58.4 ± 10.5 | 0.037 | 0.288 |
| BMI (kg/m2) | 24.2 ± 3.1 | 23.0 ± 2.4 | 0.471 | 22.4 ± 3.8 | 20.9 ± 4.1 | 0.032 | 0.273 |
| SMI (kg/m2) | 18.4 ± 5.0 | 15.7 ± 3.7 | 0.139 | 16.1 ± 3.2 | 13.6 ± 3.2 | 0.003 | 0.419 |
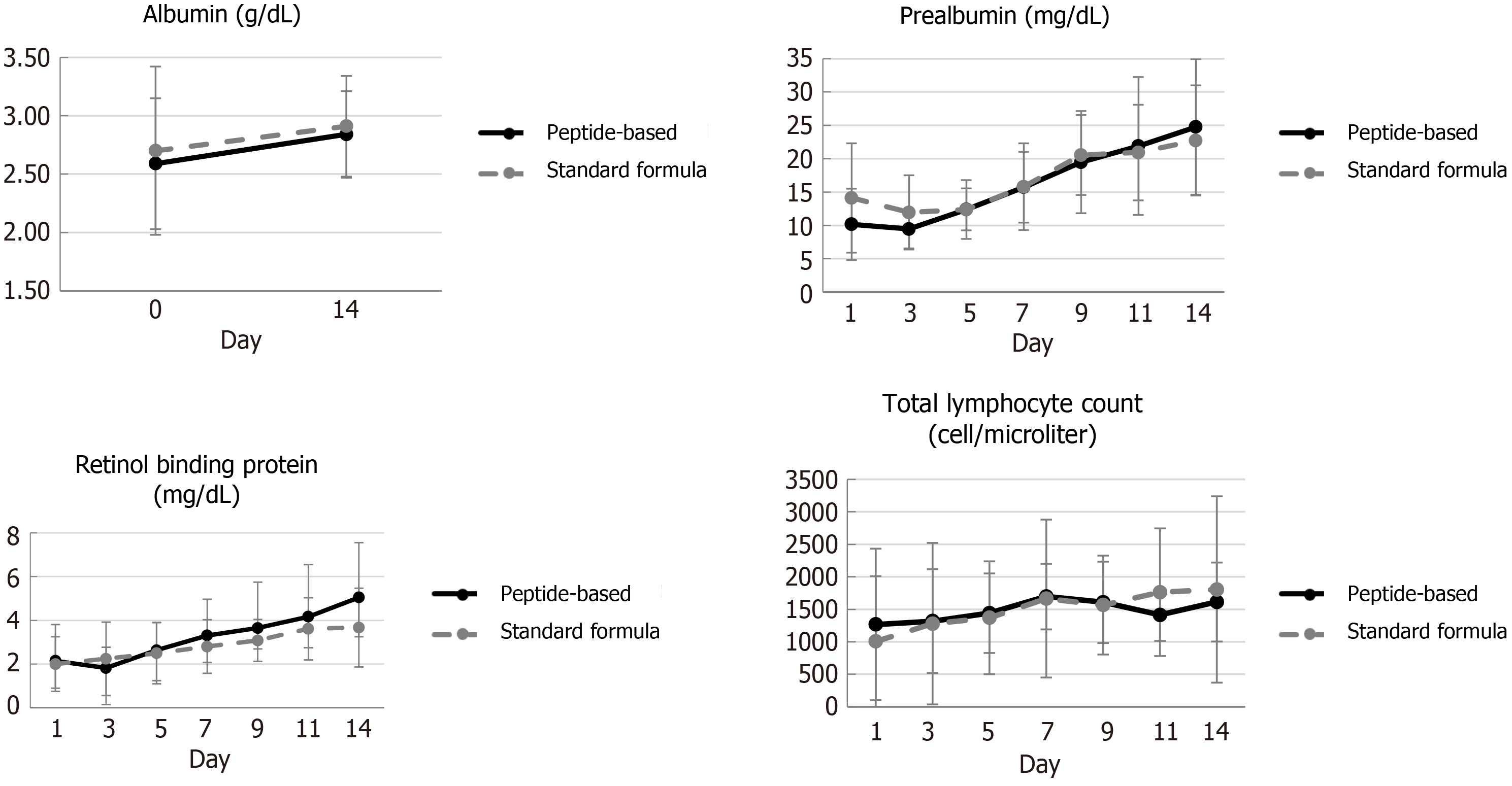
Serum albumin, prealbumin, RBP and TLC increased from baseline in all participants from both groups. However, only serum prealbumin and RBP significantly increased at day 14 (P ≤ 0.05) (Figure 2). The actual caloric and protein intake were similar between groups. Duration to achieved target calories, at 25 kcal/kg BW/d and 30 kcal/kg BW/d, in peptide-based formula was shorter than standard formula both as shown in Table 6. There was no observed readmission in either group within 90 d following hospital discharge, and the survival rate was 100% in both group at day 180.
| Day to reach goal | Peptide-based formula | Standard formula | P value |
| Step 1 (25 kcal/kg/d up) | 2.7 ± 0.6 | 3.8 ± 2.5 | 0.462 |
| Step 2 (30 kcal/kg/d up) | 7.5 ± 3.5 | 8.5 ± 0.7 | 0.733 |
There were no severe adverse event, and the frequency of GI complications was similar between groups.
Our study demonstrated that early nutrition support can lead to improvement of nutritional status for patients in SICU. Within the first week of ICU admission, all patients in this study reached their energy and protein targets according to the European Society for Parenteral and Enteral Nutrition guideline[3]. Even though, there were no differences of GRV and changes of body weight, body composition, nutritional status and biochemical parameters, including fibronectin levels, in patients receiving high-protein peptide-based formula, compared to the standard regimen. The participants in the standard formula lost their body weight, BMI and muscle mass significantly, while the participants receiving peptide-based formula could maintain their weight, BMI and muscle mass on day 14. Moreover, the participants in the peptide-based formula tended to reach their caloric target faster than the standard formula.
Peptide-based formula is the enteral formula which contains proteins that are partially hydrolyzed to dipeptides or tripeptides. Moreover, it usually includes higher MCT content compared to the standard polymeric formula which can be absorbed in GI tract and used as source of energy immediately[13]. Previous studies indicated that peptide-based formula could ameliorate feeding intolerance in critically ill patients[14] particularly in malnourished patients who underwent abdominal surgery[15]. In our study, the average GRV were similar between patients receiving either the peptide-based formula or the standard regimen. The discrepancy of the result might be explained by the fact that there were only few patients who had moderate to severe malnutrition in our study. Moreover, clinical characteristics of our study parti
Even though, there were no differences of body weight, body composition, nutritional status and biochemical parameters between groups of patients on day 14. Interestingly, the participants in the standard formula lost their weight, BMI and muscle mass significantly, while the participants receiving peptide-based formula could maintain their weight, BMI and muscle mass during baseline through day 14. Muscle wasting is a crucial factor on the recovery of critically ill patients. Sarcopenia can lead to functional disability which can persists for years after discharge from the intensive care unit[17,18]. In a recent clinical study, it was found that patients who receiving peptide-based formula lost less weight and lean mass, compared to the isocaloric and isonitrogenous enteral nutrition[19]. The study suggests that a peptide-based formula is more effective in term of maintaining body weight and muscle mass in patients undergoing surgery. Notably, both groups included the same amount of protein, but the peptide-based formula, composed of whey protein and leucine which could accelerate muscle protein synthesis[20]. Recent study demonstrated that peptide-based formula could prevent significant muscle loss, in comparison with standard formula or β-hydroxymethy β-butyrate-rich product, in obese patients who underwent Roux-en-Y gastric bypass[21]. Moreover, the peptide-based formula contains leucine, a branch-chain amino acid that the World Health Organization recommends people get 39 micrograms/kg of body weight/d[22]. This may be helpful for ICU patients, as shown by the previous research, which presented leucine concentrations that lower normal range lead to decreased cumulative survival rate[23].
The average calorie and protein intake were similar between groups however participants in the peptide-based formula had a tendency to reach their energy goals earlier, compared to the control group. the study’s finding were similar to the previous study which indicated that peptide-based formulas are more effective in achieving nutritional targets within a 7-d period compared to the intact-protein enteral nutrition formulas[24].
There was no significant difference in the change in plasma fibronectin levels between groups. This could be attributed to the fact that the level of plasma fibronectin varied greatly and was unpredictable. Previous research has shown that this value can be affected by several factors, such as the type of disease, severity of the infection, type of cancer, and blood transfusion[25-27]. Unlike previous studies[28,29], we only evaluated fibronectin levels after surgery, as we could not assess fibronectin levels before ICU admission[28,29]. Considering the factors mentioned above, the researcher concluded that the serum fibronectin value used in this study has a large variation, making it difficult to compare the degree of improvement among patients. Furthermore, it may not be specific enough to accurately measure the effect of the inter
This study has several strengths. Firstly, it was a multicenter, double-blind randomized controlled trial. Secondly, we used the isocaloric, isonitrogenous polymeric standard formula as a control. Thirdly, the feeding protocol was prog
This study’s results suggest that early nutritional support is a crucial aspect of health care for patients in the SICU. The high-protein, peptide-based enteral formula was not only well-received but also effective in helping critically ill patients meet their caloric and protein intake targets. This peptide-based enteral formula plays a significant role in preserving body weight and muscle mass. These findings have substantial implications for muscle strength, physical performance, and an individual’s ability to participate in physical medicine and rehabilitation.
We thank all participants in the studies, the nurses and laboratory staffs at Ramathibodi Hospital, Surin Hospital and Chonburi Hospital for data collection.
| 1. | Heyland DK. Nutritional support in the critically ill patients. A critical review of the evidence. Crit Care Clin. 1998;14:423-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, Griffiths R, Kreyman G, Leverve X, Pichard C, ESPEN. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr. 2009;28:387-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 691] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 3. | Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, Nitenberg G, van den Berghe G, Wernerman J; DGEM (German Society for Nutritional Medicine), Ebner C, Hartl W, Heymann C, Spies C; ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN Guidelines on Enteral Nutrition: Intensive care. Clin Nutr. 2006;25:210-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 987] [Cited by in RCA: 821] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 4. | Mentec H, Dupont H, Bocchetti M, Cani P, Ponche F, Bleichner G. Upper digestive intolerance during enteral nutrition in critically ill patients: frequency, risk factors, and complications. Crit Care Med. 2001;29:1955-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 354] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Blaser AR, Starkopf J, Kirsimägi Ü, Deane AM. Definition, prevalence, and outcome of feeding intolerance in intensive care: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2014;58:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Magnusson MK, Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol. 1998;18:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 207] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Grossman JE. Plasma fibronectin and fibronectin therapy in sepsis and critical illness. Rev Infect Dis. 1987;9 Suppl 4:S420-S430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Silk DB. Progress report. Peptide absorption in man. Gut. 1974;15:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Meredith JW, Ditesheim JA, Zaloga GP. Visceral protein levels in trauma patients are greater with peptide diet than with intact protein diet. J Trauma. 1990;30:825-8; discussion 828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Brinson RR, Kolts BE. Diarrhea associated with severe hypoalbuminemia: a comparison of a peptide-based chemically defined diet and standard enteral alimentation. Crit Care Med. 1988;16:130-136. [PubMed] |
| 11. | Heimburger DC, Geels VJ, Bilbrey J, Redden DT, Keeney C. Effects of small-peptide and whole-protein enteral feedings on serum proteins and diarrhea in critically ill patients: a randomized trial. JPEN J Parenter Enteral Nutr. 1997;21:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Hackbarth R, Sarnaik AP, Meert K, Deshmukh DR, Arciniegas E. Changes in plasma fibronectin in children after elective repair of congenital heart defects. J Thorac Cardiovasc Surg. 1993;105:31-36. [PubMed] |
| 13. | Mohamed Elfadil O, Shah RN, Hurt RT, Mundi MS. Peptide-based formula: Clinical applications and benefits. Nutr Clin Pract. 2023;38:318-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 14. | Wang K, Zhang Z. Peptide-based enteral nutrition for critically ill patients. J Transl Crit Care Med. 2021;3:2. [DOI] [Full Text] |
| 15. | Liu MY, Tang HC, Hu SH, Chang SJ. Peptide-based enteral formula improves tolerance and clinical outcomes in abdominal surgery patients relative to a whole protein enteral formula. World J Gastrointest Surg. 2016;8:700-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Montejo JC, Miñambres E, Bordejé L, Mesejo A, Acosta J, Heras A, Ferré M, Fernandez-Ortega F, Vaquerizo CI, Manzanedo R. Gastric residual volume during enteral nutrition in ICU patients: the REGANE study. Intensive Care Med. 2010;36:1386-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Mayer KP, Thompson Bastin ML, Montgomery-Yates AA, Pastva AM, Dupont-Versteegden EE, Parry SM, Morris PE. Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit Care. 2020;24:637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 18. | Schefold JC, Wollersheim T, Grunow JJ, Luedi MM, Z'Graggen WJ, Weber-Carstens S. Muscular weakness and muscle wasting in the critically ill. J Cachexia Sarcopenia Muscle. 2020;11:1399-1412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 19. | Alexander DD, Bylsma LC, Elkayam L, Nguyen DL. Nutritional and health benefits of semi-elemental diets: A comprehensive summary of the literature. World J Gastrointest Pharmacol Ther. 2016;7:306-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (2)] |
| 20. | Wamiti J, Kogi-Makau W, Onyango FE, Ngala S. Leucine supplementation in the management of protein energy malnutrition: A review article. East African Med J. 2017;94 Suppl 1:20-24. |
| 21. | Comas Martínez M, Fidilio Meli E, Palmas Candia F, Cordero E, Hernández I, Vilallonga R, Burgos R, Vila A, Simó R, Ciudin A. Protein Supplementation with Short Peptides Prevents Early Muscle Mass Loss after Roux-en-Y-Gastric Bypass. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 22. | Joint WHO/FAO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition. World Health Organ Tech Rep Ser. 2007;1-265. [PubMed] |
| 23. | Wang MY, Wang CH, Chen WS, Chu CM, Wu HP, Liu MH, Lin YT, Kao KC, Liang CY, Chen WH, Wang HJ, Lee SC. U-Shape Relationship between Plasma Leucine Level and Mortality in the Intensive Care Unit. Dis Markers. 2022;2022:7389258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Wang YQ, Li YH, Li YT, Li HX, Zhang D. Comparisons between short-peptide formula and intact-protein formula for early enteral nutrition initiation in patients with acute gastrointestinal injury: a single-center retrospective cohort study. Ann Transl Med. 2022;10:573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 25. | Stathakis NE, Fountas A, Tsianos E. Plasma fibronectin in normal subjects and in various disease states. J Clin Pathol. 1981;34:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Boughton BJ, Simpson A, Baar S, Ala F, Casson J, Gower J. The concentration of plasma fibronectin in burns patients treated with fresh frozen plasma or plasma protein fraction. Resuscitation. 1984;12:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Gironi A, Niccolai J, Fabbri LP, Niccolai M, Chisci C. [Determination of plasma fibronectin (Fn) levels. Comparison between normal subjects and patients with various pathological conditions]. Quad Sclavo Diagn. 1982;18:392-405. [PubMed] |
| 28. | Chadwick SJ, Mowbray JF, Dudley HA. Plasma fibronectin and complement in surgical patients. Br J Surg. 1984;71:718-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Gauperaa T, Giercksky KE, Revhaug A, Rekvig OP. Fibronectin, complement and immunoglobulins in serum after surgery. Br J Surg. 1985;72:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |