Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1709
Revised: April 26, 2024
Accepted: May 14, 2024
Published online: June 27, 2024
Processing time: 116 Days and 22.4 Hours
Locally advanced gastric cancer (LAGC) is a common malignant tumor. In recent years, neoadjuvant chemotherapy has gradually become popular for the treatment of LAGC.
To investigate the efficacy of oxaliplatin combined with a tigio neoadjuvant chemotherapy regimen vs a conventional chemotherapy regimen for LAGC.
Ninety patients with LAGC were selected and randomly divided into control and study groups with 45 patients in each group, according to the numerical table method. The control group was treated with conventional chemotherapy, and the study group was treated with oxaliplatin combined with tigio-neoadjuvant che
The ORR in the study group was 80.00%, which was significantly higher than that of the control group (57.78%). In the study group, SRR was 75.56%, which was significantly higher than that of the control group (57.78%). There were 15.56% adverse reactions in the study group and 35.56% in the control group. These differences were statistically significant between the two groups.
The combination of oxaliplatin and tigio before surgery as neoadjuvant chemotherapy for patients with LAGC can effectively improve the ORR and SRR and is safe.
Core Tip: This study identified the following highlights. The objective response rate in the study was 80.00%, which was significantly higher than that of the control group (57.78%). In the study group, 75.56% of the tumors were resected, which was significantly higher than that of the control group (57.78%). There were 15.56% adverse reactions in the study group and 35.56% in the control group. These differences were statistically significant between the two groups.
- Citation: Wang T, Zhang LY. Evaluation of oxaliplatin and tigio combination therapy in locally advanced gastric cancer. World J Gastrointest Surg 2024; 16(6): 1709-1716
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1709.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1709
According to the Global Cancer Statistics Report 2022, stomach cancer is the fifth most common malignancy worldwide and one of the leading causes of cancer-related deaths[1]. As of 2022, there were approximately 1089103 new cases of gastric cancer (GC) worldwide and approximately 768793 deaths[2]. GC is usually diagnosed at advanced stages in Chinese patients, and surgical resection remains the primary choice of treatment. However, in locally advanced GC (LAGC), only a small percentage of cases are surgically resected, and the proportion of radical resections is even low[3]. After exploratory laparotomy, the opportunity for surgery is often lost because the tumor invades adjacent organs, has extensive infiltration and metastasis, or only palliative resection can be performed, and the postoperative survival rate is low[4].
With the advancement of diagnostic and treatment technology in recent years, comprehensive treatment of GC has made some progress[5]. However, there is still a 20% 5-year survival rate for LAGC, and the postoperative recurrence rate and mortality rate remain high[6]. It is estimated that around 60% of the patients with GC will have local recurrence or distant metastasis even after radical resection (R0 resection)[7]. The proportion of patients with GC in stages II-III in our country is as high as 58.0%[8]. To improve the rate of R0 resection and reduce the incidence of postoperative recurrence and metastasis, the addition of neoadjuvant therapy undoubtedly brings new hope for the survival and benefits of patients with GC.
Studies have shown that neoadjuvant chemotherapy is a type of systemic chemotherapy for patients before surgery that plays a role in shrinking tumors and facilitating follow-up treatment[9,10]. Compared with conventional treatment, neoadjuvant chemotherapy has more significant clinical efficacy, higher drug safety, better disease control effects, and higher application value in advanced tumors[11,12]. Japan is advocating a chemotherapy regimen based on tigio (S-1)[13]. Tigio, a derivative of fluorouracil, is an oral anticancer agent with definite efficacy in the adjuvant treatment of GC. Five-year survival data from a Japanese trial of tigio-assisted chemotherapy for GC confirmed an improvement in 5-year OS in patients receiving tigio-assisted chemotherapy (71.7% vs 61.1%)[14]. However, due to the limited efficacy of single-drug chemotherapy, multi-drug combination regimens are often used clinically. In the ARTIST2 trial, it was shown that postoperative adjuvant (oxaliplatin + tigio) or SOX + radiotherapy could effectively extend disease-free survival in D2-resectable stage II/ III GC patients compared to tigio monotherapy[15]. Currently, regarding the neoadjuvant treatment of LAGC, a large-scale phase III clinical trial in China has confirmed the remarkable efficacy of the neoadjuvant scheme, and thus determined that oxaliplatin plus S-1 is the first choice for neoadjuvant chemotherapy of LAGC in China[16].
In this study, we compared the effectiveness of oxaliplatin combined with a tigio neoadjuvant chemotherapy regimen to a conventional chemotherapy regimen for LAGC to further improve the clinical efficacy in patients with LAGC.
Ninety patients with clinically diagnosed LAGC between June 2022 and June 2023 were included in this study. Patients were randomly divided into a study group (45 patients) and a control group (45 patients). Both groups underwent B-ultrasonography and magnetic resonance imaging to detect abdominal lymph node metastasis, lesion infiltration, and organ metastasis. Upper gastrointestinal barium meal test revealed normal digestion.
The inclusion criteria[17] were as follows: pathologically diagnosed LAGC, aged between 18 and 75 years, did not receive chemotherapy or radiotherapy, no distant metastasis, signed the informed consent form, the expected survival time was more than 6 months, and the Karnofsky Performance Status (KPS) score was > 60 points. The exclusion criteria were as follows: those whose physical signs did not meet the standards for chemotherapy, history of GC-related diagnosis and treatment, combined with other malignant tumors, and pregnant and lactating women.
The hospital ethics committee approved informed consent forms for all chemotherapy patients.
The 90 enrolled patients underwent routine and complete examinations, routine blood tests, liver and kidney function tests, electrocardiography, and cardiac ultrasonography. Antiemetics, stomach and liver protection, and other treatments were routinely administered before medication; specifically, 30 min before medication, patients were instructed to take intramuscular diphenhydramine and intravenously administered cimetidine (300.0 mg), and dexamethasone (7.5 mg) at 21:00 the previous night and 6 h in the morning of chemotherapy, and patients were administered antiemetic treatment with drugs, such as granisetron and metoclopramide.
In the control group, conventional chemotherapy was administered, that is, on the 1st d of chemotherapy, epirubicin 75 mg/m2 + cisplatin 40 mg/m2. The treatment course was 21 d.
The study group was treated with neoadjuvant chemotherapy[15], specifically, oxaliplatin and tigio combined treatment, oxaliplatin intravenous infusion on the first day of treatment at an infusion dose of 130 mg/m2 (the first day), and tigio oral therapy at the same time at a dose of 80 mg/m2 twice a day. Both groups were treated for 21 d, and the duration of treatment for both groups was more than two courses.
Primary observation indicators: Evaluation of the objective response rate (ORR). According to RECIST1.0 standards[18]: (1) The tumor disappears completely and a complete response (CR) is achieved; (2) partial response (PR), the lesion shrinks by ≥ 50%; (3) the tumor is stable, and the lesions shrink by < 50% or increase by < 25%; and (4) tumor progression, with lesions increasing by ≥ 25%. The calculation method of clinical remission rate in each group is: (Complete remission + partial remission)/number of cases × 100%.
Surgical resection rate (SRR): Patients undergoing surgical resection/ total patients treated per group × 100%.
Secondary observation indicators: Adverse reactions: The incidence of adverse reactions in the two groups before and after treatment was analyzed. KPS scores: Before and after treatment, the KPS scores were compared between the two groups.
This group used SPSS 26.0 for the analysis and processing of the research data. Measurement data were expressed as (mean ± SD), t-tests were used to compare measurements, and count data were expressed as percentages (%). Chi-square tests were used to compare count data between groups, with P < 0.05 indicating statistically significant differences between the groups.
The research flowchart is presented in Figure 1. A total of 25 men and 20 women participated in this study. Average age was 48.8 ± 9.5 years and body mass index (BMI) was 23.50 ± 3.12. Of these patients, 4 had diabetes, 18 had hypertension, 9 had hyperlipidemia, and 2 had arrhythmia; 16 were smokers and 20 were alcoholics. A total of 24 men and 21 women participated in this study. Their average age was 49.5 ± 9.8 years and BMI was 24.01 ± 2.85. Among them, 5 had diabetes, 14 had hypertension, 11 had hyperlipidemia, and 1 had arrhythmia; 18 were smokers and 22 were alcoholics. There were no statistically significant differences in the general information between the two groups during the study period (P > 0.05), indicating comparability. Table 1 presents the results of the study.
| Index | Study group (n = 45) | Control group (n = 45) | χ2/t | P value |
| Sex | 0.044 | 0.832 | ||
| Male | 25 | 24 | ||
| Female | 20 | 21 | ||
| Age (yr) | 48.80 ± 9.50 | 49.50 ± 9.80 | 0.639 | 0.524 |
| BMI (kg/m2) | 23.50 ± 3.12 | 24.01 ± 2.85 | 0.796 | 0.427 |
| Complications (n) | 0.795 | 0.672 | ||
| Diabetes | 4 | 5 | ||
| Hypertension | 18 | 14 | ||
| Hyperlipidemia | 9 | 11 | ||
| Arrhythmia | 2 | 1 | ||
| Smoking history (n) | 0.189 | 0.664 | ||
| Yes | 16 | 18 | ||
| No | 29 | 27 | ||
| Drinking history (n) | 0.179 | 0.673 | ||
| Yes | 20 | 22 | ||
| No | 25 | 23 |
Statistical analysis of the recent treatment efficacy in the two groups of patients revealed that among the 45 patients in the study group, 11 were classified as CR, 25 as PR, 6 cases as stable disease (SD), and 3 cases as progressive disease (PD). The ORR was 80.00% (36/45), and the disease control rate (DCR) was 93.33% (42/45). In the control group, there were 9 patients with CR, 17 with PR, 12 with SD, and 7 with PD. The ORR was 57.78% (26/45) and the DCR was 84.44% (38/45). The clinical efficacy was higher in the study group than that in the control group, and statistically significant differences were observed between the two groups (P < 0.05) (Table 2).
| Index | Study group (n = 45) | Control group (n = 45) | χ2 | P value | ||
| n | % | n | % | |||
| CR | 11 | 24.44 | 9 | 20.00 | ||
| PR | 25 | 55.56 | 17 | 37.78 | ||
| SD | 6 | 13.33 | 12 | 26.67 | ||
| PD | 3 | 6.67 | 7 | 15.56 | ||
| ORR (CR + PR) | 36 | 80.00 | 26 | 57.78 | 11.519 | 0.011 |
| DCR (CR + PR + SD) | 42 | 93.33 | 38 | 84.44 | 4.215 | 0.016 |
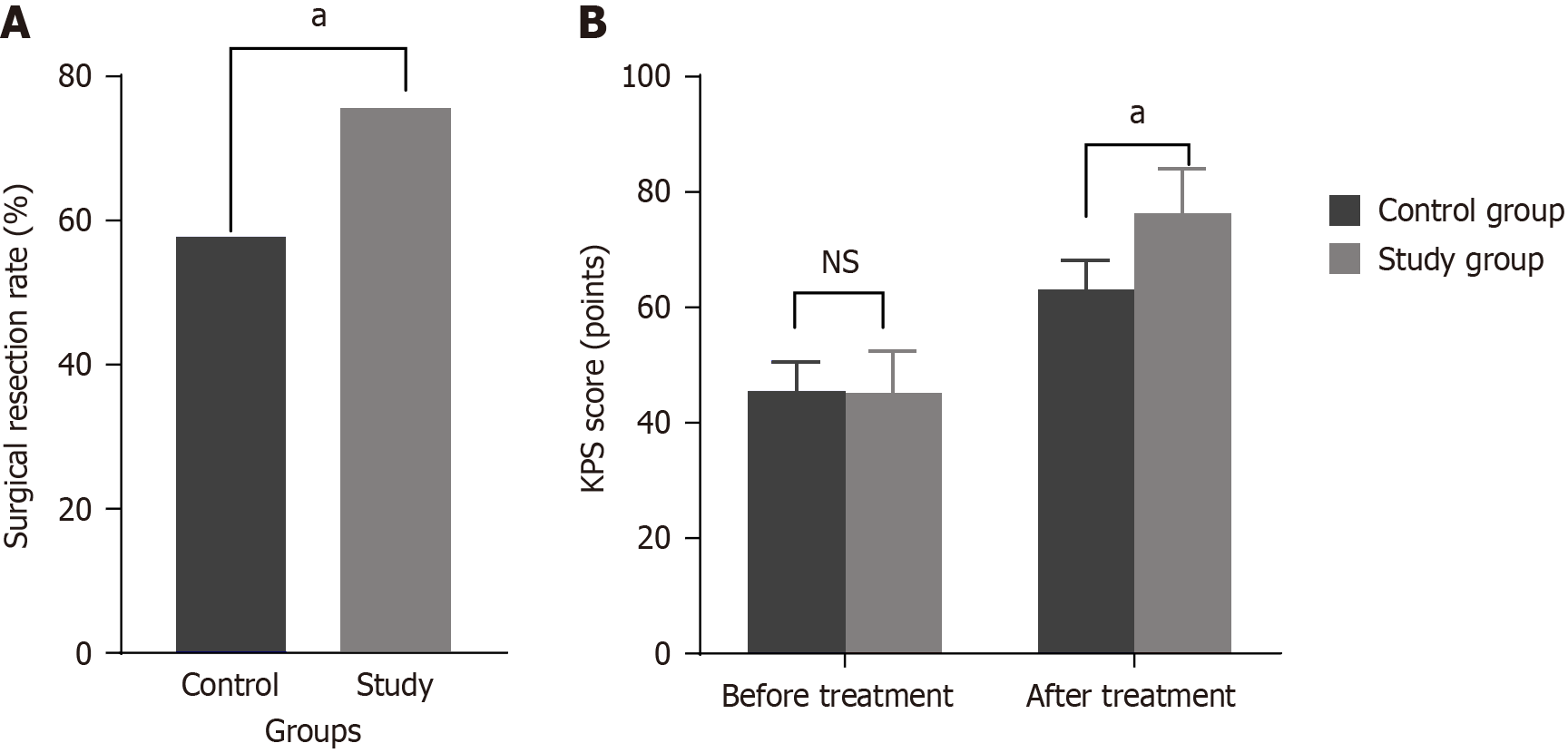
The study group had a significantly higher resection rate of 75.56% than that of the control group (57.78%). The differences between the groups were statistically significant (P < 0.05). The results are presented in Figure 2A.
Compared with that before treatment, a significant improvement in KPS scores was observed in the study group (P < 0.05). The results are presented in Figure 2B.
Based on the statistical results, 15.56% of the study group experienced adverse reactions compared to 35.56% of the control group (P < 0.05), indicating a statistically significant difference. as shown in Table 3.
| Index | Study group (n = 45) | Control group (n = 45) | χ2 | P value | ||
| n | % | n | % | |||
| Abnormal liver function | 1 | 2.22 | 5 | 11.11 | ||
| Nausea and vomiting | 3 | 6.67 | 6 | 13.33 | ||
| Diarrhoea | 2 | 4.44 | 3 | 6.67 | ||
| Decreased white blood cells | 1 | 2.22 | 2 | 4.44 | ||
| Total | 7 | 15.56 | 16 | 35.56 | 10.512 | < 0.001 |
LAGC is a common malignant tumor in clinical practice. Most patients are asymptomatic in the early stages and are often treated at a later stage because of gastrointestinal reactions[19]. After gastroscopy and pathological examination, these tumors are often found in the middle and late stages, thus losing the best period for surgery[20]. Neoadjuvant chemotherapy has become a new treatment method for some patients with middle and advanced malignant tumors in recent years, aiming to control and shrink the progression of the lesions through chemotherapy and then achieve therapeutic purpose through surgical treatment[21]. This type of therapy mainly uses a combination of two or three drugs; however, the number of drugs must be selected according to the patient’s body indicators and tolerance before treatment[22].
The results of this study showed that the ORR of the study group was 80.00% after treatment, which was significantly higher than that of the control group (57.78%) (P < 0.05). Compared with the control group, the study group had a resection rate of 75.56%, while the control group had a resection rate of 57.78%, and the two groups differed significantly
According to this study, the study group experienced adverse reactions at a rate of 15.56% compared with the control group’s 35.56%, which was statistically significant (P < 0.05). There was a significantly greater improvement in KPS score in the research group than that in the control group, with P < 0.05, which is in general agreement with the findings of Cui et al[26] and Dimpel et al[27]. This may be because the combined application of oxaliplatin and tigio can play a synergistic role in enhancing the antitumor effect. Oxaliplatin is a platinum-based anticancer drug that inhibits DNA replication and transcription, thereby inhibiting the proliferation of tumor cells. Tigio is an oral fluorouracil analog that inhibits the growth of tumor cells by inhibiting enzymes, such as thymidylate synthase, which interferes with DNA synthesis. The combined use of these two drugs is advantageous and improves their therapeutic effects. Simultaneously, the oral administration of tigio is more convenient than intravenous administration, and its metabolites are less toxic to normal cells, which can reduce the incidence of adverse reactions.
This study has several limitations. First, this was a single-center study, and a selection bias may have influenced the results. As a retrospective study, there were some limitations, such as the lack of a thorough research plan and information bias. Second, this study did not include indicators of blood drawing in patients before and after surgery. If more detailed biochemical indicators are available and their data statistics and analyses are performed, it may further explain why neoadjuvant chemotherapy does not increase the incidence of recent postoperative complications. Third, the sample size of this study was small, and the representation of the whole population was limited; therefore, this study still needs to be verified by a prospective study with a larger sample size.
In conclusion, for patients with LAGC, compared with conventional chemotherapy, oxaliplatin combined with tigio as a preoperative neoadjuvant chemotherapy regimen can effectively increase the ORR and SRR, has certain safety, and improve its clinical therapeutic effect.
| 1. | Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 678] [Article Influence: 226.0] [Reference Citation Analysis (0)] |
| 2. | Röcken C. Predictive biomarkers in gastric cancer. J Cancer Res Clin Oncol. 2023;149:467-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 96] [Reference Citation Analysis (5)] |
| 3. | Rao X, Zhang C, Luo H, Zhang J, Zhuang Z, Liang Z, Wu X. Targeting Gastric Cancer Stem Cells to Enhance Treatment Response. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 4. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 957] [Article Influence: 319.0] [Reference Citation Analysis (0)] |
| 5. | Zhou Y, Zhou Y, Lin X, Lin S, Li W. New strategy in hemorrhagic gastric cancer: A case report of complete pathological remission after neoadjuvant chemotherapy. Medicine (Baltimore). 2023;102:e32789. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Kim HD, Ryu MH, Kang YK. Adjuvant treatment for locally advanced gastric cancer: an Asian perspective. Gastric Cancer. 2024;27:439-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 7. | Zhang J, Cui Y, Wei K, Li Z, Li D, Song R, Ren J, Gao X, Yang X. Deep learning predicts resistance to neoadjuvant chemotherapy for locally advanced gastric cancer: a multicenter study. Gastric Cancer. 2022;25:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 8. | Yin Y, Lin Y, Yang M, Lv J, Liu J, Wu K, Liu K, Li A, Shuai X, Cai K, Wang Z, Wang G, Shen J, Zhang P, Tao K. Neoadjuvant tislelizumab and tegafur/gimeracil/octeracil (S-1) plus oxaliplatin in patients with locally advanced gastric or gastroesophageal junction cancer: Early results of a phase 2, single-arm trial. Front Oncol. 2022;12:959295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 9. | Yıldız İ, Özer L, Şenocak Taşçı E, Bayoglu İV, Aytac E. Current trends in perioperative treatment of resectable gastric cancer. World J Gastrointest Surg. 2023;15:323-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (0)] |
| 10. | Wang KX, Cui TY, Yang XD, Wang GQ, Jiang QS, Sun H, Jiang NY, Yong XM, Shi CB, Ding YB, Chen XF, Fang YY. Study on Efficacy and Safety of Low-Dose Apatinib Combined with Camrelizumab and SOX Regimen as First-Line Treatment of Locally Advanced and Unresectable Gastric/Gastroesophageal Junction Cancer: A Protocol for an Open-Label, Dose Escalation and Extension Phase Ib Clinical Trial. Onco Targets Ther. 2021;14:4859-4865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Song R, Cui Y, Ren J, Zhang J, Yang Z, Li D, Li Z, Yang X. CT-based radiomics analysis in the prediction of response to neoadjuvant chemotherapy in locally advanced gastric cancer: A dual-center study. Radiother Oncol. 2022;171:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Sisic L, Crnovrsanin N, Nienhueser H, Jung JO, Schiefer S, Haag GM, Bruckner T, Schneider M, Müller-Stich BP, Büchler MW, Schmidt T. Perioperative chemotherapy with 5-FU, leucovorin, oxaliplatin, and docetaxel (FLOT) for esophagogastric adenocarcinoma: ten years real-life experience from a surgical perspective. Langenbecks Arch Surg. 2023;408:81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K; ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1943] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 14. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1089] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 15. | Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, Kang JH, Oh SY, Hwang IG, Ji JH, Shin DB, Yu JI, Kim KM, An JY, Choi MG, Lee JH, Kim S, Hong JY, Park JO, Park YS, Lim HY, Bae JM, Kang WK; ARTIST 2 investigators. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial(☆). Ann Oncol. 2021;32:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 16. | Yu J, Wang Z, Li Z, Liu Y, Fan Y, Di J, Cui M, Xing J, Zhang C, Yang H, Yao Z, Zhang N, Chen L, Liu M, Xu K, Tan F, Gao P, Su X. Health-Related Quality of Life in Patients With Locally Advanced Gastric Cancer Undergoing Perioperative or Postoperative Adjuvant S-1 Plus Oxaliplatin With D2 Gastrectomy: A Propensity Score-Matched Cohort Study. Front Oncol. 2022;12:853337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Schulz C, Kullmann F, Kunzmann V, Fuchs M, Geissler M, Vehling-Kaiser U, Stauder H, Wein A, Al-Batran SE, Kubin T, Schäfer C, Stintzing S, Giessen C, Modest DP, Ridwelski K, Heinemann V. NeoFLOT: Multicenter phase II study of perioperative chemotherapy in resectable adenocarcinoma of the gastroesophageal junction or gastric adenocarcinoma-Very good response predominantly in patients with intestinal type tumors. Int J Cancer. 2015;137:678-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Schirren R, Novotny A, Friess H, Reim D. Histopathologic Response Is a Positive Predictor of Overall Survival in Patients Undergoing Neoadjuvant/Perioperative Chemotherapy for Locally Advanced Gastric or Gastroesophageal Junction Cancers-Analysis from a Large Single Center Cohort in Germany. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Shitara K, Lordick F, Bang YJ, Enzinger P, Ilson D, Shah MA, Van Cutsem E, Xu RH, Aprile G, Xu J, Chao J, Pazo-Cid R, Kang YK, Yang J, Moran D, Bhattacharya P, Arozullah A, Park JW, Oh M, Ajani JA. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2023;401:1655-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 330] [Article Influence: 165.0] [Reference Citation Analysis (0)] |
| 20. | Liu L, Wang C, Li F, Zhang X, Cheng X, Lin S, Liu Y, Yang C, Li W. The safety and efficacy of laparoscopic gastrectomy for patients with locally advanced gastric cancer following neoadjuvant chemotherapy. Sci Rep. 2022;12:10384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Lin W, Huang Z, Du Z, Wang Y, Zuo T. Case Report: Clinical application of continuous arterial infusion chemotherapy in neoadjuvant therapy for locally advanced gastric cancer. Front Oncol. 2023;13:1214599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Li N, Wang X, Tang Y, Zhao D, Chi Y, Yang L, Jiang L, Jiang J, Liu W, Fang H, Liu Y, Song Y, Wang S, Jin J, Li Y. A prospective phase I study of hypo-fractionated neoadjuvant radiotherapy for locally advanced gastric cancer. BMC Cancer. 2018;18:803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Ding PA, Liu Y, Guo HH, Yang PG, Tian Y, Fan LQ, Tan BB, Li Y, Zhao Q. [Application of laparoscopic exploration combined with abdominal exfoliative cytology in the diagnosis and treatment of locally advanced gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Gervaso L, Pellicori S, Cella CA, Bagnardi V, Lordick F, Fazio N. Biomarker evaluation in radically resectable locally advanced gastric cancer treated with neoadjuvant chemotherapy: an evidence reappraisal. Ther Adv Med Oncol. 2021;13:17588359211029559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Deng J, Zhang W, Xu M, Zhou J. Imaging advances in efficacy assessment of gastric cancer neoadjuvant chemotherapy. Abdom Radiol (NY). 2023;48:3661-3676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Cui Y, Zhang J, Li Z, Wei K, Lei Y, Ren J, Wu L, Shi Z, Meng X, Yang X, Gao X. A CT-based deep learning radiomics nomogram for predicting the response to neoadjuvant chemotherapy in patients with locally advanced gastric cancer: A multicenter cohort study. EClinicalMedicine. 2022;46:101348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 102] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 27. | Dimpel R, Novotny A, Slotta-Huspenina J, Langer R, Friess H, Reim D. UICC Staging after Neoadjuvant/Perioperative Chemotherapy Reveals No Significant Survival Differences Compared to Primary Surgery for Locally Advanced Gastric Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |