Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1691
Revised: May 1, 2024
Accepted: May 21, 2024
Published online: June 27, 2024
Processing time: 124 Days and 18.5 Hours
Given the current organ shortage crisis, split liver transplantation (SLT) has emerged as a promising alternative for select end-stage liver disease patients.
To introduce an ex-vivo liver graft splitting approach and evaluate its safety and feasibility in SLT.
A retrospective analysis was conducted on the liver transplantation data from cases performed at our center between April 1, 2022, and May 31, 2023. The study included 25 SLT cases and 81 whole liver transplantation (WLT) cases. Total ex-vivo liver splitting was employed for SLT graft procurement in three steps. Patient outcomes were determined, including liver function parameters, postoperative complications, and perioperative mortality. Group comparisons for categorical variables were performed using the χ²-test.
In the study, postoperative complications in the 25 SLT cases included hepatic artery thrombosis (n = 1) and pulmonary infections (n = 3), with no perioperative mortality. In contrast, among the 81 patients who underwent WLT, complications included perioperative mortality (n = 1), postoperative pulmonary infections
Our findings suggest that the total ex-vivo liver graft splitting technique is a safe and feasible approach, especially under the expertise of an experienced transplant center. The approach developed by our center can serve as a valuable reference for other transplantation centers.
Core Tip: Split liver transplantation has become a routine procedure at many transplant centers, and there are currently two main approaches for the generation of split-liver allografts: In-situ splitting and ex-vivo splitting. While in-situ splitting, which involves liver division within the organ donor’s body before procurement, is the prevailing technique adopted by most transplant centers, the utilization of ex-vivo splitting, wherein the liver is divided after procurement, remains limited. Our findings suggest that the ex-vivo liver graft splitting technique is a safe and feasible approach, especially under the expertise of an experienced transplant center.
- Citation: Zhao D, Xie QH, Fang TS, Zhang KJ, Tang JX, Yan X, Jin X, Xie LJ, Xie WG. How to apply ex-vivo split liver transplantation safely and feasibly: A three-step approach. World J Gastrointest Surg 2024; 16(6): 1691-1699
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1691.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1691
Given the current organ shortage crisis, split liver transplantation (SLT) has emerged as a promising alternative for select patients with end-stage liver disease[1-4], offering clinical outcomes akin to those achieved through whole liver transplantation (WLT)[5-7]. The techniques for SLT involve primarily splitting off the left lateral section and the right trisegment, followed by further partitioning into the left and right hemi-livers or liver segments, contingent on the compatibility conditions between the donor and recipient[8,9].
There are currently two main approaches for the generation of split-liver allografts: In-situ splitting and ex-vivo splitting. While in-situ splitting, which involves liver division within the organ donor’s body before procurement, is the prevailing technique adopted by most transplant centers, the utilization of ex-vivo splitting, wherein the liver is divided after procurement, remains limited[4,10,11]. Despite its potential benefits, ex-vivo splitting is currently employed by only a few specialized centers. Ding et al[12] previously reported that out of 11 liver grafts, only 2 (18.2%) underwent ex-vivo splitting. Similarly, Xu et al[13] performed only 20 (14.3%) SLT procedures out of the 140 liver transplantations.
Interestingly, SLT has become a routine procedure at our transplant center, and the total ex-vivo liver graft splitting technique has become our preferred approach. Despite the significance of ex-vivo liver graft splitting, there are few detailed reports on this splitting technique. To address this knowledge gap, our present study presents a comprehensive summary of our center’s practice and technical approach to ex-vivo liver graft splitting, aiming to evaluate its safety and feasibility and provide a reference for surgeons in other transplant centers.
Clinical data from 122 liver transplantation cases performed at Shenzhen Third People’s Hospital were initially collected between April 1, 2022, and May 31, 2023. The study enrolled 81 cases of WLT, 16 cases of living-donor liver transplantation, and 25 cases of SLT. A total of 106 cases, comprising of SLT and WLT recipients, were eventually included in our study. Comprehensive data, including clinical records, surgical reports, laboratory findings, and imaging results, were obtained for each case. Liver function parameters, incidence of surgical complications, and perioperative mortality rate were independently analyzed for the SLT and WLT groups. All the patients provided informed consent before operation, and the study was approved by the ethics committee of Shenzhen Third People’s Hospital (No. 2022-133).
Before organ procurement, all potential organ donors received comprehensive preoperative evaluations, including complete blood counts, liver function tests, renal function tests, infectious disease pathogen screening, and inflammation marker testing. Additionally, imaging studies, such as liver ultrasound or contrast-enhanced computed tomography (CT) scans, were performed.
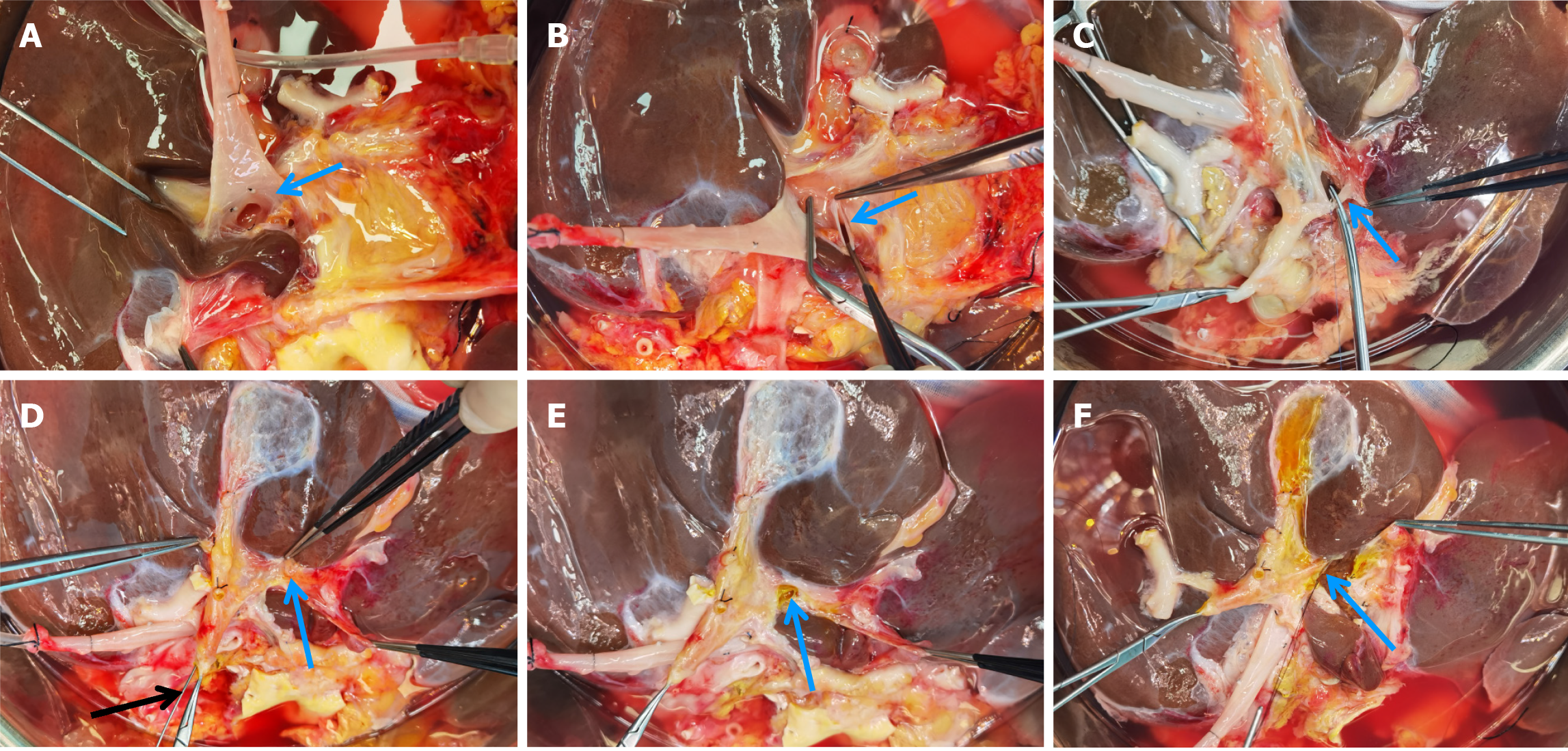
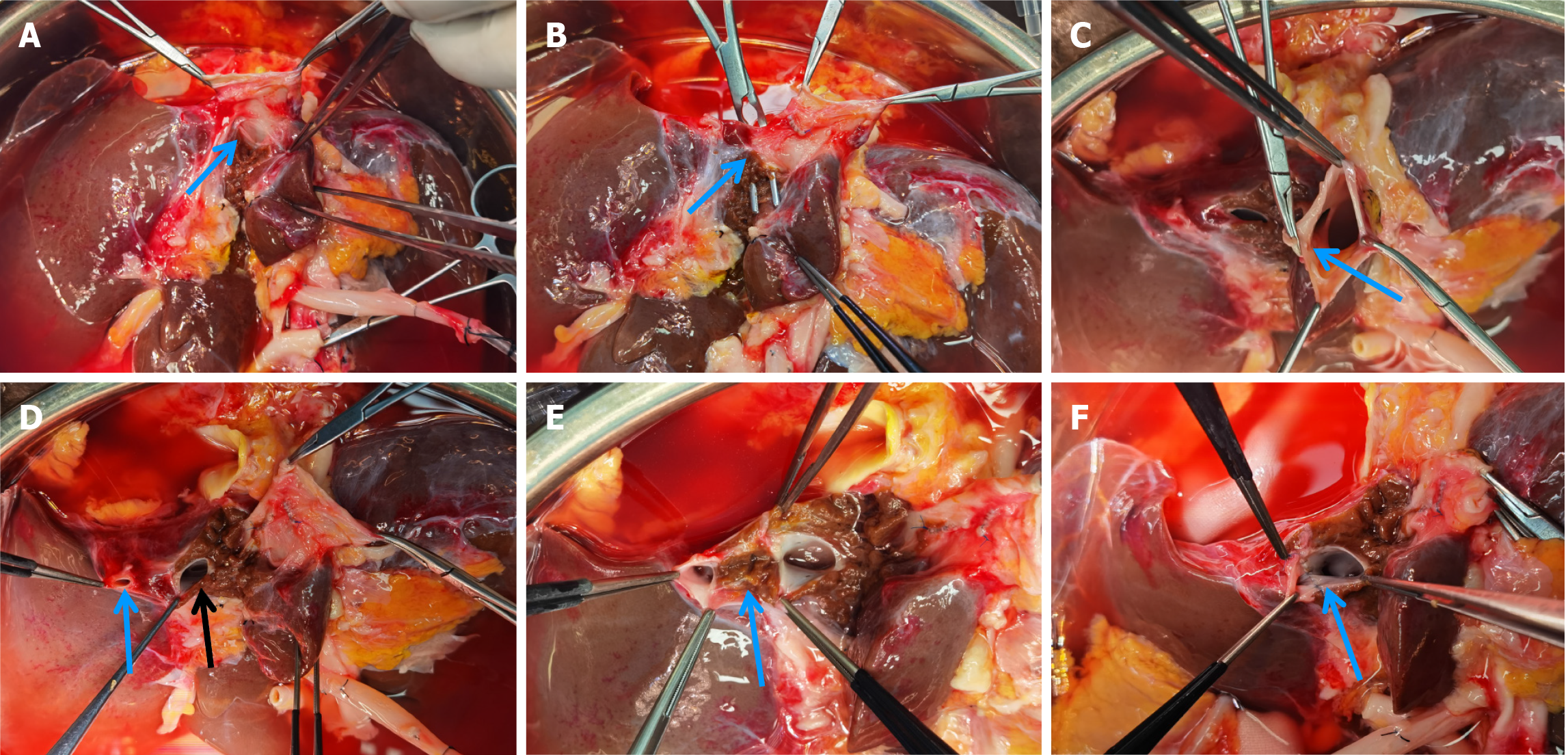
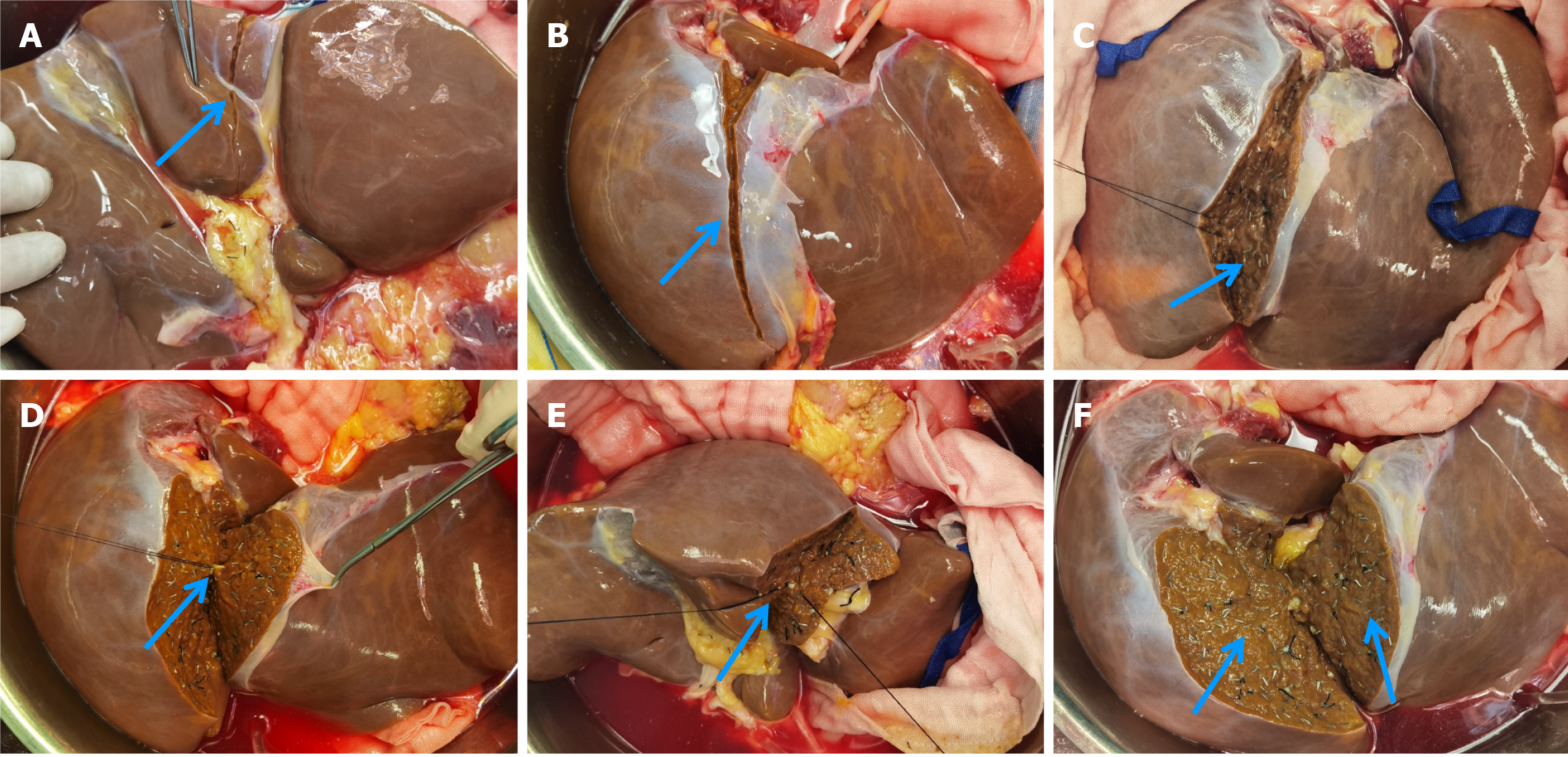
The liver graft splitting procedure was performed using the total ex-vivo splitting technique. Following liver procurement, the donor liver was partitioned while immersed in a cold storage solution. The splitting of the left lateral section and the right trisegment involved three main steps: (1) Division of the first porta hepatis: The anatomical structures of the first porta hepatis were dissected, followed by the separate division of the left branch of the portal vein and the left branch of the hepatic artery. Next, the division site of the left hepatic duct was identified under biliary probe guidance. Following bile duct resection, the splitting line on the visceral surface of the liver was marked (Figure 1); (2) Division of the second porta hepatis: The suprahepatic inferior vena cava was gently elevated, and the root of the left hepatic vein was bluntly separated to fully expose the site where the left hepatic vein joins the inferior vena cava. After dividing the left hepatic vein, the surface splitting line of the liver on the diaphragmatic aspect was marked, connecting it to the visceral surface splitting line (Figure 2); and (3) Division of liver parenchyma: A clamp-crushing technique was utilized for dividing the liver parenchyma to avoid thermal injury to liver tissues. Smaller vessels were ligated with titanium clips, while larger vessels were ligated using silk or Prolene sutures. Throughout the procedure, continuous monitoring of anatomical structures with positional changes was performed to ascertain the precise division plane and avoid injuries to critical intrahepatic structures (Figure 3). After completing the division of the right trisegment and the left lateral section, the caudate lobe on the left side of the inferior vena cava was excised. The surgical procedure depicted above is further detailed in Figures 1-3.
All statistical analyses were performed using SPSS 24.0 statistical software. The descriptive statistics are expressed as frequencies (%) for categorical variables, and median (interquartile range) for continuous variables. Group comparisons for categorical variables were performed using the χ²-test. For metric variables, the Mann-Whitney U test was used. A two-sided P-value < 0.05 was considered statistically significant.
Between April 1, 2022 and May 31, 2023, 13 liver grafts were subjected to splitting. These grafts were procured from 13 brain-dead organ donors, with all exhibiting hemodynamic stability preoperatively, with minimal or no use of vasoactive drugs. The median age of the liver donors was 31 years, and they had a median preoperative total bilirubin (TB) level of 20.76 μmol/L, median alanine aminotransferase (ALT) level of 43.3 U/L, median aspartate aminotransferase (AST) level of 83 U/L, and median intensive care unit (ICU) stay duration of 4 d.
The liver graft splitting procedure was conducted using the total ex-vivo splitting technique, whereby both the left lateral section and the right trisegment were divided in all cases. Following the procedure, 26 liver segments were obtained (13 left lateral sections and 13 right trisegments). Among these liver segments, 25 were allocated to our transplant center by the China Organ Transplant Response System, while one right trisegment was given to another transplant center. During the liver graft splitting procedure for the 12 cases in which the right trisegment was utilized for liver transplantation, the caudate lobe located on the left side of the inferior vena cava was consistently excised. Further details regarding the donor liver information can be found in Table 1.
| No. | Gender | Age (yr) | Type of donation | Cause of death | Preoperative Na+ concentration (mmol/L) | Preoperative TB (μmol/L) | Preoperative ALT (U/L) | Preoperative AST (U/L) | ICU stay duration (d) |
| 1 | Male | 31 | DBD | Craniocerebral injury | 143 | 57.80 | 37.0 | 41.0 | 3 |
| 2 | Female | 12 | DBD | Hypoxic-ischemic encephalopathy | 140 | 13.40 | 42.0 | 53.0 | 4 |
| 3 | Female | 42 | DBD | Craniocerebral injury | 146 | 50.80 | 45.0 | 83.0 | 5 |
| 4 | Male | 44 | DBD | Cerebral hemorrhage | 143 | 45.30 | 18.0 | 37.0 | 4 |
| 5 | Male | 36 | DBD | Craniocerebral injury | 150 | 13.80 | 131.0 | 172.0 | 4 |
| 6 | Male | 34 | DBD | Cerebral hemorrhage | 156 | 23.40 | 267.5 | 293.2 | 2 |
| 7 | Male | 12 | DBD | Hypoxic-ischemic encephalopathy | 136 | 13.00 | 43.3 | 93.1 | 11 |
| 8 | Male | 31 | DBD | Cerebral hemorrhage | 150 | 26.80 | 15.0 | 25.0 | 10 |
| 9 | Male | 25 | DBD | Craniocerebral injury | 143 | 54.50 | 177.0 | 83.0 | 4 |
| 10 | Female | 9 | DBD | Hypoxic-ischemic encephalopathy | 148 | 3.05 | 103.0 | 227.0 | 10 |
| 11 | Male | 8 | DBD | Hypoxic-ischemic encephalopathy | 149 | 10.21 | 22.7 | 22.9 | 4 |
| 12 | Female | 29 | DBD | Cerebral hemorrhage | 148 | 20.76 | 37.0 | 42.0 | 7 |
| 13 | Male | 40 | DBD | Cerebral hemorrhage | 132 | 12.20 | 166.0 | 85.0 | 5 |
All 106 Liver transplant procedures retrospectively analyzed in this study were successfully performed. The age of WLT cases was younger than that of SLT cases (49.00 vs 1.83, P = 0.001), and there were more decompensated cirrhosis recipients in WLT cases (48 vs 5, P = 0.001). Out of the 81 WLT cases, 66 were carried out using the classic in-situ liver transplantation technique, while the remaining 15 utilized the modified piggyback liver transplantation technique. As for the 25 SLT cases, 13 pediatric recipients received left lateral section grafts, and 7 adult and 5 pediatric recipients received right trisegment grafts. In the 12 patients who underwent SLT with the right trisegment graft, we conducted the removal of ischemic hepatic tissue from Segment IV while preserving the middle hepatic vein during the surgical procedure.
Among the 81 cases of WLT, one perioperative death occurred, while the remaining patients were successfully discharged. The postoperative complications primarily included pulmonary infections in 8 cases (9.9%), intra-abdominal infections in 1 (1.2%), incisional infections in 1 (1.2%), herpes zoster infection in 1 (1.2%), hepatic artery thrombosis in 3 (3.7%), portal vein thrombosis in 1 (1.2%), intra-abdominal bleeding in 5 (6.2%), graft-versus-host disease (GVHD) in 1 (1.2%), and acute kidney injury in 1 (1.2%). The patient with intra-abdominal bleeding underwent exploratory laparotomy to achieve hemostasis, while those with hepatic artery or portal vein thrombosis received surgical thrombectomy. The patient who experienced GVHD passed away on postoperative day 56 despite aggressive treatment. Subsequent follow-ups, ranging from 2 to 15 mo, revealed that 76 patients recovered well and had no abnormalities.
Among the 25 subjects that underwent SLT, one experienced hepatic artery thrombosis on postoperative day 3, which was successfully treated by surgical thrombectomy, leading to a favorable recovery. Another three patients developed postoperative pulmonary infections, but there were no instances of bile leakage or intestinal leakage, and no perioperative deaths were reported. All 25 patients were discharged without complications and showed no abnormalities during a follow-up period ranging from 4 to 15 mo.
A comparison of postoperative data between SLT and WLT revealed statistically significant differences in ALT (176.0 vs 73.5, P = 0.000) and AST (42.0 vs 29.0, P = 0.004) levels at 1 wk post-surgery. Additionally, at 2 wk post-surgery, there were statistically significant differences in TB (11.8 vs 20.8, P = 0.003) and AST (41.5 vs 26.0, P = 0.014) levels. However, no statistically significant difference was observed in the overall incidence of postoperative complications between the two groups (P > 0.05). Further details can be found in Table 2.
| Split liver transplantation (n = 25) | Whole liver transplantation (n = 81) | P value | |
| Gender (male/female) | 17/8 | 65/16 | 0.273 |
| Age (yr) | 1.83 (0.55, 43.00) | 49 (40.50, 55.00) | 0.001 |
| Underlying diseases | |||
| Decompensated cirrhosis | 5 | 48 | 0.001 |
| Liver cancer | 2 | 15 | 0.210 |
| One-week postoperative indicators | |||
| TB | 26.400 (12.950, 34.350) | 28.250 (17.525, 48.925) | 0.274 |
| ALT | 176.0 (81.5, 259.5) | 73.5 (43.5, 115.5) | 0.000 |
| AST | 42.00 (32.00, 79.00) | 29.00 (20.25, 49.25) | 0.004 |
| GGT | 139.0 (102.5, 227.5) | 118.0 (64.0, 174.0) | 0.117 |
| Two-week postoperative indicators | |||
| TB | 11.80 (7.95, 20.55) | 20.80 (15.20, 26.30) | 0.003 |
| ALT | 63.0 (29.5, 82.5) | 40.0 (21.0, 82.0) | 0.154 |
| AST | 41.5 (20.5, 61.5) | 26.0 (17.0, 41.0) | 0.014 |
| GGT | 81.0 (54.5, 182.5) | 114 (56.0, 201.0) | 0.528 |
| Postoperative complications | 0.584 | ||
| Intra-abdominal bleeding | 0 | 5 | |
| Hepatic artery thrombosis | 1 | 3 | |
| Pulmonary infections | 3 | 8 | |
| Abdominal infection | 0 | 1 | |
| Bile leakage | 0 | 0 | |
| Intestinal leakage | 0 | 0 | |
| 30-d postoperative mortality | 0 | 1 |
In the face of a critical shortage of available donor organs, SLT represents a valuable approach to address this pressing issue. SLT involves the division of a single high-quality liver into two parts, thereby saving the lives of two recipients[14]. The success of SLT hinges on ensuring that each split portion of the liver maintains intact anatomical structures, encompassing the inflow vessels (hepatic artery and portal vein), outflow vessels (hepatic veins), and biliary tract. Additionally, adherence to conventional criteria for SLT, such as the graft-to-recipient weight ratio (GRWR), is crucial. Typically, a GRWR greater than 1% for adults[8,15] and between 2% to 4% for children is recommended. In our study, all the 25 recipients of SLT met these criteria and did not experience postoperative complications such as large-for-size or small-for-size graft syndromes. However, some scholars reported that the ideal graft weight is approximately 1-3% of the recipient weight[16].
Different transplant centers primarily adopt either in-situ or ex-vivo splitting approaches. Reyes et al[17] previously reported that the survival rates of recipients undergoing in-situ and ex-vivo liver splitting were comparable and similar to the survival rates of WLT recipients, in line with the findings of our study. Our literature review revealed that the majority of transplant centers have a preference for the in-situ liver-splitting approach[8,12,18]. In-situ liver splitting involves performing the procedure within the donor’s body for liver procurement. This approach offers several advantages[19,20], including shorter cold ischemia time, simultaneous hemostasis during liver parenchymal transection, and facilitated intraoperative cholangiography. However, it may also have certain drawbacks, such as potential delays in procuring other organs and the need for coordination between transplant centers. Recent literature has explored the use of normothermic perfusion devices for liver splitting, which holds the potential to mitigate some of the limitations associated with in-situ splitting. Although this technology shows promise, it has not yet been widely adopted in clinical practice, and its clinical effectiveness requires further observation and research[21].
By contrast, ex-vivo splitting, which we primarily use, avoids these drawbacks. However, it requires a skilled surgical team familiar with ex-vivo liver anatomy to prevent damage to critical structures. Although some literature reported a higher incidence of biliary and vascular complications in adult recipients undergoing ex-vivo splitting compared to in-situ splitting[22], in our study, out of the 25 cases of SLT, only one adult recipient suffered from hepatic artery thrombosis postoperatively. Besides, there was no incidence of other biliary or vascular complications in the remaining cases. Importantly, the overall incidence of postoperative complications showed no statistically significant difference between the SLT and WLT groups (P > 0.05). As mentioned in the Methods section, recipients undergoing liver right trisegment graft surgery had the caudate lobe and ischemic segment IV of the liver excised during the procedure, likely contributing to the absence of bile leakage and intra-abdominal infections postoperatively[23,24].
Postoperative liver function tests revealed statistically significant differences between the SLT group and the WLT group in ALT (176.0 vs 73.5, P = 0.000) and AST (42.0 vs 29.0, P = 0.004) levels at 1 wk postoperatively, as well as in TB (11.8 vs 20.8, P = 0.003) and AST (41.5 vs 26.0, P = 0.014) levels at 2 wk after surgery. Herein, the higher postoperative ALT and AST levels observed in the SLT group at 1 wk and the elevated AST level at 2 wk might be associated with ischemic necrosis on the transection plane of the liver. Although the difference in TB at 2 wk showed statistical significance, both groups had median values within the normal range, indicating good postoperative liver function.
The safety of SLT relies not only on a surgical team with extensive experience but also on a comprehensive evaluation and careful selection of the donor liver prior to the surgery. Several studies[12,14,25] have emphasized the importance of choosing relatively young donors with stable hemodynamics, short ICU stays, no significant steatosis or infections, and no apparent vascular or biliary anomalies. At our center, we adhere to specific criteria for selecting split liver donors, which include individuals under 45 years of age (with a median age of 31 years in this study) exhibiting stable hemodynamics, absence of significant steatosis or infections, and no apparent vascular or biliary anomalies. It has been reported that intraoperative cholangiography is a necessary examination[14,20], but we did not perform intraoperative cholangiography in this study, because no bile duct variation was found before surgery. However, it is essential to note that cholangiography should be considered if suspicious ductal structures are encountered during the surgery. In the study, all 25 recipients had no relevant biliary complications postoperatively.
In this retrospective analysis of consecutive ex-vivo SLT cases conducted over the past year, the methods and steps of ex-vivo liver graft splitting technique were summarized in detail, and our study demonstrated that the total ex-vivo liver splitting approach with three steps is safe and feasible, especially when performed in experienced transplant centers. Importantly, this approach has been found to address concerns associated with the geographical distance between organ donor hospitals and transplant centers, as well as potential risks of prolonged surgical duration during organ procurement and potential harm to other donated organs. However, follow-up studies with large samples are warranted due to the relatively small number of cases, in order to allow more donor livers suitable for cleavage to be split and to benefit more liver transplant recipients.
We thank all the patients for cooperating with our investigation, and we thank professor Nan Ma for his contribution to the operation.
| 1. | Smith SK, Miloh T. Pediatric Liver Transplantation. Clin Liver Dis. 2022;26:521-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 2. | Perkins JD, Dick AA, Healey PJ, Montenovo MI, Biggins SW, Sibulesky L, Reyes JD. New Evidence Supporting Increased Use of Split Liver Transplantation. Transplantation. 2020;104:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Valentino PL, Emre S, Geliang G, Li L, Deng Y, Mulligan D, Rodriguez-Davalos MI. Frequency of whole-organ in lieu of split-liver transplantation over the last decade: Children experienced increased wait time and death. Am J Transplant. 2019;19:3114-3123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Herden U, Fischer L, Koch M, Li J, Achilles EG, Nashan B. Outcome following right-extended split liver transplantation in the recent transplant era: Single-center analysis of a German transplant center. Clin Transplant. 2018;32:e13288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Gambaro SE, Romero P, Pedraza N, Moulin L, Yantorno S, Ramisch D, Rumbo C, Barros-Schelotto P, Descalzi V, Gondolesi GE. Right Extended Split Liver Transplantation Compared With Whole Liver Transplantation: Lessons Learned at a Single Center in Latin America-Results From a Match Case-Control Study. Transplant Proc. 2017;49:2122-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Doyle MB, Maynard E, Lin Y, Vachharajani N, Shenoy S, Anderson C, Earl M, Lowell JA, Chapman WC. Outcomes with split liver transplantation are equivalent to those with whole organ transplantation. J Am Coll Surg. 2013;217:102-12; discussion 113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Hong JC, Yersiz H, Farmer DG, Duffy JP, Ghobrial RM, Nonthasoot B, Collins TE, Hiatt JR, Busuttil RW. Longterm outcomes for whole and segmental liver grafts in adult and pediatric liver transplant recipients: a 10-year comparative analysis of 2,988 cases. J Am Coll Surg. 2009;208:682-9; discusion 689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Zhao D, Zhang KJ, Fang TS, Yan X, Jin X, Liang ZM, Tang JX, Xie LJ. Topological approach of liver segmentation based on 3D visualization technology in surgical planning for split liver transplantation. World J Gastrointest Surg. 2022;14:1141-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Sneiders D, van Dijk ARM, Polak WG, Mirza DF, Perera MTPR, Hartog H. Full-left-full-right split liver transplantation for adult recipients: a systematic review and meta-analysis. Transpl Int. 2021;34:2534-2546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Kilic M, Seu P, Stribling RJ, Ghalib R, Goss JA. In situ splitting of the cadaveric liver for two adult recipients. Transplantation. 2001;72:1853-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Vagefi PA, Parekh J, Ascher NL, Roberts JP, Freise CE. Ex vivo split-liver transplantation: the true right/left split. HPB (Oxford). 2014;16:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Ding L, Yu X, Zhang R, Qian J, Zhang W, Wu Q, Zhou L, Yang Z, Zheng S. Full-Right Full-Left Split Liver Transplantation for Two Adult Recipients: A Single-Center Experience in China. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 13. | Xu M, Dong C, Sun C, Wang K, Zhang W, Qin H, Han C, Yang Y, Zhang F, Wang Z, Zheng W, Wei X, Gao W, Shen Z. Impact of donor age on short-term outcomes after pediatric split liver transplantation. Front Pediatr. 2023;11:1131629. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Hashimoto K, Fujiki M, Quintini C, Aucejo FN, Uso TD, Kelly DM, Eghtesad B, Fung JJ, Miller CM. Split liver transplantation in adults. World J Gastroenterol. 2016;22:7500-7506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Lee WC, Chan KM, Chou HS, Wu TJ, Lee CF, Soong RS, Wu TH, Lee CS. Feasibility of split liver transplantation for 2 adults in the model of end-stage liver disease era. Ann Surg. 2013;258:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Cuenca AG, Kim HB, Vakili K. Pediatric liver transplantation. Semin Pediatr Surg. 2017;26:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Reyes J, Gerber D, Mazariegos GV, Casavilla A, Sindhi R, Bueno J, Madariaga J, Fung JJ. Split-liver transplantation: a comparison of ex vivo and in situ techniques. J Pediatr Surg. 2000;35:283-9; discussion 289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Cherukuru R, Reddy MS, Shanmugam NP, Rajalingam R, Kota V, Gunasekaran V, Narasimhan G, Kaliamoorthy I, Rela M. Feasibility and Safety of Split-Liver Transplantation in a Nascent Framework of Deceased Donation. Liver Transpl. 2019;25:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Dalmau M, Gómez-Gavara C, Dopazo C, Molino JA, Caralt M, Bilbao I, Charco R, Hidalgo E. Left Lateral Sector In Situ Split Liver Transplantation Technique: Step-by-Step Video Demonstration. Transplant Proc. 2022;54:2511-2514. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Lau NS, Jacques A, McCaughan G, Crawford M, Liu K, Pulitano C. Addressing the challenges of split liver transplantation through technical advances. A systematic review. Transplant Rev (Orlando). 2021;35:100627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Lau NS, Ly M, Dennis C, Ewenson K, Ly H, Huang JL, Cabanes-Creus M, Chanda S, Wang C, Lisowski L, Liu K, Kench J, McCaughan G, Crawford M, Pulitano C. Liver splitting during normothermic machine perfusion: a novel method to combine the advantages of both in-situ and ex-vivo techniques. HPB (Oxford). 2023;25:543-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | Wan P, Li Q, Zhang J, Xia Q. Right lobe split liver transplantation versus whole liver transplantation in adult recipients: A systematic review and meta-analysis. Liver Transpl. 2015;21:928-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Mahamid A, Chen M, Sulimani O, Amodeo S, Facciuto L, Kozato A, Bekki Y, Schiano TD, Facciuto ME. The Importance of Segment 4 Anatomy on Outcomes Following Living Donor Left Lateral Segmentectomy. J Surg Res. 2023;285:13-19. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Shankar S, Rammohan A, Rela M, Srinivasan P. Surgical anatomy of segment four of liver and its implications in hepato-biliary surgery and liver transplantation. J Liver Transpl. 2022;6:100076. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Azoulay D, Castaing D, Adam R, Savier E, Delvart V, Karam V, Ming BY, Dannaoui M, Krissat J, Bismuth H. Split-liver transplantation for two adult recipients: feasibility and long-term outcomes. Ann Surg. 2001;233:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |