Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1629
Revised: April 23, 2024
Accepted: April 28, 2024
Published online: June 27, 2024
Processing time: 162 Days and 9.9 Hours
Upper gastrointestinal (GI) signet ring cell carcinomas (SRCC) confer a poor prognosis. The benefit of operative intervention for this patient group is controversial in terms of overall survival.
To investigate factors relating to survival in patients with upper GI SRCC.
A retrospective, tertiary, single-centre review of patients who were diagnosed with oesophageal, gastroesophageal junction and gastric SRCC was performed. The primary outcome was to compare mortality of patients who underwent operative management with those who had nonoperative management. Secon
One hundred and thirty-one patients were included. The one-year survival for the operative group was 81% and for the nonoperative group was 19.1%. The five-year survival in the operative group was 28.6% vs 1.5% in the nonoperative group. The difference in overall survival between groups was statistically significant (HR 0.19, 95%CI (0.13-0.30), P < 0.001). There was no difference in survival when ad
Well-selected patients with upper GI SRCC appear to have reasonable medium-term survival following surgery. Offering surgery to a carefully selected patient group may improve the outcome for this disease.
Core Tip: This retrospective review confirms that in a select group of patients, surgical management for upper gastro
- Citation: Grinlinton M, Furkert C, Maurice A, Angelo N, Booth M. Gastroesophageal signet ring cell carcinoma morbidity and mortality: A retrospective review. World J Gastrointest Surg 2024; 16(6): 1629-1636
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1629.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1629
Signet ring cell carcinomas (SRCC) are a rare and aggressive subtype of adenocarcinoma, found most frequently in the stomach. SRCC has specific oncogenesis and phenotypic and treatment resistance heterogeneity. The 2019 WHO classification defines poorly cohesive SRCC as a histological subtype of malignant gastric epithelial tumours[1]. It is characterised by prominent cytoplasmic mucin within the cells and an eccentrically placed crescent-shaped nucleus. The Lauren classification morphologically categorises gastric tumours as ‘intestinal’ or ‘diffuse’[2]. Intestinal tumours tend to be more organised, and arrange themselves into tubular or glandular structures. Diffuse tumours include SRCC, and are less di
Oesophageal SRCC is less common than gastric SRCC, ranging from 0.6%-9% of all oesophageal adenocarcinomas[3]. Oesophageal SRCC is an aggressive disease with poor chemoradiation response and diminished survival. The incidence of gastric SRCC has increased by more than 400% in the United States since the 1970s[4]. SRCC is estimated to account for 33%-71% of gastric adenocarcinomas[5,6].
Operative management for patients with SRCC of the oesophagus and stomach is generally viewed as the standard of care. However, survival is low and response to chemotherapy is typically poor in this subgroup of patients. Given its historical associations as a negative predictive factor in terms of mortality, the primary outcome was to identify whether operative management improves the prognosis of patients with oesophageal, gastroesophageal junction (GOJ) and gastric SRCC. Secondary outcomes were to identify exact characteristics that determine patient prognosis.
All patients diagnosed with histologically confirmed oesophageal, GOJ or gastric SRCC between January 2010 and December 2020 were included for review from a single, tertiary surgical unit. The study was approved by the New Zea
Clinical and pathological staging were performed in accordance to the American Joint Committee on Cancer Staging Manual and Handbook (Seventh Edition)[7]. Patient management was determined after discussion at a multidisciplinary meeting with a panel of experts. Appropriate patients with locally advanced cancer were treated with neoadjuvant che
The initial clinical stage was compared with the final pathologic stage to determine response to induction therapy. Histopathological response to chemotherapy was evaluated in the final surgical specimen by using tumour regression grade. Operative management depended on tumour location. 45 patients underwent a partial or total gastrectomy, 14 patients had an Ivor-Lewis oesophagectomy, three patients had a palliative operation such as a bypass procedure, and one patient had an endoscopic resection. Palliative bypass patients were included in the operative group.
Patients were analysed on an intention-to-treat basis. T-test and chi-square tests were used to determine differences in baseline characteristics between the operative and nonoperative groups. Survival analysis using the log-rank test for univariate comparisons and Cox regression for multivariate analysis was used to determine differences in overall sur
One hundred and thirty-one patients were identified in the 10-year period with oesophageal, GOJ or gastric SRCC. The majority of these were gastric (67.2%), followed by oesophageal (21.4%) and GOJ (12.2%) cancers. Patient demographics are summarised in Table 1. 52 patients were women and 79 were men. The mean patient age was 63.3 years. 63 (48%) patients underwent operative intervention. The average intensive care unit and total length of stay were 2.6 d and 16.1 d. Of the 68 patients who did not undergo resection, 47 (69.1%) were due to inoperable disease, 11 (16.2%) were deemed unfit for surgery due to comorbidities, seven (10.3%) patients declined operative management, two (2.9%) patients had an acute deterioration and died while waiting for an operation, and one (1.5%) reason was unknown. The Charlson Co
| Variables | Operative group (N = 63) | Nonoperative group (N = 68) | P value |
| Demographics | |||
| Age | 63.3 | 66.7 | |
| Age > 50 yr, n (%) | 57 (90.5) | 53 (78.0) | |
| Male sex, n (%) | 37 (58.7) | 42 (61.8) | |
| Ethnicity | |||
| European | 33 | 44 | |
| Asian | 15 | 4 | 0.013 |
| Pacific Peoples | 6 | 8 | |
| Māori | 7 | 10 | |
| MELAA | 0 | 2 | 0.004 |
| Unknown | 2 | 0 | |
| Tumour location | |||
| Oesophageal | 9 | 19 | |
| Gastro-oesophageal | 10 | 6 | |
| Gastric | 44 | 43 | |
| Pathological stage | |||
| I | 18 | 11 | |
| II | 10 | 6 | |
| III | 30 | 5 | |
| IV | 5 | 46 | < 0.001 |
| Current smoker | 9 | 11 | |
| Charlson Comorbidity Index | 6.5 ± 2.1 | 7.7 ± 2.5 | 0.0073 |
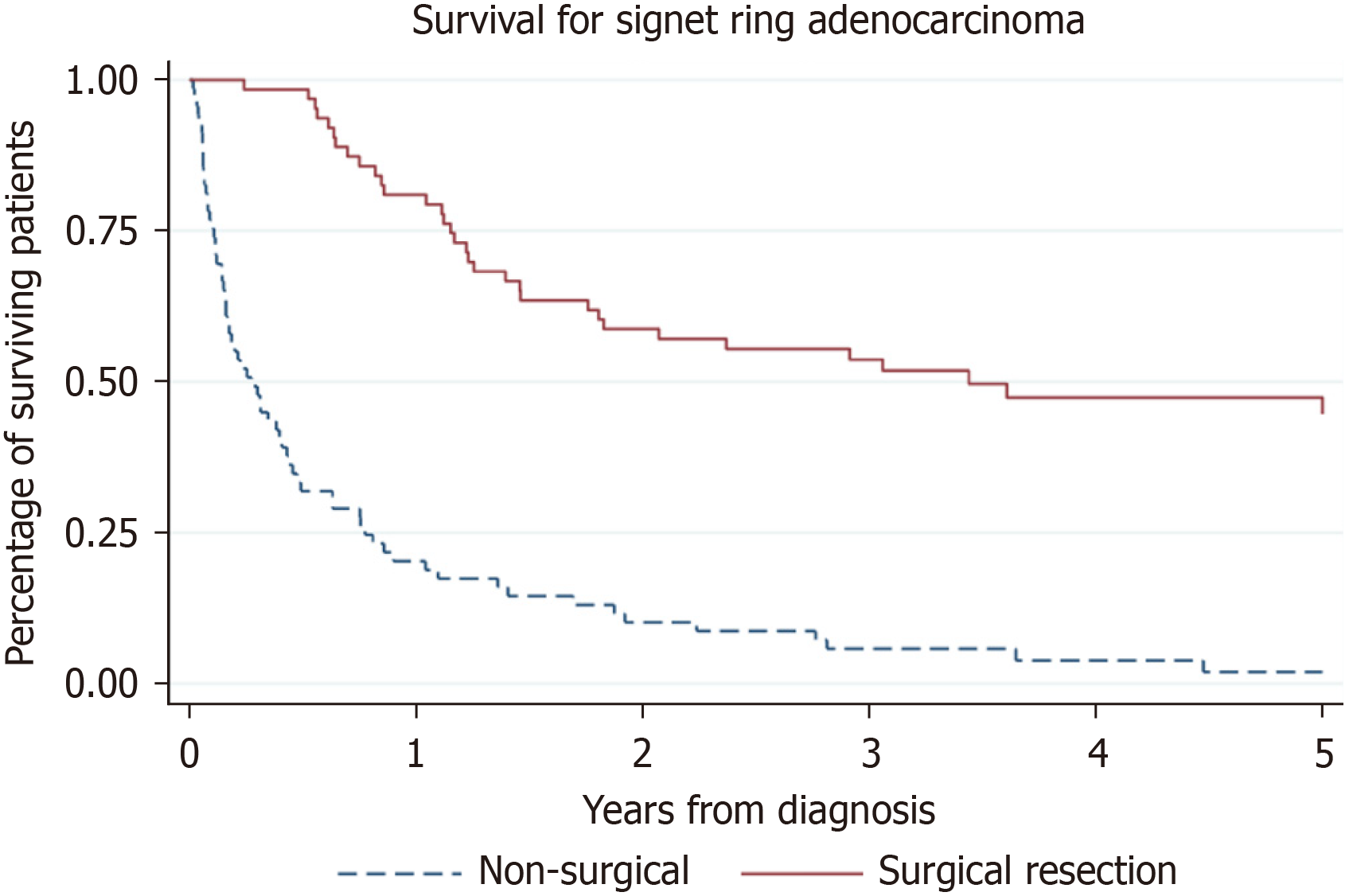
Factors were analysed to identify if they contributed to patient survival. This is summarised in Table 2. The mean overall survival for all patients from the date of diagnosis was 2.1 years, and the median survival was 10.5 months. In the operative group, the mean survival was 3.2 years and the median survival was 2.5 years. In the nonoperative group, the mean survival was 22.4 months and the median survival was 3.3 months. 26/63 (41.3%) patients were reported to have a recurrence. The mean time from the date of operation to the date of recurrence was 18.1 months. The mean length of survival from date of recurrence was 7.6 months. Only 3/63 (4.8%) patients who had a documented recurrence survived within the time period of follow-up.
| Univariate analysis - factors associated with survival | ||
| Demographic factors | Hazard ratio | P value |
| Age | 1.003 (0.99-1.02) | |
| Male | 0.93 (0.63-1.38) | |
| Smoker | 1.12 (0.63-1.98) | |
| European | 1.24 (0.84-1.84) | |
| Asian | 0.45 (0.24-0.84) | 0.013 |
| Pacific Peoples | 0.92 (0.50-1.69) | |
| Māori | 1.35 (0.78-2.34) | |
| MELAA | 11.3 (2.62-48.64) | 0.001 |
| Unknown | 0.66 (0.16-2.76) | |
| Surgery | 0.20 (0.13-0.30) | < 0.001 |
| Stage IV | 5.13 (3.35-7.87) | < 0.001 |
| Stage (overall) | 2.23 (1.78-2.79) | < 0.001 |
| Charlson Comorbidity Index | 1.27 (1.18-1.38) | < 0.001 |
| Proportion of signet ring cells | 1.07 (0.72-1.58) | |
| Neoadjuvant chemotherapy | 1.87 (0.93-3.76) | |
| Multivariate analysis - factors associated with survival | ||
| Stage | 1.62 (1.30-2.01) | < 0.001 |
| Surgery | 0.27 (0.17-0.42) | < 0.001 |
| Charlson Comorbidity Index | 1.19 (1.08-1.30) | < 0.001 |
| Asian ethnicity | 0.75 (0.39-1.43) | |
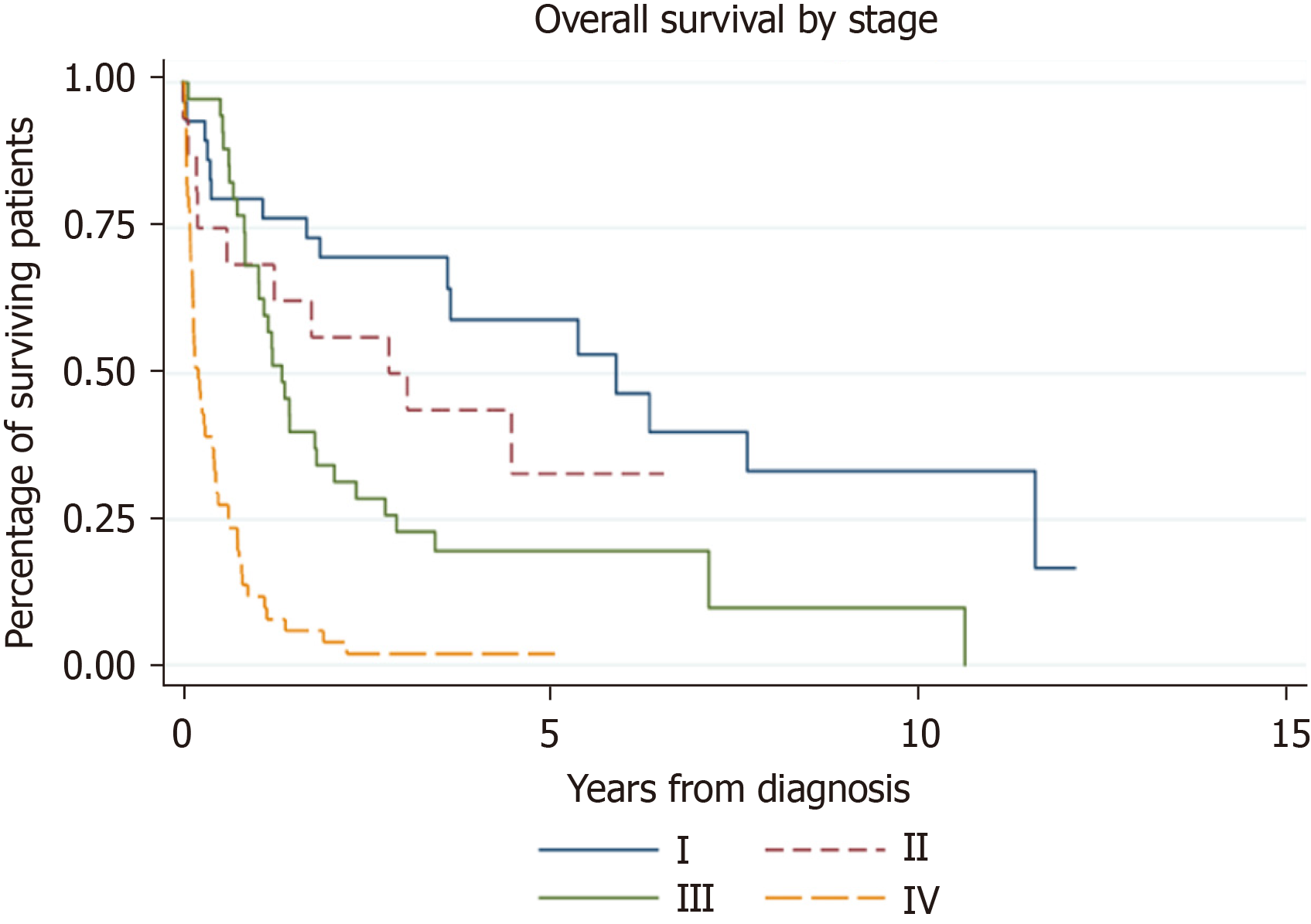
The one-year survival for the operative group was 81% vs 19.1% in the nonoperative group. The five-year survival in the operative group was 28.6% vs 1.5% in the nonoperative group. The difference in overall survival between groups was statistically significant [HR 0.19, 95%CI (0.13, 0.30), P < 0.001]. This is represented by Figures 1 and 2.
On univariate analysis, there was no statistically significant difference in survival when adjusting for age, smoking status or gender. Asian patients appeared to have improved survival when compared with other ethnicities [HR 0.45, 95%CI (0.24-0.84), P = 0.013]. There were no other statistically significant survival differences within other ethnic groups.
Survival of patients who received neoadjuvant chemotherapy prior to surgery was directly compared with those who did not. The survival effect of neoadjuvant chemotherapy in operative patients did not reach statistical significance [HR 1.87, 95%CI (0.93-3.76), P = 0.080]. The small number of patients limited further analysis.
Surgical samples were analysed to determine histological response to chemotherapy. The histopathological analysis of the 31 patients showed 13 specimens with more than 50% of residual tumour (grade 3), 12 specimens with 10%-50% residual tumour (grade 2), four with less than 10% residual tumour (grade 1), and in two patients the response was not recorded. Neoadjuvant chemotherapy was therefore only partially or completely effective in 51.6% of selected operative candidates.
On multivariate analysis, patients who underwent surgical management, those with a lower stage of disease, and those with a lower CCI had significantly improved survival when accounting for other confounding factors.
Multivariate analysis was performed comparing operative with nonoperative groups, adjusting for the presence of stage IV disease as well as CCI. This found that even when adjusting for stage IV disease, the operative group had improved survival (P = 0.031). This would suggest that surgery may confer benefit, even after the confounding effect of stage, metastatic disease and patient comorbidities is considered.
Asian ethnicity was no longer significant after multivariate analysis. This may suggest that Asian patients presented with an earlier stage of disease, that they were more likely to have operative management, or that they benefited more from surgery than other ethnicities.
This study found that operative management of gastric, oesophageal and GOJ SRCCs appeared to be beneficial in a select group of patients. Patients with upper GI SRCC appear to benefit from surgical intervention, regardless of whether the tumour arises from the oesophagus or stomach. Analysing oesophageal, gastric and GOJ SRCCs separately may give more information about tumour-specific prognosis. However, dividing these patients further into subgroups limits meaningful analysis and results would be underpowered due to small numbers. In this study there was no significant difference in survival depending on location of the tumour. This suggests that the current paradigm of primary operative management in this group of patients is appropriate both in gastric and oesophageal tumours.
SRCC is a rare pathological entity that is increasing in frequency worldwide. SRCC histology is an independent predictor of poor prognosis when compared with adenocarcinoma not otherwise specified (NOS) of the GI tract. Patients diagnosed with SRCC are typically younger and present with more advanced disease. Surgery and systemic therapy are understood to improve survival, although this is less successful than in NOS patients[8].
There is a paucity of literature directly comparing oesophageal and gastric SRCC. Piessen et al[9] found that gastric SRCCs were more likely to have metastasised to peritoneal tissues at the time of diagnosis, but are associated with a better prognosis than oesophageal SRCCs.
Gastric SRCCs are aggressive tumours with a poor prognosis. Studies have found that gastric SRCCs are more infiltrative, diagnosed at a more advanced stage, have a greater incidence of lymph node metastases and peritoneal spread, and are more chemoresistant when compared with gastric adenocarcinoma NOS[5,6,9,10].
A recent study found that signet ring histology was an independent predictor for poor survival, and that perioperative chemotherapy for gastric SRCC provided no survival benefit[6]. Neoadjuvant chemotherapy also appeared to have a li
Despite these findings, there is ongoing controversy in the literature about whether SRCC is consistently a negative prognostic factor. Research is often restricted to noncomparative retrospective reviews, which can limit meaningful interpretation. A meta-analysis by Nie et al[11] found that SRCC may behave differently in early vs advanced gastric cancer, portending a better prognosis in early disease and a worse prognosis in advanced disease.
Multiple reports in Asian countries have found that SRCC features in gastric cancer were not found to be predictive of poor prognosis, and may even be associated with a better survival[12,13]. This finding is seen specifically in early gastric cancer, and may be because of lead time bias, or younger age at presentation[14].
A large database study in the United States of 10246 patients with gastric cancer found that patients with SRCC were more likely to present at stage 3-4, have lymph node spread and distant metastases, but did not have a worse prognosis[15]. The study also noted that Asian ethnicity appeared to confer a survival advantage. This was also seen in our cohort in the univariate, but not multivariate analysis. These results may suggest molecular or genetic factors that confer a survival benefit, rather than environmental factors such as screening programs and improved access to endoscopy.
Oesophageal SRCC is less well studied than gastric SRCC due to its rarity. Multiple studies support the finding that oesophageal SRCC carries a worse prognosis when compared with adenocarcinoma NOS, regardless of stage at pre
This study is limited by its retrospective nature and the fact that it was carried out at a single institution. Known con
Improved survival was seen even after accounting for stage, metastatic disease and CCI. This would suggest that there is a true possible survival benefit of surgery independent of patient factors, however selection bias may account for this.
Another limitation of this study is its record of patient deaths. This is dependent on the accuracy of the institutional regional electronic records used to access patient information. If a patient had moved to a different location, their death may not have been captured. However, this limitation applied equally to both operative and nonoperative groups, which reduces potential bias.
Despite these limitations, this study is one of the few in Australasia to analyse the outcomes of patients with this rare disease. This is one of the largest cohorts described for this illness in Aotearoa New Zealand. This adds to the sparse body of existing surgical literature on upper GI SRCC. The results of this study aim to improve evidence-based local clinical practice.
This study supports the decision to pursue operative management in a well-selected group of patients with upper GI SRCC. Operative intervention appeared to result in an improved survival for these patients. The survival advantage extended to at least five years. Age, smoking status and gender had no correlation with survival. Earlier tumour stage, and a lower CCI were other significant factors associated with improved survival. Despite the aggressive nature, high recurrence rate and subsequent mortality of SRCC, operative management should be considered as the first line strategy in appropriate patients with upper GI SRCC. Long term survival is possible in appropriately selected patients.
We would like to acknowledge the work of the Pathology Department at North Shore Hospital, and the patients who made this study possible.
| 1. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2438] [Article Influence: 487.6] [Reference Citation Analysis (3)] |
| 2. | LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4323] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 3. | Bleaney CW, Barrow M, Hayes S, Ang Y. The relevance and implications of signet-ring cell adenocarcinoma of the oesophagus. J Clin Pathol. 2018;71:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 301] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250:878-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 6. | Messager M, Lefevre JH, Pichot-Delahaye V, Souadka A, Piessen G, Mariette C; FREGAT working group - FRENCH. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg. 2011;254:684-93; discussion 693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Frederick LG, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC cancer staging manual. Springer Science & Business Media; 2002. Available from: https://www.facs.org/media/j30havyf/ajcc_7thed_cancer_staging_manual.pdf. |
| 8. | Franko J, Le VH, Tee MC, Lin M, Sedinkin J, Raman S, Frankova D. Signet ring cell carcinoma of the gastrointestinal tract: National trends on treatment effects and prognostic outcomes. Cancer Treat Res Commun. 2021;29:100475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Piessen G, Messager M, Lefevre JH, Goéré D, Mabrut JY, Meunier B, Brigand C, Hamy A, Glehen O, Mariette C; FREGAT Working Group – FRENCH. Signet ring cell adenocarcinomas: different clinical-pathological characteristics of oesophageal and gastric locations. Eur J Surg Oncol. 2014;40:1746-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Liu X, Cai H, Sheng W, Yu L, Long Z, Shi Y, Wang Y. Clinicopathological Characteristics and Survival Outcomes of Primary Signet Ring Cell Carcinoma in the Stomach: Retrospective Analysis of Single Center Database. PLoS One. 2015;10:e0144420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Nie RC, Yuan SQ, Li YF, Chen YM, Chen XJ, Zhu BY, Xu LP, Zhou ZW, Chen S, Chen YB. Clinicopathological Characteristics and Prognostic Value of Signet Ring Cells in Gastric Carcinoma: A Meta-Analysis. J Cancer. 2017;8:3396-3404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Kao YC, Fang WL, Wang RF, Li AF, Yang MH, Wu CW, Shyr YM, Huang KH. Clinicopathological differences in signet ring cell adenocarcinoma between early and advanced gastric cancer. Gastric Cancer. 2019;22:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Gronnier C, Messager M, Robb WB, Thiebot T, Louis D, Luc G, Piessen G, Mariette C; FREGAT working group-FRENCH. Is the negative prognostic impact of signet ring cell histology maintained in early gastric adenocarcinoma? Surgery. 2013;154:1093-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21:11428-11438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 178] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (3)] |
| 15. | Taghavi S, Jayarajan SN, Davey A, Willis AI. Prognostic significance of signet ring gastric cancer. J Clin Oncol. 2012;30:3493-3498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 16. | Yendamuri S, Huang M, Malhotra U, Warren GW, Bogner PN, Nwogu CE, Groman A, Demmy TL. Prognostic implications of signet ring cell histology in esophageal adenocarcinoma. Cancer. 2013;119:3156-3161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Nafteux PR, Lerut TE, Villeneuve PJ, Dhaenens JM, De Hertogh G, Moons J, Coosemans WJ, Van Veer HG, De Leyn PR. Signet ring cells in esophageal and gastroesophageal junction carcinomas have a more aggressive biological behavior. Ann Surg. 2014;260:1023-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Enlow JM, Denlinger CE, Stroud MR, Ralston JS, Reed CE. Adenocarcinoma of the esophagus with signet ring cell features portends a poor prognosis. Ann Thorac Surg. 2013;96:1927-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |